ISSN: 0973-7510
E-ISSN: 2581-690X
Magnesium (Mg) is an essential macronutrient that can be obtained through mineralization of mine spoils. The leftover mine spoils of magnesite mines still contain a reliable amount of Mg in it. The Mg present in raw magnesite spoils is in its carbonate form and hence it has to be mineralized to convert it into plant available soluble forms of Mg. The effect of B. cereus and B. stercoris in the mineralization of Mg has been studied in synthetic mineral salts medium (MSM). To obtain maximum mineralization results it is important to know the optimal conditions of the organisms under which they can grow and produce more Mg. The mineralizing capability of the individual organisms and their combined effect as a consortium under various concentrations of carbon source, pH, temperature and soil organic matter has been studied. It has been seen that the organisms grow and mineralize better when 1% of glucose has been supplemented as carbon source. The optimal pH and temperature were found to be pH 7 and 35°C, respectively. The addition of anthraquinone-2-sulphonic acid (ADQS) as soil organic matter enhances the mineralization Mg in synthetic medium. Rendering to SEM and EDX analysis, the mineralization of Mg in the synthetic medium was established.
Mineralization, Mine Spoils, Magnesium, B. cereus and B. stercoris
Magnesite is an essential mineral ore, which is extensively used in manufacturing refractory materials used by steel industry. Despite its usefulness, the extractive operations and mineral processing wastes of mining are associated with a variety of environmental and social impacts.1,2 Salem district is a major sector of magnesite mining in Tamil Nadu, India. As the demand for magnesite increases, it results in a large excavation of landscape and maximize the dumping of wastes as spoils or mine tailing. The ore processing wastes of magnesite mine spoils comprise with high variety of minerals. The characteristic features of mine wastes might also assist in determining their suitability for alternative uses.3 One such use of mine spoils is the extraction of leftover minerals from the spoils to form stable outcomes that comprise of readily available minerals to plants. For exploitation of mine wastes, the extraction process can be achieved through mineralization.
The degradation of an initial compound to attain a mineral compound, is a well-defined process of mineralization. The composition of the initial compound determines the release of mineral compounds through mineralization.4,5 Mineralization in soil is greatly enhanced by the activity of microbes which oxidize or decompose the initial compounds into easily available minerals to plants.6 This process can be termed as biomineralization. Harnessing microbes improves rehabilitation outcomes and transform mine spoils.7,8
Through mineralization of mine spoils many plants are obtained available minerals. One such essential mineral obtained is Mg. It is a macronutrient that plays an important role in plant shoot-root formation9 and photosynthetic process.10 The effect of various crops and plant species 11-14 and its role in plant physiology15,16 has been widely studied. Mg deficiency causes various physiological and morphological deformities in plants,17-20 and may also cause enzymatic alterations in plants.21
This essential mineral has almost become a forgotten element in crop production.22 To overcome this situation, in this study, the initial compound that is taken into consideration for mineralization is the Mg carbonate present in the mine tailing or spoils of magnesite mines. The water insoluble carbonate forms of Mg have been solubilized or mineralized into water soluble plant available forms of Mg. This is attained with the help of two bacterial species namely, Bacillus cereus and Bacillus stercoris are isolated from the natural soil. The ability of these two organisms individually as well as a consortium to mineralize Mg has been studied. Moreover, the optimum conditions under which maximum mineralization and growth can be obtained is being discussed.
Bacterial species and purification
Two potential bacterial isolates, Bacillus cereus (MS2) and Bacillus stercoris (MS76) that are capable of mineralizing Mg carbonate into available Mg were obtained from Bioremediation Laboratory of Periyar University, Salem. The strains were preserved as pure isolates in nutrient agar slants for further studies.
Antagonistic activity between the potential strains
The bacterial isolates B. cereus and B. stercoris were tested for their antagonistic activity against each other through agar well diffusion method. Muller Hinton Agar (MHA) plates were prepared, poured into sterile Petri dishes and solidified. To one of the plates, B. cereus was swabbed using a sterile cotton swab and a well punctured in the agar plate was inoculated with B. stercoris culture. Similarly, on another plate the cultures were inoculated vise-versa. The plates were then incubated at 35°C for 24 hours and the results were observed.
