ISSN: 0973-7510
E-ISSN: 2581-690X
Water Hyacinth (Eichhornia crassipes) is a weedy lignocellulosic material which represents the best substrate for the production of bioethanol in tropical countries due to its more availability and more biomass yield. For the fermentation and bioethanol production from water hyacinth, the different bacterial and fungal inoculants were used. Water hyacinth was obtained from ukkadam-valankulam lake, Coimbatore. These leaves were washed properly to remove soil and contaminants and dried for 3 hours at 120°C. It was grained into a fine powder and sieved using 1.5µm in diameter nylon sieve. The sieved material was cultured using different microbial culture such as Aspergillus oryzae, Aspergillus niger and Saccharomyces cerevisiae. After 21 days incubation, the culture was distilled using rotary vacuum evaporator and the produced bioethanol was analyzed by Fourier-transform infrared spectroscopy (FTIR) technique. The previous report showed, less yield only obtained using water hyacinth, potato peels, cassava peel and millet husks but this study produced highest yield using water hyacinth. This study indicated that water hyacinth is mostly available aquatic plant for the production of ethanol. The aim of current work is to understand the usage of water hyacinth as main substrate for bioethanol production using fungal inoculum. The microbial inoculants used for current study reported more potential for the production of bioethanol. This current work gives more economical value to water hyacinths and it can be cleared from all the waterways.
Bioethanol, Water Hyacinth, Microbial Inoculants, Eichhornia crassipes
Energy sources are needed more for the growth of industry in an environment. Bioethanol is a cost-effective fuel compared to petrol. The end product of fermentation is an alcohol which gives more attention to world for energy crisis and an interest in an enhancement for renewable energy sources. So, alternative renewable fuels are required to reduce the dependency on the use of petroleum base fuel.1 Here is an interest in starch utilization for alcohol production due to its renewable and easy availability at global level. Bioethanol is an alternative source for the energy to decreases emission of Green House Gases and different pollutants to make high demand for agricultural products. The expectation of bioethanol increase is about 28% in worldwide by 2026. Because the current production of bioethanol was about 115 billion litres.2 Water hyacinth (Eichhornia crassipes) represented a best substrate for the production of bioethanol in tropical countries due to its more availability and more biomass yield. Water hyacinth (Eichhornia crassipes) is an invasive plant which grows well in a water-rich environment. It is a floating plant found in rivers, reservoirs, lakes, swamps, etc.3 Eichhornia crassipes is a plant which grows well in water, family Pontideraceae found in South America.4,5 It is an invasive weed. Grow at high density (over 60 kg/m2), it having economical, ecological and health impact. Some shows negative effects. This plant can grow in fresh and seawater.6 Though the whole plant of water hyacinth can be used but the metal contamination may be found in the roots of water hyacinth. So, some researchers could not use the roots of water hyacinth to avoid contamination.7 It forms complex root structure due to highest sedimentation. The plant decreases the growth of phytoplankton and submerged vegetation in dense mats. Highest growth of this plant destroys the aquatic animals. The fast spread of the weed can be controlled by traditional and mechanical methods.8 Water hyacinth can prevent the entry of sunlight into waterbodies and also reduce the O2 level. It disturbs the aquatic ecology.9 Chemical methods of control can pollute the water. Recently, the control of weed growth and its fastest spread gone failed and only solution is of Eradication through utilization of weed in a useful manner.10 After harvesting of water hyacinth biomass, if it isn’t discarded properly, leads to issues in environment where it is deposited. Due to this context, many works have been done to convert water hyacinth into valuable products.11 Water hyacinth has been used to encourage the student to become entrepreneurship.12
Therefore, this research focused on producing bioethanol by the fungal culture Aspergillus niger, Aspergillus oryzae and Saccharomyces cerevisiae using water hyacinth as substrate. The fungi can have the ability to hydrolyze lignocellulose and convert the substrate into hydrogen and Ethanol. Thus, the fungi are used highly for production of bioethanol. A filamentous ascomycete fungus called Aspergillus niger that is found in the environment. A. niger is opportunistic human pathogen, and also important in fermentation. It has a good tendency to produce bioethanol. Aspergillus niger is white in color at first day of growth and becomes black heads on third day of growth, texture shows cottony appearance, septate mycelium, conidiophore arises from fertile branch and sporangium appears in globose shape.13 Thus, it is a capable substitute feedstock for production of bioethanol. The rice wine is produced by the koji mold Aspergillus oryzae. The koji mold saccharifies starch. The yeast ferments glucose to ethanol.14 Aspergillus oryzae gives high yield of ethanol production than Rhizopus oryzae. The bioethanol is produced by simultaneous saccharification by fungi and fermentation by the sake yeast Saccharomyces cerevisiae. Saccharomyces cerevisiae is most validated than thousand validated yeasts which belongs to the kingdom – Fungi. This is a eukaryotic, unicellular yeast, it grows best on sugar supplemented medium and abundant in fruits, plants and soil. It will tolerate up to twenty percent ethanol concentration in a fermentation medium. It is the best one organism for ethanol production.15 The Saccharomyces cerevisiae is the only yeast used in bioethanol production due to its high yield, more tolerance and capability to ferment many saccharides compared to other microorganisms. The aim of current work is to study the bioethanol production from Water Hyacinth-substrate and pure colony of microbial inoculum. Bioconversion of this weed to bioethanol could be properly performed using appropriate technology.
