ISSN: 0973-7510
E-ISSN: 2581-690X
Low density polyethylene (LDPE) is widely used plastic and its use has increased over the past few decades due to its extensive properties. The increased use of plastic generates an increasing amount of plastic waste making waste management more challenging and ultimately contributing to plastic pollution. One possible solution to this issue is the biodegradation of LDPE by utilizing microorganisms which can be advantageous economically and environmentally. There are no studies specifically addressing the biodegradation of vegetable packaging LDPE films by bacteria isolated from waste disposal sites. In this study, bacteria that can degrade vegetable packaging LDPE films were isolated from waste disposal site located in Langdiyawas, Jaipur, Rajasthan. The isolates were screened for LDPE biodegradation using clear zone assay. In the presence of LDPE powder, isolates IRB1 and IRB13 were able to grow and produce clear zone surrounding the colony. Weight loss analysis has been done after 120 days of incubation to assess the biodegrading capability of the isolates. Isolates IRB1 and IRB13 significantly reduced the weight of LDPE film, resulting in weight loss of 19.94 ± 2.15% and 25.08 ± 1.18%, respectively. The efficacy of isolates was further confirmed using biofilm formation, hydrophobicity, fourier transform infrared (FTIR) spectroscopy, scanning electron microscopy (SEM), and energy dispersive X-ray (EDX) analysis. IRB1 and IRB13 have been identified as Enterobacter sp. and Bacillus sp., respectively by using 16S rRNA gene sequencing and phylogenetic analysis. Both isolates have shown promising results towards LDPE biodegradation and could aid in the management of plastic waste, hence reducing plastic pollution.
Low Density Polyethylene, Biodegradation, Weight Loss, Biofilm Formation, Hydrophobicity
Polyethylene is a synthetic plastic that is used extensively in commercial production. It is an ethylene polymer with the formula (-CH2-CH2)n, where “n” represents the number of carbon atoms.1 The major sector where polyethylene has been employed more extensively is the packaging industry.2 Since LDPE has excellent sealing, stiffness, moisture barrier, and transparency qualities, it is the most widely used polymer film for vegetable packaging.3-6 LDPE is the main constituent of municipal solid trash and makes up 60% of the plastic bags produced overall within the polyethylene family.7
The widespread use of LDPE poses a serious risk to both marine and terrestrial ecosystems. The body of an animal or bird becomes entangled in plastic waste that is discarded into the environment, eventually leading to the organism’s death.8 In addition, it produces a mosquito breeding ground and clogs the sewage system. When plastic debris is improperly disposed of, the environment’s natural beauty deteriorates, which can lead to lost tourism earnings.9
Since LDPE is hydrophobic and has no functional groups, it is extremely resistant to the environmental degradation.10,11 As a result, this polymer has a long environmental shelf life. About 25 million tonnes of this polymer accumulate in the environment annually.12,13 The majority of plastics are incinerated or thrown into landfills. Incineration produces a lot of harmful emissions and there are less and fewer landfills available to safely dispose of plastic waste.14-16
Plastic degradation is very challenging and time taking process, so it is essential that these pollutants should be eliminated using a range of methods.17 There are a number of traditional and customary approaches of managing plastic waste, however these systems have a number of drawbacks.18 Consequently, embracing the use of biological techniques is crucial in addressing the environmental issues associated with plastic pollution.8 Furthermore, plastic biodegradation through microbes provides a comprehensive, economical and environmentally safe way to handle plastic waste and helps in the reduction of plastic pollution.19,20
The process of degrading substances through the action of microorganisms is known as biodegradation. It is a multistep process that includes biodeterioration, depolymerization, assimilation, and mineralization.21 Numerous bacterial genera, including Pseudomonas, Bacillus, Sphingobacterium, Acinetobacter, Micrococcus etc. that were isolated from diverse sources are involved in the breakdown of LDPE.22-25 Frequent adherence of microorganisms to polymer surface causes mineralization of the plastic polymer via enzymatic action.26-28
Microbial cells ingest and metabolize plastic that has undergone enzymatic breakdown. Environmental pollution and growing waste that cannot be replenished or degraded, promotes research on biodegradation.29
There is no research explicitly addressing the bacterial breakdown of LDPE films used in vegetable packaging. The vegetable packaging LDPE films utilized in this study offer a novel viewpoint on microbial deterioration. The precise degradation pathways of these films are still poorly understood, despite the fact that they contribute significantly to the plastic waste. The molecular pathways, enzymes, and possible pertinent degradation mechanisms involved in the degradation of vegetable packaging LDPE film require further investigation.
Further research into bacterial strains from waste disposal sites has significant potential for addressing plastic waste in a sustainable manner. These bacteria are important in reducing the negative environmental effects of plastic waste because they have evolved naturally to degrade synthetic polymers. The objective of this research is the degradation of the vegetable packaging LDPE films through bacterial strains collected from waste disposal site and molecular identification of LDPE degrading bacterial strains.