Utilization of carbon source
B. cereus, B. stercoris and a consortium of both bacterial strains were tested for their growth under different carbon sources. About 100 ml of Mineral Salt Medium (MSM) containing (g/l) 1.7g of NaCl, 0.3g of KCl, 0.15g of NH4Cl, 0.2g of K2HPO4, 0.07g of Na2SO4, 2g of NaHCO3 and 0.4g of Yeast extract was prepared. The medium was sterilized with the supplementation of two different carbon sources namely, 1% starch and 1% glucose. To the sterile MSM, 1% of B. cereus, B. stercoris and bacterial consortium were inoculated respectively. A control flask was maintained without any carbon source. All the flasks were incubated under shaking condition at 120 rpm and 35°C. At an interval of every 24 hours, 10 ml of sample was drawn aseptically and were centrifuged at 4000 rpm for 20 minutes. The pellets obtained were dissolved in double distilled water and the suspension made were observed as bacterial growth at 600nm using UV-vis spectrophotometer. The results were noted for a period of 10 days.
Synthesis of magnesium carbonate
Magnesium carbonate was synthesized by adding equal volumes of 0.5M of magnesium sulphate solution and 0.5M of sodium carbonate solution. The solutions were mixed thoroughly and was left to stand for 2 hours. When aqueous magnesium sulphate reacts with aqueous sodium carbonate, a white precipitate of magnesium carbonate will be formed along with aqueous sodium sulphate. The chemical reaction that takes place during this process as expressed,
MgSO4(aq) + Na2CO3(aq) = MgCO3(p) + Na2SO4(aq)
The precipitate thus formed was collected, air dried and were powdered. This powdered magnesium carbonate was insoluble in water and used it as mine sector resource for further studies. The magnesium oxide synthetically obtained was similar feature as the mine wastes magnesite collected from Salem District of Tamil Nadu, India.
Standardization of Mg
Mg in the sample solution was known using standard Eriochrome Black T (EBT) method.23 The reagents required were ammonia buffer (25 ml of 25% ammonia solution, 25 ml of d.H2O and 0.5g of NH4Cl) and Eriochrome Black T indicator (0.1% of EBT dissolved in methanol). To obtain a standard graph, 9.1 ml of known concentration of Mg sample was taken to which 0.8 ml of buffer solution and 0.1 ml of the EBT indicator solution was added. Reagents added in dd.H2O was kept as blank solution. The intensity of the color variation for each concentration of Mg and their absorbance at 530nm was noted using UV-vis spectrophotometer. Thus, a standard graph with repression value (R2) of 0.9 was obtained.
Optimal conditions for mineralization of Mg and growth of bacteria
Optimal conditions for Mg mineralization and its corresponding bacterial growth were determined under various concentrations of glucose (0.5, 1.0 and 1.5%), pH (3, 5, 7, 9 and 11), temperature (25, 30, 35, 40 and 45oC) and different soil organic matter (Humic acid and ADQS-anthraquinone-2-sulphonic acid).
About 0.5% of synthesized magnesium carbonate was incorporated in MSM along with 1% of bacterial inoculum and were subjected to grow and mineralize under various conditions as stated above. This study was carried out under batch condition for a period of 10 days. In which, after every 24 hours, 10 ml of samples were drawn aseptically and were centrifuged at 4000rpm for 20 minutes. The supernatant of the centrifuged samples was tested for the amount of soluble concentration of Mg and its concentration of variations each day by following the EBT method of determination. The pellets obtained were dissolved in double distilled H2O, and the growth of the organism was determined at 600nm using UV-vis spectrophotometer. The mineralizing ability and growth conditions were examined separately for B. cereus, B. stercoris and bacterial consortium.
SEM and EDX Analysis of Mg obtained from MSM
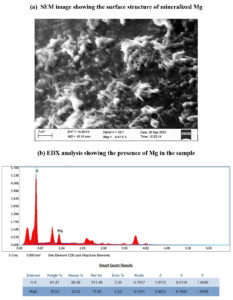
Under optimum condition the bacterial consortium has showed better results in mineralizing Mg in the MSM supplemented with MgCO3. Therefore, the structure and presence of soluble Mg obtained from the medium by the action of bacterial consortium was tested through scanning electron microscopy (SEM) and energy dispersive X-ray (EDX) analysis. Using SEM, the size of the Mg mineral, morphology and texture of the crude crystals were observed. Subsequently, EDX was used to determine the presence of Mg in elemental form in the specimens.