Substrate Preparation
The Water Hyacinth – Aquatic Plant was collected from Ukkadam-Valankulam Lake, a natural pond in Coimbatore, TamilNadu, India. Sand and other impurities were removed from the water hyacinth plant by thoroughly washing it several times under running water. The plant was cut into little pieces then dried at 120°C for 4 hours in a laboratory hot air oven dryer. It was grinded into fine powder by using milling machine and filtered using 1.5µm in diameter nylon. The powdered leaves were placed into clean, sterile plastic containers, then properly covered and appropriately labelled.
Isolation of microorganisms
Test organism used as Aspergillus niger, Aspergillus oryzae. Both were isolated from paddy field soil sample which was collected from nearby clinical laboratory and inoculated on the selective media (Potato dextrose agar and Rose Bengal agar). The organism Saccharomyces cerevisiae (dry yeast) was brought in local supermarket.
Identification of isolated organisms
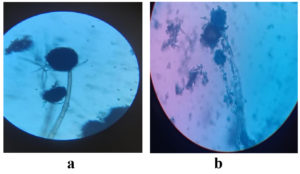
The isolated microorganism was macro morphologically and micro morphologically identified based on their following morphological features: colony growth, colony color, conidial morphology, pigment production, form, size, and surface roughness.
Broth Inoculum Preparation
Broth cultures of Aspergillus oryzae and Aspergillus niger were made ready in the flask containing 50 ml of potato dextrose liquid medium with the standard protocol described by Baker et al.16
Lacto Phenol Cotton Blue Staining
Lacto Phenol Cotton Blue (LPCB) staining is an easy staining method for the microscopic study and detection of isolated fungi.
Fermentation and Saccharification
50 gms of the filtered water hyacinth powder was mixed in each flask containing 500 ml of distilled water. The flasks were sealed with cotton plug, then sterilized for 15 mins at 120°C and 15 lbs. The sterile flask containing WH powder was inoculated with the following fungal cultures: 4.5 ml Aspergillus oryzae + 4.5 ml Aspergillus niger and 2g Saccharomyces cerevisiae in respective flasks. These were covered properly with aluminum foil and kept for incubation at 28°C for 21 days under anaerobic conditions. After incubation, the samples were filtered through Whatman No.4 filter sheet to separate the bioethanol from the residue.
Distillation of bioethanol
The filtrate from fermentation was carried out for distillation process in the laboratory by using rotary vacuum evaporator. The solvent mixture was then loaded into an evaporating flask of the rotary evaporator. The sample was heated at 70°C to get the bioethanol. Ethanol being volatile at this temperature evaporates and condenses into the receiving flask of the rotary evaporator.
Substrate Preparation, Isolation and Identification of Organism
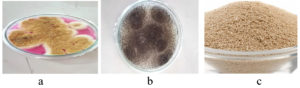
The Powdered Water Hyacinth substrate was used for ethanol production (Figure 1). Aspergillus niger, Aspergillus oryzae and Saccharomyces cerevisiae were isolated and used as inoculum (Figure 2). These inoculum were stored in Potato Dextrose broth (Figure 3). These fungal cultures were morphologically identified and shown in Figure 4. The cultural characteristics of these fungal inoculum were tabulated in Table 1.