Materials
LDPE films (50 microns) used for vegetables packaging were collected from a nearby shop and cut into 2×2 cm pieces for the biodegradation experiment. LDPE powder was obtained from Thermo Fisher Scientific.30
Sample collection and isolation of bacteria
Soil samples were collected from a waste disposal site located in Langdiyawas, Jaipur, Rajasthan. This site is located at latitude 26°35’3″ N and longitude 76°30’5″ E. At a depth of three to five centimeters, soil samples were collected and transferred to the sterile zip-lock bags. After sample collection, it was immediately sent to the laboratory and stored at 4 °C for later usage.31 1 gm soil was used to isolate soil bacteria using the serial dilution method. Bacteria were isolated on nutrient agar media through spread plate technique. For each sample, three replicas were maintained and incubated at 37 °C for 24 hrs. The isolated colonies were subcultured to obtain pure colonies through streak plate method.30,32
Clear zone assay
The clear zone approach was employed to screen bacteria that can degrade LDPE. 5.0 g/L sodium chloride (NaCl), 0.15 g/L potassium chloride (KCl), 2.0 g/L ammonium chloride (NH4Cl), 0.1 g/L dipotassium phosphate (K2HPO4), 3.0 g/L potassium dihydrogen phosphate (KH2PO4), 0.2 g/L magnesium sulfate (MgSO4), 0.1 g/L calcium chloride dihydrate (CaCl2.2H2O), agar powder (15 g/L), and distilled water were added to prepare the mineral salt media (MSM). The media was supplemented with LDPE powder (1.0 g/L) after sterilization to prevent deformation. Isolates were grown at 30-35 °C for two to four weeks. Following the incubation period, agar plates were flooded for 20 minutes with a 0.1% (w/v) Coomassie blue R-250 solution in 10% (v/v) acetic acid and 40% (v/v) methanol. Then, plates were flooded with 10% (v/v) acetic acid and 40% (v/v) methanol for 20 minutes after the Coomassie blue solution R-250 was drained out. The isolates produced clear zone in the blue background were chosen as a LDPE utilize.33
Characterization of microorganisms
After screening, additional morphological and biochemical analysis were carried out in compliance with Bergey’s manual of determinative bacteriology to further characterize the isolates that formed the clear zone.34
Analysis of LDPE biodegradation
Percent weight loss analysis
In the current study, the biodegradation efficiency of bacterial isolates was examined using untreated vegetable packaging LDPE films, in contrast to the majority of prior studies that employed pretreated films to analyze the microbial degradation of LDPE. LDPE films (2×2 cm) were air-dried in a laminar air flow chamber for 15 minutes after being dried for the whole night at 60 °C, weighed, disinfected in 70% ethanol for thirty minutes in order to conduct a biodegradation experiment.35 Each Erlenmeyer flask containing 100 mL of sterile mineral salt medium (MSM) and 5 mL of bacterial culture. 20 mg (5 LDPE films of 4 mg) of LDPE films were aseptically added to each flask of the experiment. The bacteria were grown in MSM with LDPE films in a shaking incubator for four months at 30 °C and 120 rpm. To measure the weight loss during incubation, one LDPE film was taken out of each flask containing the reaction mixture at a 30-day interval.36,37 To compare the reduction in weight and for future reference, a negative control was kept with LDPE film in MSM medium without inoculation.38 The LDPE films were removed from the medium and washed with a 2% v/v sodium dodecyl sulphate (SDS) solution. To get rid of any contaminants, the films were rinsed with distilled water, dried for an entire night at 45 °C, and then weighed. In addition, after every fifteen days, the media was refreshed. The % weight loss of LDPE films was computed using the formula below37:
Weight loss (%) = Initial weight – Final weight/Initial weight × 100
Measurement of cell-surface hydrophobicity
The measurement of the hydrophobicity of bacterial cells towards the hydrophobic surface of LDPE was conducted using the bacterial adhesion to hydrocarbon (BATH) test.39 The log phase fresh culture of the bacterial isolates was used in this experiment. They were centrifuged at 5000 rpm and then washed twice with phosphate urea magnesium (PUM) buffer that included the following per litre: 1.8 g urea, 7.26 g KH2PO4, 17 g K2HPO4, and 0.2 g MgSO4. Following that, bacterial cells were suspended in PUM buffer until their optical density (OD) at 600 nm was within the range of 1.0 and 1.2. In a series of test tubes, an aliquot of this mixture (1.2 mL of each) was mixed with a gradually larger volume of hexadecane (0-0.2 mL). To aid in phase separation, test tubes were agitated for 10 minutes and then let to stand for 2 minutes. Spectrophotometry was then used to calculate the aqueous suspension’s OD600. A buffer devoid of cells served as the reference blank.40
Cell surface hydrophobicity (%) = 100 × {(initial OD – final OD)/initial OD}
Biofilm formation assay
The serial dilution method of plate counting was employed to quantify the growth of microorganisms. Planktonic microorganisms were incubated for 30, 60 and 90 days before counting their colony-forming units (CFU). Following a specific protocol, bacteria that attached to the LDPE films were counted after 30, 60 and 90 days. The films were taken out of the solution, washed with sterile phosphate buffer (pH 7) to get rid of any bacteria that could have been loosely adhering, and then vortexed three times for five minutes in the sterile phosphate buffer. After separating suspensions from the three tubes, the cells were extracted using centrifugation at 8000 x g for five minutes. Cell pellets were added in 1 mL of sterile phosphate buffer. Number of cells were counted using traditional spreading plate-culture method on nutrient agar.41
Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray (EDX) analysis
Surface alterations on LDPE films were analyzed using a scanning electron microscope (SEM) along with energy dispersive X-ray (EDX) device.42 SEM examination was performed on the control and treated LDPE films to look for surface imperfections such as pits, fissures, and cracks after 120 days of incubation.43 The SEM analysis was done after washing LDPE films with 2% (w/v) of aqueous SDS and distilled water and then gently shaken for a few minutes.44 EDX analyzer was used to identify the relative concentration of elements (% by weight) present in the control and treated LDPE films.42 Samples were submitted to Accuphychem Analytics in Chomu, Rajasthan, for SEM and EDX analysis.