The bacterial strains, Bacillus cereus (MS2) and Bacillus stercoris (MS76) that are proficient to Mg mineralization in synthetic MSM were studied under batch conditions. To obtain maximum mineralization in natural resources, it is important to know the optimal conditions of the organisms under which they can produce more Mg. The mineralizing capability of the MS2 and MS76 and their effect as a consortium under various concentrations of carbon source, pH, temperature and soil organic matter has been studied.
Antagonistic activity between the potential isolates
The plates inoculated with the bacterial isolates were observed after 24 hours. No zone of clearance was observed in the plates, resulting that the organisms are capable of growing in symbiotic relationship with each other. Therefore, B. cereus (MS2) and B. stercoris (MS76) were also used as a consortium of bacterial species in further studies.
Utilization of carbon source
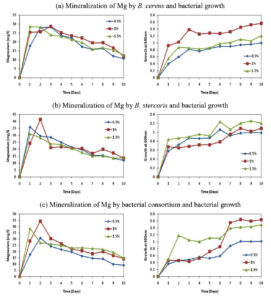
The growth of bacterial isolates was noted on daily basis using UV-vis spectrophotometer at 600nm and the results were shown in Figure 1. The results showed that the growth of B. cereus, B. stercoris and consortium were better and higher in glucose when starch was supplemented as a carbon source. The growth of the organisms without carbon source was relatively lesser. Hence, glucose was selected as a sole carbon source for both the isolates as well as for bacterial consortium.
Optimum glucose concentration on mineralization of Mg and bacterial growth
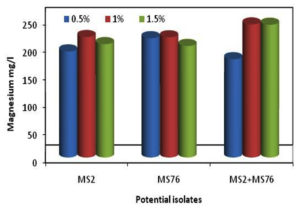
The bacterial isolates B. cereus, B. stercoris and consortium were subjected to various concentrations of glucose (0.5, 1.0 and 1.5%) mineralization of Mg. The Figure 2 shows the mineralization of Mg, in which it was seen that the Mg concentration in the samples was constantly increasing for the first three days and later the concentration of Mg started to decrease. But the growth of the organisms was constantly increasing and attained stationary phase after the 7th day of incubation as shown in Figure 2. While calculating the total mineralization of Mg in 10 days, B. cereus, B. stercoris and consortium were able to produce more Mg when 1% of glucose was supplemented (Figure 3). The other concentrations of glucose (0.5 and 1.5%) showed comparatively less Mg concentration. Hence, 1% of glucose supplementation is noted as the optimal concentration of glucose as carbon source.
Figure 2. Effect of glucose at varying concentrations on the mineralization of Mg and bacterial growth
Figure 3. Total Mg minerals obtained from MSM supplemented with glucose at varying concentrations after 10th day
Optimum pH on mineralization of Mg and bacterial growth
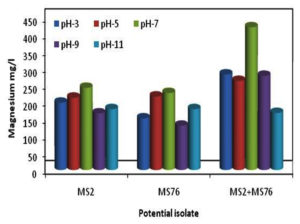
The isolates were tested at various pH (3, 5, 7, 9 and 11) for their ability to mineralize Mg and their growth parameters. As shown in Figure 4, pH 7 showed greater Mg concentration compared to other pH levels. The growth of the organisms as shown in Figure 4 revealed that the growth was also maximum at pH 7. B. cereus showed less growth at pH 5 and least mineralization at pH 9. In the case of B. stercoris showed less growth at pH 3 and less mineralization at pH 9. Consortium showed least growth and mineralization at pH 11 (Figure 5). The optimum pH for magnesium mineralization and growth of the organisms is
pH 7.