Table (1):
Identification of Aspergillus niger and Aspergillus oryzae
Isolate |
Aspergillus niger |
Aspergillus oryzae |
|---|---|---|
Size |
400-3000 µm |
500-2500 µm |
Colony colour |
black |
Pale yellowish green |
Reverse colony form |
Radial and biserrate |
Surface cottony appearance |
Colony edge |
Smooth and hyaline |
Rough |
Conidial surface |
Globose vesicle and biseriate |
Uniseriate |
Mycelial form |
Septate and hyaline hyphae |
Smooth |
Mycelial colour |
Dark brown colour |
Yellow green |
Figure 1. Sample Processing: a – Water hyacinth, b – Water hyacinth was chopped into small pieces, c – Water hyacinth powder
Figure 2. Microbial Inoculum: a – Aspergillus oryzae on rose Bengal agar plate, b – Aspergillus niger on PDA plate, c – Saccharomyces cerevisiae
Figure 3. Fungal Inoculum on potato dextrose broth medium: a – Aspergillus oryzae, b – Aspergillus niger
Fermentation, Saccharification and Distillation of Bioethanol
The fermentation process was carried out using Water Hyacinth as substrate and shown in Figure 5. This was finally distilled using Rotary Vacuum Evaporator (Figure 6).
Figure 5. Fermentation Process a – Water hyacinth kept for fermentation, b – Filtrate of water hyacinth after fermentation
Analytical method for bioethanol production
Different analytical methods were used for further analysis of bioethanol after distillation such as litmus test to determine pH of the distillate solution, determine the presence of ethanol using iodoform test, FTIR analysis of distillate solution, analysis of bioethanol by gas chromatography and confirmatory test for produced bioethanol.
Litmus test (Figure 7) is used to test whether the compound in the given solution has any acidic or basic content present in it. If the pH is alkaline, the red paper turns blue. Similarly, blue paper turns red when the pH turns acidic. Hence, the ethanol solution is neutral (neither acts as an acid or a base) in nature and it does not bring any changes in the color of litmus whether it is blue or red.
Iodoform test
The Bioethanol extract was further confirmed through an Iodoform method (Figure 8). 1ml of Bioethanol extract and 1ml of 10 percent sodium hydroxide (NaOH) was mixed in a sterile test tube and noted the yellow color precipitate upon addition of few drops of 10% iodine solution and followed by heating for 2 minutes.
Analysis of bioethanol by FTIR (Fourier-Transform Infrared Spectroscopy)
The bioethanol produced was analyzed by using Fourier Transform Infrared Spectroscopy (FTIR) (Figure 9 and Table 2). In this study, FTIR spectrophotometer produced some peaks of waves as bioethanol which confirmed the presence of bioethanol in our test sample extracts.
Table (2):
FT-IR spectral data of prepared bioethanol and authentic ethanol
| Peak No. | FTIR Analysis-Absorption band (cm-1) wave number | ||
|---|---|---|---|
| Prepared bioethanol | Authentic ethanol | Peak assignments | |
| 1. | 3308.70 | 3352 | -OH stretching |
| 2. | 1636.15 | 1481 | O-H bending |
| 3. | 1015.60 | 1047 | C-O stretching of C-O-H |
| 4. | 460.40 | 480 | C-C-O bending |
Analysis of Bioethanol by Gas Chromatography (GC) method
Volatile and semi-volatile individual particles could be separated through Gas chromatography (GC) (Figure 10 and Table 3). It is an analytical powerful technique for the separation, identification and quantification of each single chemical components from complex materials. The GC-MS analysis of the bioethanol was performed according to the method of Dizhbite et al.17
Table (3):
Gas chromatography of bioethanol extracted from water hyacinth
No. |
Compound |
Retention Time (In mins) |
|---|---|---|
1. |
Benzhydrazide, N2- (2-methoxy-5- nitrobenzyl |
9.27 |
2. |
Silicone oil |
12.98 |
3. |
4-Hexen-3-one,2,2-dimethyl |
16.0 |
4. |
Heptadecane |
19.64 |
5. |
Heptachlor-exo-epoxide |
23.68 |
6. |
Alpha-Endosulfan |
25.78 |
7. |
Tetratriacontane |
30.13 |
8. |
Eicosane |
32.87 |
9. |
Methoxychlor |
38.38 |
Confirmation test for produced bioethanol
The extracted bioethanol sample was further carried out for confirmation test by using potassium dichromate technique (Figure 11).18 The test reagent which reacted with -OH group indicated the presence of an alcohol in an extract. The orange solution is turning into green color if the primary or secondary alcohol content is present in the extract upon addition of few drops of potassium dichromate (VI) Solution acidified with dilute sulfuric acid into the test tube containing our test sample extract followed by the tube was warmed in a hot water bath for 30 mins.