Fourier Transform-Infrared (FTIR) spectroscopy
FTIR spectroscopy is used to identify different functional groups that are present in a substance.45 This study employed a FTIR spectrometer to examine the films’ infrared radiation in the frequency range of 4000-400 cm-1. Films were sent to the Materials Research Centre, Malaviya National Institute of Technology (MNIT), Jaipur, for FTIR examination after the incubation period of 120 days. PerkinElmer Spectrum Version 10.4.00 was used to carry out FTIR analysis.36 FTIR was conducted for both control and treated LDPE films.46
Molecular identification and phylogenetic analysis of LDPE degrading bacteria
Bacterial Genomic DNA Isolation Kit (Qiagen) was utilized to extract DNA. The 16S rRNA gene of the isolated bacteria was partially amplified by polymerase chain reaction (PCR) using the genomic DNA as the template and the universal primers 27F and 1492R that are specific to the 16S rRNA gene. The amplification was carried out in a 50 µL mixture that included 10 ng of genomic DNA, 1 µL of Forward and Reverse, 25 µL of 2X Mastermix with Taq polymerase, and nuclease-free water. The PCR settings that were employed were as follows: a 10-minute initial activation at 90 °C, 30 cycles of 45 s at 90 °C, 30 s at 58 °C, and 45 s at 72 °C, and a 10-minute extension at 72 °C. The amplicons were delivered to Eurifins in Bangalore for sequencing.47,48 To assemble and trim the forward and reverse sequencing reads into single contigs, DNA Sequence Assembler version 5.15.0 was used. BLAST (Basic Local Alignment Search Tool) was used to align contigs with the National Centre for Biotechnology Information (NCBI) database. References and similar sequences were retrieved from the NCBI database in order to conduct a phylogenetic analysis. MEGA 11’s ClustalW programme was used to further align the sequences. Neighbor-joining method was used to generate the phylogenetic tree. Statistical significance was determined using 1000 bootstrap replications.35,48,49
Statistical analysis
A one-way ANOVA was performed using GraphPad Prism (version 10.4.1) to evaluate variations in weight loss among bacterial isolates after 120 days of incubation. The generated data were expressed as mean ± standard deviation. Statistical analysis was performed to show significant differences at P-value lower than 0.05 (P < 0.05).
Sample collection, isolation of bacteria and clear zone assay
Nineteen bacterial isolates were obtained from soil samples that were collected from waste disposal site located in Langdiyawas, Jaipur (Figure 1). Based on their ability to grow on solid MSM with LDPE powder, all the nineteen bacterial isolates were evaluated for LDPE degradation capability. Two isolates, i.e. IRB1 and IRB13, out of the nineteen were able to grow in the presence of LDPE powder and produced a detectable clear zone surrounding the colony. For additional research, these two isolates involved in the formation of a clear zone were chosen.50
Characterization of microorganisms
For the morphological and biochemical analysis, isolates IRB1 and IRB13 that formed a clear zone were selected.51 Table 1 and 2 summarize the morphological and biochemical test results.
Table (1):
Morphological and biochemical characterizations of IRB1 isolate
| No. | Morphological characterizations | Biochemical characterizations | ||
|---|---|---|---|---|
| 1. | Shape | Circular | Methyl Red test | Negative |
| 2. | Margin | Entire | Voges-Proskauer test | Positive |
| 3. | Elevation | Flat | Indole test | Negative |
| 4. | Size | Small | Citrate test | Positive |
| 5. | Texture | Smooth | Urease test | Positive |
| 6. | Appearance | Glistening | Triple Sugar Iron test | Acid/Acid (G) |
| 7. | Pigmentation | Off white | Oxidase test | Positive |
| 8. | Optical property | Translucent | Catalase test | Positive |
| 9. | Gram staining | Negative Rods | Nitrate Reduction test | Negative |
Table (2):
Morphological and biochemical characterizations of IRB13 isolate
| No. | Morphological characterizations | Biochemical characterizations | ||
|---|---|---|---|---|
| 1. | Shape | Irregular | Methyl Red test | Negative |
| 2. | Margin | Curved | Voges-Proskauer test | Positive |
| 3. | Elevation | Flat | Indole test | Positive |
| 4. | Size | Large | Citrate test | Positive |
| 5. | Texture | Dry | Urease test | Positive |
| 6. | Appearance | Dull | Triple Sugar Iron test | Alkali/Acid (G), H2S |
| 7. | Pigmentation | Off white | Oxidase test | Negative |
| 8. | Optical property | Opaque | Catalase test | Negative |
| 9. | Gram staining | Positive Rods | Nitrate Reduction test | Negative |
Analysis of LDPE biodegradation
Percent weight loss analysis
The bacterial isolates were added to the MSM in the biodegradation experiment along with five 4 mg pieces of LDPE film. Weight loss % was determined after 120 days of incubation period. IRB1 and IRB13 resulted in maximum weight loss 19.94 ± 2.15% and 25.08 ± 1.18%, respectively of LDPE films while no weight loss was observed in negative control.35,36 IRB1 and IRB13 were chosen as the potential degrader of LDPE and taken for further analysis.