Optimum temperature on mineralization of Mg and bacterial growth
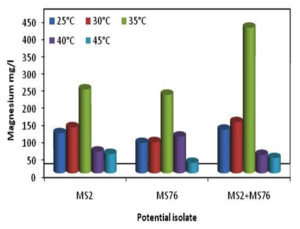
The study carried out among the various temperatures (25, 30, 35, 40 and 45°C) with B. cereus, B. stercoris and bacterial consortium, the results showed maximum mineralization rates of Mg under 35°C and least mineralization at 45°C (Figure 6). The growth of the organisms was also maximum under 35°C and all the other temperatures showed relatively lesser growth. Figure 7 depicts the overall concentration of Mg obtained in 10 days. From the results it can be supposed that 35°C is the optimum temperature for the bacterial growth and Mg mineralizing capability.
Optimum soil organic matters on mineralization of Mg and bacterial growth
In addition to carbon source, humic acid (HA) and anthraquinone-2-sulphonic acid (ADQS) were added as soil organic matter. The addition of these substances was done to see whether these substances could increase the growth of the organism and corresponding Mg concentration in the medium. It was noticed that the mineralization of Mg increased when ADQS was added along with the carbon source (Figure 8 and Figure 9). The addition of ADQS also increased the growth of the organisms. Hence, the results illustrated that Mg concentration and the growth of the organisms can be increased with the addition of ADQS.
SEM and EDX analysis of Mg from MSM
The structure and presence of soluble Mg obtained from the MSM medium by the action of bacterial consortium were tested through SEM and EDX analysis. Figure 10 displays that SEM and EDX analysis of the sample showing the presence and surface structure of mineralized Mg. SEM observation at 3µm showed that the presence of Mg mineral of strong crystals with rough texture. EDX measurement revealed that the samples obtained from the MSM contained Mg, O and trace elements. As depicted in the image, the Mg peak obtained through EDX analysis confirms that the presence of Mg in the sample after mineralization.
B. cereus and B. stercoris are the two potential bacterial isolates that are capable of mineralizing MgCO3 into soluble forms of Mg are being used in the present study. It has been known that even in the previous studies, Bacillus sp. has been a dominant species in solubilizing minerals24 and is the most common species that are capable of solubilizing agriculturally important minerals.25-27 Bacillus sp. is also used in solubilizing magnesium, calcium and zinc.28 Moreover, Bacillus sp. is a major type of bacteria that can survive under harsh environmental condition and can promote crop productivity.28
It has been found B. cereus and B. stercoris can form a symbiotic relationship with each other and hence they can also be used as consortium. The organisms showed better growth when glucose was supplemented in the medium. Hence, glucose was selected as sole carbon source. The concentration of glucose under which enhanced growth and magnesium was obtained was when 1% of glucose was supplemented. Similarly, enhanced results were obtained for B. cereus and B. stercoris when the medium was maintained at pH 7 and the temperature of 35°C. The optimal condition of B. cereus growth as reported by Oualha et al.29 is pH from 7 to 8 and a wide range of temperature ranging from 30°C to 42°C. The optimum growth parameter for B. stercoris has been reported as pH 7 and 35°C.30 Moreover, it has also been said that the mobility of Mg in soils and plants are strongly influenced by pH. The pH around 7 to 7.5 is reported to be effective and acidic pH (<6) reduces the mobility of magnesium.17
In the present study, it has been seen that the mineralizing ability of B. cereus, B. stercoris and the consortium was increased with the addition of soil organic matter anthraquinone-2-sulphonic acid (ADQS). In a previous study, the dissolution of heavy metals has been enhanced in the presence of glucose and ADQS.31,32 While noticing the results of B. cereus, B. stercoris and the consortium under various conditions, all the results showed an increasing concentration of magnesium till the 3rd day and after which the concentration decreases. But the growth of the organisms is constantly in its increase phase and attains stationary phase after the 7th day. This might be because of that the mineralized magnesium must have been utilized by the organisms for their growth. Groisman et al.,33 has reported that bacteria have special Mg2+ transporter mechanisms that sense the levels of Mg2+ and maintain homeostasis in the bacterial cell. The presence of Mg in the mineralized sample was tested with SEM and EDX. The results represented in the image, the Mg gathering and the peak obtained through SEM and EDX confirm that the presence of Mg in the sample after mineralization.