Based on our research work, the whole plant parts of water hyacinth contain higher amount of cellulose. So, it was screened to use as substrate for bioethanol production. The used inoculum A. oryzae, A. niger and S. cerevisae showed highest ethanol production upon fermentation of aquatic plant water hyacinth. Singh et al.,19 resulted that the enzyme production was done using water hyacinth as substrate and Aspergillus niger as inoculum. Then the enzymatic hydrolysate was further concentrated. Then it was fermented by three cultures such as one with Saccharomyces cerevisiae, Another with Scheffersomyces stipitis and third one with co-culture of both. This resulted bioethanol concentrations as 4.3 g/l, 6.2 g/l and 9.8 g/l, respectively. Production of ethanol was higher in co-culture about 56% than S. cerevisiae – single culture and S. stipitis – single culture. Litmus test was performed by dipping the litmus paper into the sample. It showed no color change in the litmus paper which indicates a positive result. American Herbal Product Association, 2017 also reported that the ethanol is a neutral compound and won’t change the colour of the litmus paper. Triiodomethane test is most common qualitative technique used for the analysis of presence of ethanol. This test is also used to differentiate ethanol from methanol. When the ethanol was mixed with iodine and sodium hydroxide, it produced a yellow color precipitate because of the formation of Iodoform. The positive result was obtained when the methyl group of alcohol binding with a hydrogen atom and hydroxyl group of carbon. Hence it could be stated that there were alcohol compounds in the sample. This was agreed with the result of Pandey et al.20 where samples having high reducing sugar produced positive results. The crude extract color turned from pink into green. The green color formation is good evidence for the presence of ethanol in crude extract. The grade for bioethanol depends on the purity of bioethanol which should be higher than 99.5vol %. The water-rich bioethanol mixture can be purified by distillation method.21 The structure of bioethanol is confirmed by the analysis of distillate solution using FTIR method and this result agreed with the results of Rawinder et al.,22 who done same work but different raw material. The GC-MS analysis of our bioethanol showed the chromatogram at the retention time of 9.27 to 38.38 minutes. Similar work was reported by Bhalt et al.,23 that the production of ethanol using the millet bran – agricultural waste by A. flavus FPDN1 and the retention time is 3.31 minutes at the concentration of 88% ethanol. The GC-MS (RT) analysis of bioethanol showed the functional group of ethanol was found on 7.84% peak area and 2.58 min retention time.24 Belete et al.,25 portrayed that the yellow-orange colour of dichromate was turned into green when the 5ml of bioethanol was mixed with 1ml of 2 percent potassium dichromate solution. This is due to the presence of ethanol in the test sample. Thus, the product was definitely produced from water hyacinth weed upon fermentation process.
This study showed that the water hyacinth aquatic plant was best material for production of bioethanol. It also decreases the emissions of small particles from chemical fuels and green house gases. It decreases GHG emissions by approximately 71% compared to fossil fuels. It also decreases emissions of other pollutants to improve the air quality. It has low toxicity and is easily biodegradable. This study also showed the choice of microbial inoculant for the better bioethanol production. The result concluded that ethanol production is observed using inoculant (A. oryzae + A. niger + S. cerevisae). Current study indicated that it is economically feasible method for high yield production of bioethanol using aquatic plant such as water hyacinth. Compared to landfill water hyacinth Bioethanol production is a best choice of economics, which means it can improve social welfare. Instead of disposing water hyacinth by landfill, it may be contributed to decrease in GHG emission by producing bioethanol. The bioethanol cost, rate of yield is more feasible economically. For the selection of feedstocks for producing bioethanol output targets; we strongly suggest selecting water hyacinth.
ACKNOWLEDGMENTS
The authors are sincerely grateful to the Management Authorities, Karpagam Academy of Higher Education, Coimbatore, TamilNadu, India, for the constant encouragement and support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Nguyen DQ, An-Tran TT, Pham TT, Le VT, Mai TP. Investigation of water hyacinth as a feedstock for bioethanol production by simultaneous saccharification and fermentation process. Chem Eng Trans. 2021; 83:289-294.
- International Energy Agency. Renewable Energy Market Update 2021. https://www.iea.org/reports/renewable-energy-market-update-2021.
- Jirawattanasomkul T, Minakawa H, Likitlersuang S, et al. Use of water hyacinth waste to produce fibre-reinforced polymer composites for concrete confinement: Mechanical performance and environmental assessment. J Clean Prod. 2021; 292:126041.
Crossref - Elenwo E, Akankali J. The Estimation of Potential Yield of Water Hyacinth: A Tool for Environmental Management and an Economic Resource for the Niger Delta Region. J Sustain Dev Stud. 2016; 9(2):115-137.