Measurement of cell-surface hydrophobicity
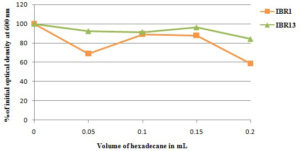
Hydrophobicity of the microbial cell has been identified as an essential feature that facilitates the microorganism’s adhesion to LDPE through hydrophobic interaction.52 The initial rate-limiting step in the production of biofilms is microbial colonization on the surface of the polymer. An effective biofilm is frequently formed following successful microbial colonization of a surface.53 The isolate IRB1 and IRB13 both displayed maximum hydrophobicity of 89.4% and 96.3%, respectively, at 0.1 and 0.15 ml of hexadecane, according to the BATH assay results (Figure 2).54 Hydrophobic microorganisms may easily grow and break down organic contaminants due to the hydrophobic nature of their cell surfaces. Increased cell surface hydrophobicity produced strong biofilms that endure for a longer period of time and are difficult to break down.55
Biofilm formation assay
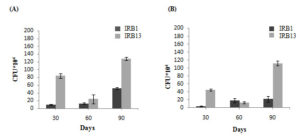
Microbial biofilm formation is linked to increased breakdown of polymers such as LDPE.56,57 Consequently, serial dilution method was used to count the number of bacteria adhered to the LDPE surface and developing planktonically in the culture suspension during course of the 90 days culture period. The data clearly demonstrated that within the first 30 days of incubation, the isolates started the biofilm formation (Figure 3A). The isolate IRB13 displayed the highest biofilm density (1.28 × 106 CFU/mL) after 90 days of incubation, followed by IRB1 (0.52 × 106 CFU/mL). The isolates appear to have shown strong adaptability to carbon deprivation circumstances in the presence of LDPE film, as evidenced by development of a dense biofilm on the surface of LDPE. As anticipated, the planktonic bacterial population’s growth pattern matches the biofilm pattern on the LDPE surface (Figure 3B). After 90 days of incubation period, the planktonic cells of the bacterial isolates IRB13 (1.12 × 106 CFU/mL) and IRB1 (0.22 × 106 CFU/mL) showed a maximum increase in cell density. This rise could be the result of detached bacterial cells from the biofilm. It appears that both bacterial isolates were capable to form biofilms, as evidenced by the greater CFUs they displayed on the film surface compared to planktonic suspension.41 Biofilm production by Bacillus cereus was described by Liu et al.58 Biofilm formation by Bacillus siamensis was reported by Tarafdar et al.59
Figure 3. Growth pattern of (A) Bacterial biofilm. (B) Planktonic cells in liquid medium. All the experimental work has been done in triplicates
Scanning electron microscopy (SEM) and Energy dispersive X-ray (EDX) analysis
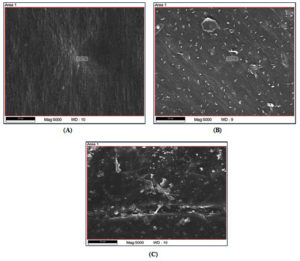
Through SEM analysis of LDPE films, the ability of the bacterial isolates to degrade LDPE film was verified. The structural alterations and surface characteristics were identified after 120 days of incubation period. Evaluation of control and treated LDPE films revealed surface modifications on the surface of polymer. The control plastic film, on the other hand, did not experience any structural alterations and had a very smooth surface free of cavities, erosions, and cracks (Figure 4A).60 The SEM investigation showed that the treated plastic films’ surface had cavities, erosions, and cracks (Figure 4B and C).