The present experiment was conducted to know the optimal condition under which B. cereus, B. stercoris and consortium could produce more magnesium. It was found that glucose at 1%, pH7 and a temperature of 35°C with addition of ADQS can grow and produce more magnesium in the surrounding medium. Thus, a consortium of B. cereus and B. stercoris are capable of producing magnesium in the surrounding environment in the presence of magnesium carbonate and under all the optimum conditions. SEM and EDX clearly shown on its confirmation that the significant mineralization of Mg from synthetic medium. Therefore, utilizing B. cereus and B. stercoris can serve as an alternative mechanism to rehabilitate mine spoils as well as to extract an essential macronutrient magnesium from the mine spoils under favorable conditions. It is concluded that, although Mg is naturally present in soils its low solubility makes Mg as a less available element in the surrounding. Hence, it is necessary to increase Mg solubility as a mineral for the growth and development of plants and other living organisms.
ACKNOWLEDGMENTS
The authors would like to thank FIST grants of the Department of Science and Technology, New Delhi, India, and the University Research Fellowship, Periyar University, Salem, India, for financial support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
The research was helped with FIST grants of the Department of Science and Technology, New Delhi (Ref No. SR/FST/LSI-640/2015(C) Dt. 30/5/2016) and University Research Fellowship granted by Periyar University, Salem.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Azapagic A. Developing a framework for sustainable development indicators for the mining and minerals industry. J Clean Prod. 2004;12(6):639-662.
Crossref - Franks DM, Boge DV, Cote CM, Mulligan DR. Sustainable development principles for the disposal of mining and mineral processing wastes. Resour Policy. 2011;36(2):114-122.
Crossref - Mhlongo SE, Amponsah-Dacosta F, Mphephu NF. Rehabilitation prioritization of abandoned mines and its application to Nyala magnesite mine. J African Earth Sci. 2013;88:53-61.
Crossref - Knapp JS, Bromley-Challoner KCA. Recalcitrant organic compounds. Handbook of Water and Wastewater Microbiology. 2003:559-595.
Crossref - Hussain I, Lamiel C, Sahoo S, et al. Factors affecting the growth formation of nanostructures and their impact on electrode materials. Materials Today Physics. 2022;27:100844.
Crossref - Sharma K, Garg VK. Solid state fermentation for vermicomposting:a step toward sustainable and healthy soil. Curr Dev Biotech Bioeng. 2018:373-413.
Crossref - Zornoza R, Acosta JA, Faz A, Baath E. Microbial growth and community structure in acid mine soils after addition of different amendments for soil reclamation. Geoderma. 2016;272:64-72.
Crossref - da Silva GOA, Southam G, Gagen E.J. Accelerating soil aggregate formation:a review on microbial processes as the critical step in a post-mining rehabilitation context. Soil Research. 2022;61(3):209-223.
Crossref - Cakmak I. Magnesium in crop production, food quality and human health. Plant Soil. 2013;368(1-2):1-4.
Crossref - Hauer-Jakli M, Trankner M. Critical leaf magnesium thresholds and the impact of magnesium on plant growth and photo-oxidative defense:a systematic review and meta-analysis from 70 years of research. Front Plant Sci. 2019;10:00766.
Crossref - Huang Y, Jiao Y, Nawaz, MA, et al. Improving magnesium uptake, photosynthesis and antioxidant enzyme activities of watermelon by grafting onto pumpkin rootstock under low magnesium. Plant Soil. 2016;409:229-246.
Crossref - Yang N, Jiang J, Xie H, et al. Metabolomics reveals distinct carbon and nitrogen metabolic responses to magnesium deficiency in leaves and roots of Soybean [Glycine max (Linn.) Merr.]. Front Plant Sci. 2017;8:1-12.
Crossref - da Silva DM, de Souza KRD, Vilas Boas LV, Alves YS, Alves JD. The effect of magnesium nutrition on the antioxidant response of coffee seedlings under heat stress. Sci Hortic. 2017;224:115-125.
Crossref - Rehman H, urAlharby HF, Alzahrani Y, Rady MM. Magnesium and organic biostimulant integrative application induces physiological and biochemical changes in sunflower plants and its harvested progeny on sandy soil. Plant Physiol Biochem. 2018;126:97-105.