- Rodrigues A, Odero M, Hayombe P, et al. Converting Water Hyacinth to Briquettes: A Beach Community Based Approach. IJSBAR. 2014; 15(1):358-378.
- Lata N, Dubey V. Preliminary phytochemical screening of Eichhornia crassipes: the world’s worst aquatic weed. J Pharm Sci. 2010; 3(6):1240-1242.
- Moses T, Barku V, Kyereme C, Odoi F. Composition of water hyacinth (Eichhornia crassipes) plant harvested from the volta lake in Ghana and its potential value as a feed ingredient in rabbit rations. Adv Anim Vet Sci. 2021; 9(2):230-237.
Crossref - Anuja S, Aggarwal N, Anita S, Anita Y. Beyond biocontrol: water hyacinth-opportunities and challenges. J Environ Sci Technol. 2016; 9(1):26-48.
Crossref - Madikizela LM. Removal of organic pollutants in water using water hyacinth (Eichhornia crassipes). J Environ Manage. 2021; 295:113-153.
Crossref - Ayanda OI, Ajayi T, Asuwaju FP. Eichhornia crassipes (Mart.) Solms: Uses, Challenges, Threats, and Prospects. The Scientific World Journal. 2020; 1-12.
Crossref - Li F, He X, Srishti A, et al. Water Hyacinth for Energy and Environmental Applications: A Review. Bioresour Technol. 2021; 327:124809.
Crossref - Syamsi AN, Fitrihidajati H. Validitas lembar kegiatan peserta didik (LKPD) berbasis problem-based learning (PBL) pada materi perubahan lingkungan untuk melatihkan keterampilan berpikir kritis siswa kelas X SMA. Berkala Ilmiah Pendidikan Biologi (BioEdu). 2021; 10(2):39-402.
Crossref - Saraswathi K, Vadamalaikrishnan K, Jayaraman P. The Effect of Chromium and Herbicide on the Growth of Aspergillus terreus Isolated from Soil Environment. J Pure Appl Microbiol. 2020; 14(3):2093-2104.
Crossref - Nout MJR, Aidoo KE. Asian fungal fermented food in Industrial Applications. Springer, New York, NY, USA. 2002; 10: 23-47.
Crossref - Baig MZ, Dharmadhikari SM, Ismail S. Technological Processes for Conversion of Lignocellulosic Biomass to Bioethanol. J Pure Appl Microbiol. 2017; 11(4):1863-1881.
Crossref - Bayman P, Baker JL, Doster MA, Michailides TJ, Noreen E. Ochratoxin Production by the Aspergillus ochraceus Group and Aspergillus alliaceus. Applied and Environmental Microbiology. 2002; 68(5): 2326-2329.
Crossref - Dizhbite T, Telysheva G, Dobele G, Arshanitsa A, Kampars V. Py-GC/MS for characterization of non-hydrolyzed residues from bioethanol production from softwood. J Analy Appl Pyrol. 2011; 90(2):126-132.
Crossref - Caputi A. Book Note and Review: Personnel: Management-Employee Communication in Action. ILR Review.1959; 12(2): 315.
Crossref - Singh A, Bishnoi NR. Comparative study of various pretreatment techniques for ethanol production from water hyacinth. Ind Crops Prod. 2013; 44:283-289.
Crossref - Pandey A, Tiwari S, Tiwari KL, Jhadav SK. Bioconversion of Lignocellulosic Azolla into Bioethanol. J Adv Phytotech Enviro Sanit. 2013; 2(2):59-64.
- Carravetta V, Gomes AHDA, Marinho RDST, et al. An atomistic explanation of the ethanol-water azeotrope. Phys Chem Chem Phys. 202242(24):26037-26045.
Crossref - Rawinder K, Himanshu S. Bio-ethanol production from rice husk using simultaneous saccharification and fermentation and optimization of pretreatment methods. J Der Pharma Chemica. 2017; 9(7):1-7.
- Bhalt N, Dharmesh A, Thakor P. Production of xylanase by Aspergillus flavus (FPDN1) on pearl millet bran: optimization of culture conditions and application in bioethanol production. Int J Res Chem Environ. 2012; 2:204–210.
- Du M, Huang S, Wang J. The volatiles from fermentation product of Tuber formosanum. J Forest. 2014; 4(4):426–429.
Crossref - Hirphaye BY, Mezgebe FH, Tura AM, Fanta GM. Bio-ethanol production potential of Water Hyacinth (Eichhornia crassipes) as alternative energy feedstocks. Research square. 2022; 1-17.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.