Figure 4. Scanning electron microscopy of LDPE films. (A) Control LDPE film. (B) IRB1 treated LDPE film. (C) IRB13 treated LDPE film
Table (3):
Percentage elemental composition of control and treated LDPE films
Elements |
Control film |
IRB1 |
IRB13 |
|---|---|---|---|
Carbon |
74.78 |
71.10 |
63.29 |
Nitrogen |
5.33 |
5.89 |
6.31 |
Oxygen |
4.17 |
5.03 |
12.74 |
Silicon |
0.57 |
0.40 |
0.72 |
Sulfur |
0.15 |
0.29 |
0.52 |
Chlorine |
0.06 |
0.16 |
1.25 |
Gold |
25.48 |
28.36 |
26.38 |
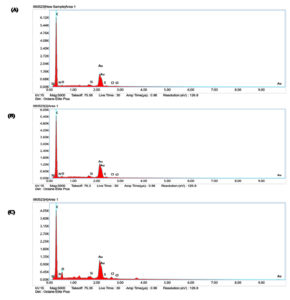
Table 3 displays the elemental composition of the treated and control LDPE films. It was shown that as the biodegradation process proceeds, the elemental oxygen content increases. This finding demonstrated the occurrence of an oxidation reaction on the LDPE surface. Furthermore, a significant decrease in the percentage of elemental carbon was also observed in the treated films. Highest reduction was observed in LDPE film treated with IRB13 followed by IRB1. It was evident from the results that these bacterial isolates utilized the LDPE films as a source of carbon. The EDX spectra of the control and treated LDPE films are displayed in Figure 5.42
Figure 5. EDX spectra of (A) Control LDPE film; (B) LDPE film treated with IRB1; (C) LDPE film treated with IRB13
Fourier Transform-Infrared (FTIR) spectroscopy
The changes in the chemical structure of LDPE film produced during the polymer’s breakdown process were assessed using FTIR in the 4000-400 cm-1 frequency range.61 The alteration and new peak found in the treated LDPE were confirmed by FTIR when compared to the control (Figure 6 A, B and C). The IRB13-treated LDPE film displayed a new peak at 1217.05 cm-1, which was induced by the oxidized fraction of the -OH group.62 FTIR study clearly showed the N–H stretching of the aldehyde group at 3390.17 cm-1.63 C=O stretching of the aldehyde group was observed at 1735.75 cm-1 in LDPE treated with IRB13. Bacteria that established ketone or aldehyde groups produced the carboxyl absorption band, which was visible between 1710-1750 cm-1.44 FTIR measurement of LDPE films treated with IRB1 revealed a reduction in peak wavelength from 2915.14 cm-1 (control) to 2914.83 cm-1 (test) which indicated breakdown of C-H bonds.63
Figure 6. FTIR spectra of (A) Control LDPE film; (B) LDPE film treated with IRB1; (C) LDPE film treated with IRB13
Another peak was seen in the LDPE control film at 2847.94 cm-1; however, the peak intensity of the LDPE treated with IRB1 and IRB13 decreased to 2848.03 cm-1 and 2848.17 cm-1, respectively. Comparable findings were also documented when Acinetobacter baumannii biodegraded LDPE.64 Observable differences in biodegradation of polyethylene before and after exposure to bacteria have been found by other researchers using FTIR analysis.44 Das and Kumar observed emergence and removal of several functional groups in their study on LDPE degradation using a strain of Bacillus amyloliquefaciens.27 To confirm degradation of LDPE, Zhang et al. employed FT-IR spectra. Additionally, they noticed that the FTIR data showed the emergence of ether and carbonyl groups associated with microplastic particles.65
Molecular identification and phylogenetic analysis of LDPE degrading bacteria
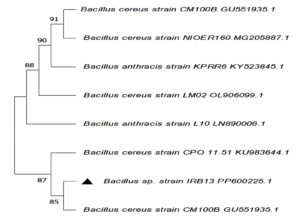
Bacterial isolates that degrade LDPE were identified by 16S rRNA gene sequencing. BLAST similarity searches were used to compare the obtained nucleotides with reference sequences, and from Genbank, the closely related sequences were retrieved.66 MEGA 11, a bioinformatics tool, was used to perform phylogenetic analysis of two bacterial isolates IRB1 and IRB13. Gene sequences for bacterial isolates IRB1 and IRB13 were successfully submitted to GenBank with accession numbers PP600223 and PP600225, respectively. The neighbor-joining method was used to construct a phylogenetic tree based on 16S rRNA gene sequences (Figure 7 and 8). IRB1 and IRB13 shown clustered with Enterobacter hormaechei and Bacillus cereus with up to 98.74% and 99.14% similarity, respectively.67 Jayan et al. reported the biodegradation of LDPE by Bacillus cereus isolated from soil samples taken from a plastic waste dump.68 Yao et al. demonstrated LDPE breakdown by Bacillus sp.69 Ndahebwa Muhonja et al. reported LDPE biodegradation by Bacillus sp. isolated from the Dandora dumpsite in Nairobi, Kenya.70 The degradation of LDPE by Enterobacter hormaechei was demonstrated by Makut et al. using soil samples taken from municipal waste dumps in a few major cities in North Central Nigeria.71 According to Ren et al., Enterobacter sp. isolated from the gut of wax moth (Galleria mellonella) was able to break down polyethylene.72
Figure 7. Construction of phylogenetic relationship tree of Enterobacter sp. IRB1 using the neighbor-joining method
LDPE is extensively used synthetic plastic in vegetable packaging which is continuously accumulating into the environment leading to environmental pollution. So, there is an urge to find a greener approach to reduce the plastic waste. Biodegradation of LDPE through bacteria provides an environmental-friendly solution to solve this issue and reduce environmental contamination. This study showed the LDPE degradation potential of Enterobacter sp. IRB1 and Bacillus sp. IRB13 which were isolated from Langdiyawas waste disposal site near Jaipur, Rajasthan. Screening of the isolates with LDPE powder as the only carbon source was done initially using the clear zone assay. The weight loss percentage was determined to further assess the biodegrading ability of the isolates. The bacterial strain Enterobacter sp. IRB1 and Bacillus sp. IRB13 exhibited the greatest reduction in weight i.e. 19.94 ± 2.15% and 25.08 ± 1.18%, respectively of LDPE film. The degradation was confirmed by biofilm formation, cell surface hydrophobicity, FTIR, SEM, and EDX analysis. Thus, these bacterial strains potentially be a suitable option for the vegetable packaging LDPE biodegradation, offering a sustainable way to lessen the effects of plastic pollution. More research is required to fully understand the function of enzymes and the mechanism of plastic breakdown.