Crossref - Guo W, Nazim H, Liang Z, Yang D. Magnesium deficiency in plants:an urgent problem. Crop J. 2016;4(2):83-91.
Crossref - Chen ZC, Peng WT, Li J, Liao H. Functional dissection and transport mechanism of magnesium in plants. Semin Cell Dev Biol. 2018;74:142-152.
Crossref - Gransee A, Fuhrs H. Magnesium mobility in soils as a challenge for soil and plant analysis, magnesium fertilization and root uptake under adverse growth conditions. Plant Soil. 2013;368:5-21.
Crossref - da Silva DM, Brandao IR, Alves JD, de Santos MO, de Souza KRD, de Silveira HRO. Physiological and biochemical impacts of magnesium-deficiency in two cultivars of coffee. Plant Soil. 2014;382(1-2):133-150.
Crossref - Trankner M, Jakli B, Tavakol E, et al. Magnesium deficiency decreases biomass water-use efficiency and increases leaf water-use efficiency and oxidative stress in barley plants. Plant Soil. 2016;406(1-2):409-423.
Crossref - Li CP, Qi YP, Zhang J, et al. Magnesium-deficiency-induced alterations of gas exchange, major metabolites and key enzymes differ among roots, and lower and upper leaves of Citrus sinensis seedlings. Tree Physiol. 2017;37(11):1564-1581.
Crossref - Uzilday RO, Uzilday B, Yalcinkaya T, Turkan I. Mg deficiency changes the isoenzyme pattern of reactive oxygen species-related enzymes and regulates NADPH-oxidase-mediated ROS signaling in cotton. Turkish J Biol. 2017;41(6):868-880.
Crossref - Cakmak I, Yazici AM. Magnesium:a forgotten element in crop production. Better Crops. 2010;94:23-25.
- Sirotiak M, Bartosova A, Blinova L. UV-vis spectrophotometric determinations of selected elements in modelled aqueous solutions. J Evniron Prot Safety Educ Manag. 2014;2:75-87.
- Devi R, Kaur T, Kour D, et al. Mineral solubilizing and mobilizing microbiomes:a sustainable approach for managing minerals’ deficiency in agricultural soil. J App Micro. 2022;133(3):1245-1272.
Crossref - Nagpal S, Sharma P, Kumawat KC. Microbial bioformulations:revisiting role in sustainable agriculture. Biofertilizers. 2021:329-346.
Crossref - Dukare A, Paul S, Kumar R, Sharma V. Microbial based inoculants in sustainable agriculture: current perspectives and future prospects. Biofertilizers. 2021:167-181.
Crossref - Salwan R, Sharma V. Plant beneficial microbes in mitigating the nutrient cycling for sustainable agriculture and food security. Plant Nut Food Sec Era Clim Change. 2022:483-512.
Crossref - Biswas S, Shivaprakash M.K. Comparative studies of concomitant release of secondary and micronutrients by potassium solubilizing bacteria (KSB) from different minerals. Indian J Pure App Biosci. 2019;7(4):341-345.
Crossref - Oualha M, Bibi S, Sulaiman M, Zouari N. Microbially induced calcite precipitation in calcareous soils by endogenous Bacillus cereus, at high pH and harsh weather. J Envi Management. 2020;257:109965.
Crossref - Majhi K, Let M, Halder U, Bandopadhyay R. Copper absorption potentiality of Bacillus stercoris GKSM6 and Pseudomonas alcaliphila GKSM11 isolated from Singhbhum copper mines. Geomicrobiol J. 2022;40(2):193-202.
Crossref - Ayyasamy PM, Lee S. Redox transformation and biogeochemical interaction of heavy metals in Korean soil using different treatment columns in the presence of Shewanella sp. Chemosphere. 2009;77(4):501-509.
Crossref - Ayyasamy PM, Chun S, Lee S. Desorption and dissolution of heavy metals from contaminated soil using Shewalla sp. (HN-41) amended with various carbon sources and synthetic soil organic matters. J Haz Materials. 2009;161(2-3):1095-1102.
Crossref - Groisman EA, Hollands K, Kriner MA, Lee EJ, Park SY, Pontes MH. Bacterial Mg2+ homeostasis, transport and virulence. Annu Rev Genet. 2013;47:625-46.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.