ACKNOWLEDGMENTS
The authors would like to thank the Department of Microbiology, JECRC University, Rajasthan, India, for providing the related support to compile this work.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
DKN conceived the study. DKN and HS designed the study. HS performed data collection and experiments. DKN interpreted the data. HS wrote the manuscript. Both authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Sandhu R, Shakya M. Comparative study of synthetic plastics and biodegradable plastics. Glob J Bio Sci Biotechnol. 2019;8(1):107-112.
- Singh P, Sharma VP. Integrated plastic waste management: environmental and improved health approaches. Procedia Environ Sci. 2016;35:692-700.
Crossref - Kassim A, Workneh TS. Influence of postharvest treatments and storage conditions on the quality of Hass avocados. Heliyon. 2020;6(6):1-9.
Crossref - Nanda S, Sahu S, Abraham J. Studies on the biodegradation of natural and synthetic polyethylene by Pseudomonas spp. J Appl Sci Environ Manage. 2010;14(2):57-60.
Crossref - Goswami TK, Mangaraj S. Advances in polymeric materials for modified atmosphere packaging (MAP). Multifunctional and Nanoreinforced Polymers for Food Packaging. 2011:163-242.
Crossref - Chowdhury SR, Sabharwal S. Molecular-scale design of a high performance organic-inorganic hybrid with the help of gamma radiation. J Mater Chem. 2011;21(19):6999-7006.
Crossref - Gajendiran A, Subramani S, Abraham J. Effect of Aspergillus versicolor strain JASS1 on low density polyethylene degradation. IOP Confer Ser: Mater Sci Eng. 2017;263(2):1-8.
Crossref - Kumar SS, Raut S. Microbial degradation of low density polyethylene (LDPE): A review. J Environ Chem Eng. 2015;3(1):462-473.
Crossref - Muthukumar A, Veerappapillai S. Biodegradation of plastics-a brief review. Int J Pharm Sci Rev Res. 2015;31(2):204-209.
- Tribedi P, Sarkar S, Mukherjee K, Sil AK. Isolation of a novel Pseudomonas sp. from soil that can efficiently degrade polyethylene succinate. Environ Sci Pollut Res. 2012;19(6):2115-2124.
Crossref - Bhatia M, Girdhar A, Tiwari A, Nayarisseri A. Implications of a novel Pseudomonas species on low-density polyethylene biodegradation: an in vitro to in silico approach. Springerplus. 2014;3(1):497.
Crossref - Soni R, Kapri A, Zaidi MGH, Goel R. Comparative biodegradation studies of non-poronized and poronized LDPE using indigenous microbial consortium. J Environ Polym Degrad. 2009;17(4):233-239.
Crossref - Zahra S, Abbas SS, Mahsa MT, Mohsen N. Biodegradation of low-density polyethylene (LDPE) by isolated fungi in solid waste medium. Waste Manag. 2010;30(3):396-401.
Crossref - Al-Salem SM, Lettieri P, Baeyens J. Recycling and recovery routes of plastic solid waste (PSW): a review. Waste Manag. 2009;29(10):2625-2643.
Crossref - Crowley D, Staines A, Collins C, et al. Health and environmental effects of land filling and incineration of waste-A literature review. Reports 3. 2003. https://arrow.tudublin.ie/schfsehrep/3
- Jumaah OS. Screening of plastic degrading bacteria from dumped soil area. IOSR J Environ Sci Toxicol Food Technol. 2017;11(5):93-98.
Crossref - Mazaheri H, Nazeri S. Biodegradation and detoxification of low-density polyethylene (LDPE) by Stenotrophomonas sp. and Alcaligenaceae bacterium. Bull Environ Contam Toxicol. 2024;112(1):19.
Crossref - Sharma S, Chatterjee S. Microplastic pollution, a threat to marine ecosystem and human health: a short review. Environ Sci Pollut Res. 2017;24(27):21530-21547.
Crossref - Crystal Thew XE, Lo SC, et al. Enhancing plastic biodegradation process: strategies and opportunities. Crit Rev Biotechnol. 2024;44(3):477-494.
Crossref - Rajapandi JD, Rajamanickam U. Low-density polyethylene management by using selective bacterial strains from garbage soil. Biologia. 2024;79(3):985-1001.
Crossref - Gu JD. Microbiological deterioration and degradation of synthetic polymeric materials: recent research advances. Int Biodeterior Biodegrad. 2003;52(2):69-91.
Crossref - Shilpa, Basak N, Meena SS. Biodegradation of low-density polythene (LDPE) by a novel strain of Pseudomonas aeruginosa WD4 isolated from plastic dumpsite. Biodegradation. 2024;35(5):641-655.
Crossref - Khampratueng P, Rice D, Anal AK. Biodegradation of low-density polyethylene by the bacterial strains isolated from the dumping site community. Discov Appl Sci. 2024;6(7):348.
Crossref - Kim YJ, Lee JS, Park JA, Kim HO, Lim KS, Ha SJ. Biodegradation of low-density polyethylene by Acinetobacter guillouiae PL211 isolated from the waste treatment facility. Microbiol Biotechnol Lett. 2024;52(2):189-194.
Crossref - Gupta KK, Sharma KK, Chandra H. Micrococcus luteus strain CGK112 isolated from cow dung demonstrated efficient biofilm-forming ability and degradation potential toward high-density polyethylene (HDPE). Arch Microbiol. 2022;204(7):402.
Crossref - Silva RMP, Rodriguez A, De Oca JMGM, Moreno DC. Biodegradation of crude oil by Pseudomonas aeruginosa AT18 strain. Tecnologia Quimica. 2006;26(1):70-77. http://www.redalyc.org/articulo.oa?id=445543749010
- Das MP, Kumar S. An approach to low-density polyethylene biodegradation by Bacillus amyloliquefaciens. 3 Biotech. 2015;5(1):81-86.
Crossref - Chaisu K, Siripholvat V, Chiu CH. New method of rapid and simple colorimetric assay for detecting the enzymatic degradation of poly lactic acid plastic films. Int J Life Sci Biotechnol Pharm. 2015;4(1):57-61.
- Usha R, Sangeetha T, Palaniswamy M. Screening of polyethylene degrading microorganisms from garbage soil. Libyan Agric Res Cen J Intl. 2011;2(4):200-204.
- Deepika S, Madhuri RJ. Biodegradation of low density polyethylene by microorganisms from garbage soil. J Exp Biol Agric Sci. 2015;3(1):1-5.
- Bardaji DKR, Furlan JPR, Stehling EG. Isolation of a polyethylene degrading Paenibacillus sp. from a landfill in Brazil. Arch Microbiol. 2019;201(5):699-704.
Crossref - Maniyar JP, Doshi DV, Bhuyan SS, Mujumdar SS. Bioemulsifier production by Streptomyces sp. S22 isolated from garden soil. Indian J Exp Biol. 2011;49:293-297.
- Gupta KK, Devi D, Rana D. Isolation and screening of low density polyethylene (LDPE) degrading bacterial strains from waste disposal sites. World J Pharm Res. 2016;5(11):1633-1643.
Crossref - Holt JG, Krieg NR, Sneath PHA, Staley JT, Williams ST. Gram-Positive Cocci. Bergey’s Manual of Determinative Microbiology. Hensyl, W.R. (ed), 9th ed., Williams and Wilkins. 1994:527-558.
- Nademo ZM, Shibeshi NT, Gemeda MT. Isolation and screening of low-density polyethylene (LDPE) bags degrading bacteria from Addis Ababa municipal solid waste disposal site “Koshe”. Ann Microbiol. 2023;73(1):1-11.
Crossref - Nadeem H, Alia KB, Muneer F, et al. Isolation and identification of low-density polyethylene degrading novel bacterial strains. Arch Microbiol. 2021;203(9):5417-5423.
Crossref - Gupta KK, Devi D. Characteristics investigation on biofilm formation and biodegradation activities of Pseudomonas aeruginosa strain ISJ14 colonizing low density polyethylene (LDPE) surface. Heliyon. 2020;6(7):e04398.
Crossref - Kyaw BM, Champakalakshmi R, Sakharkar MK, Lim CS, Sakharkar KR. Biodegradation of low density polythene (LDPE) by Pseudomonas species. Indian J Microbiol. 2012;52(3):411-419.
Crossref - Rosenberg M, Gutnick D, Rosenberg E. Adherence of bacteria to hydrocarbons: A simple method for measuring cell-surface hydrophobicity. FEMS Microbiol Lett. 1980;9:29-33.
Crossref - Harshvardhan K, Jha B. Biodegradation of low-density polyethylene by marine bacteria from pelagic waters, Arabian Sea, India. Mar Pollut Bull. 2013;77(1-2):100-106.
Crossref - Han YN, Wei M, Han F, et al. Greater biofilm formation and increased biodegradation of polyethylene film by a microbial consortium of Arthrobacter sp. and Streptomyces sp. Microorganisms. 2020;8(12):1-15.
Crossref - Oluwole OA, Oluyege JO, Olowomofe TO. Biodegradation of polyethylene based films used in water packaging by dumpsite bacteria. Bioremed J. 2024;28(1):1-13.
Crossref - Rafiq S, Fathima F, Shahina SKJ, Ramesh KV. Biodegradation of low density polyethylene (LDPE) by halophilic bacteria isolated from solar saltpans, Kovalam, Chennai. Nat Environ Pollut Technol. 2018;17(4):1367-1371.
- Gajendiran A, Krishnamoorthy S, Abraham J. Microbial degradation of low-density polyethylene (LDPE) by Aspergillus clavatus strain JASK1 isolated from landfill soil. 3 Biotech. 2016;6(52):e04398.
Crossref - Sudhakar M, Doble M, Murthy PS, Venkatesan R. Marine microbe mediated biodegradation of low and high density polyethylenes. Int Biodeterior Biodegrad. 2008;61(3):203-213.
Crossref - Soleimani Z, Gharavi S, Soudi M, Moosavi-Nejad Z. A survey of intact low-density polyethylene film biodegradation by terrestrial Actinobacterial species. Int Microbiol. 2021;24(1):65-73.
Crossref - Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA 5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731-2739.
Crossref - Kumar S, Steche G, Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870-1874.
Crossref - Li KB. ClustalW-MPI: clustalW analysis using distributed and parallel computing. Bioinform. 2003;19(12):1585-1586.
Crossref - Hussein AA, Al-Mayaly IK, Khudeir SH. Isolation, screening and identification of low density polyethylene (LDPE) degrading bacteria from contaminated soil with plastic wastes. Mesop Environ J. 2015;1(4):1-14.
- Soud SA. Biodegradation of polyethylene LDPE plastic waste using locally isolated Streptomyces sp. J Pharm Sci Res. 2019;11(4):1333-1339.
- Tribedi P, Sil AK. Cell surface hydrophobicity: a key component in the degradation of polyethylene succinate by Pseudomonas sp. AKS2. J Appl Microbiol. 2014;116(2):295-303.
Crossref - Maric S, Vranes J. Characteristics and significance of microbial biofilm formation. Period Biol. 2007;109:115-121.
- Mathur G, Mathur A, Prasad R. Colonization and degradation of thermally oxidized high-density polyethylene by Aspergillus niger (ITCC No. 6052) isolated from plastic waste dumpsite. Bioremediat J. 2011;15(2):69-76.
Crossref - Mirani ZA, Naz S, Khan F, et al. Antibacterial fatty acids destabilize hydrophobic and multicellular aggregates of biofilm in S. aureus. J Antibiot. 2017;70(2):115-121.
Crossref - Orr IG, Hadar Y, Sivan A. Colonization, biofilm formation and biodegradation of polyethylene by a strain of Rhodococcus ruber. Appl Microbiol Biotechnol. 2004;65:97-104.
Crossref - Tribedi P, Gupta AD, Sil AK. Adaptation of Pseudomonas sp. AKS2 in biofilm on low-density polyethylene surface: an effective strategy for efficient survival and polymer degradation. Bioresour Bioprocess. 2015;2:14
Crossref - Liu X, Dong X, Wang D, Xie Z. Biodeterioration of polyethylene by Bacillus cereus and Rhodococcus equi isolated from soil. Int Microbiol. 2024;27:1-12.
Crossref - Tarafdar A, Lee JU, Jeong JE, et al. Biofilm development of Bacillus siamensis ATKU1 on pristine short chain low-density polyethylene: A case study on microbe-microplastics interaction. J Hazard Mater. 2020;409:1-45.
Crossref - Skariyachan S, Taskeen N, Kishore AP, Krishna BV, Naidu G. Novel consortia of Enterobacter and Pseudomonas formulated from cow dung exhibited enhanced biodegradation of polyethylene and polypropylene. J Environ Manage. 2021;284:1-19.
Crossref - Dang TCH, Nguyen DT, Thai H, et al. Plastic degradation by thermophilic Bacillus sp. BCBT21 isolated from composting agricultural residual in Vietnam. Adv Nat Sci: Nanosci Nanotechnol. 2018;9(1):1-12.
Crossref - Esmaeili A, Pourbabaee AA, Alikhani HA, Shabani F, Kumar L. Colonization and biodegradation of photo-oxidized low-density polyethylene (LDPE) by new strains of Aspergillus sp. and Lysinibacillus sp. Bioremed J. 2014;18(3):213-226.
Crossref - Gupta KK, Devi D. Biofilm mediated degradation of commercially available LDPE films by bacterial strains isolated from partially degraded plastic. Remed J. 2020;30(4):39-47.
Crossref - Pramila R, Ramesh KV. Potential biodegradation of low density polyethylene (LDPE) by Acinetobacter baumannii. Afr J Microbiol Res. 2015;7(3):24-28.
Crossref - Zhang J, Gao D, Li Q, et al. Biodegradation of polyethylene microplastic particles by the fungus Aspergillus flavus from the guts of wax moth Galleria mellonella. Sci Total Environ. 2020;704:135931.
Crossref - Abraham J, Ghosh E, Mukherjee P, Gajendiran A. Microbial degradation of low density polyethylene. Environ Prog Sustain. 2017;36(1):147-154.
Crossref - Khandare SD, Chaudhary DR, Jha B. Bioremediation of polyvinyl chloride (PVC) films by marine bacteria. Mar Pollut Bull. 2021;169:1-11.
Crossref - Jayan N, Skariyachan S, Sebastian D. The escalated potential of the novel isolate Bacillus cereus NJD1 for effective biodegradation of LDPE films without pre-treatment. J Hazard Mater. 2023;455:131623.
Crossref - Yao Z, Seong HJ, Jang YS. Degradation of low density polyethylene by Bacillus species. Appl Biol Chem. 2022;65(1):1-9.
Crossref - Muhonja CN, Magoma G, Imbuga M, Makonde HM. Molecular characterization of low-density polyethene (LDPE) degrading bacteria and fungi from Dandora dumpsite, Nairobi, Kenya. Int J Microbiol. 2018;2018(1):4167845.
Crossref - Ogu CJ, Makut MD, Obiekezie SO, Okey-Ndeche NF. Biodegradation of low-density polyethylene (IDPE) by bacteria isolated from dump sites in some metropolitan cities in north central Nigeria. World J Adv Eng Technol Sci. 2023;9(2):223-234.
Crossref - Ren L, Men L, Zhang Z, et al. Biodegradation of polyethylene by Enterobacter sp. D1 from the guts of wax moth Galleria mellonella. Int J Environ Res Public Health. 2019;16(11):1941.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.