ISSN: 0973-7510
E-ISSN: 2581-690X
The incidence of oro-dental disorders has emerged as a serious threat to the healthcare sector owing to the increasing complexity of the oral microbiome. Significant advances in biomaterial research have led to the advent of a plethora of drug delivery systems including nanocarriers, dendrimers, hydrogels and other kinds of stimuli-responsive polymeric biomaterials. Anti-microbial peptides (AMPs) have engendered considerable interest in the past decades as potential alternatives to traditional disinfecting agents and also emerged as potent antibiofilm agents. Among the most viable approaches in targeted drug delivery, hydrogels incorporated with AMPs are emerging as bio-functional platforms yielding increased stability and bioavailability. The antibacterial and antibiofilm activities of Nisin are studied using microbiological methods followed by the synthesis of Nisin loaded PVA-Alginate hydrogel for dental treatment. The physicochemical characteristics of Nisin loaded hydrogel were done by swelling behavior, release kinetics assay, FTIR spectroscopic methods and cytotoxicity studies. Nisin showed antibacterial activity towards clinical isolates of drug-resistant bacteria and the antibiofilm and anti-adhesion studies demonstrated that Nisin could control the bacterial count in the test sample. The polymerization of Nisin into hydrogels was done and the physico-chemical characterization of Nisin loaded hydrogel network could be envisaged as a potential drug delivery platform for oral infections. Nisin loaded PVA-Alginate biocompatible hydrogel exhibited apparent swellable, flexible, nonhaemolytic materials and active antimicrobial and nontoxic materials. Physicochemical properties of these Nisin loaded PVA-Alginate biocompatible hydrogels have great potential in biomaterial-based drug delivery systems in controlling the growth and proliferation of major oro-dental pathogens. This could be exploited for the temporary biocompatible dental filling materials to treat the caries. Exploring potential nisin loaded hydrogel delivery systems will provide a brighter future of more friendly, effective and personalized treatment to deal with dental caries.
Periodontitis, Anti-biofilm Activity, Drug Delivery, Anti-microbial Peptides, Biocompatible and Biomaterials
Anti-microbial peptides (AMPs) are emerging as drug candidates for the management of oro-dental disorders owing to their significant therapeutic potential. Therapeutic approaches to overcome microbial resistance to conventional antibiotics which is an area of active clinical research. AMP-based drugs are used in the treatment of dental caries, periodontitis and other oral infections and have yielded promising results in overcoming bacterial resistance.1 In spite of the emergence of a large number of synthetically designed potent AMPs, their clinical translation is considerably limited due to low bioavailability imparted by endogenous protease degradation, non-specific binding to proteins and decreased stability.
Among the most viable approaches in targeted drug delivery, hydrogels incorporated with AMPs are emerging as biofunctional therapeutic systems.2 Drug loaded hydrogels possess adequate biocompatibility and are reported to be an ideal drug release system in dental therapeutic applications. Hydrogels loaded with antibiotic and growth factors exhibit enhanced therapeutic efficacy as these delivery systems aid the targeted administration of drugs. Bioactive molecules incorporated in hydrogels function as a suitable template for dental pulp regeneration.3 Biomaterials for the controlled and directed delivery of specific drugs and for reducing microbial resistance have been envisaged as a potential approach in dentistry.4 Localized delivery systems loaded with AMPs have been implicated in the treatment of dental caries and periodontal infections.5
The therapeutic efficacy of Nisin as a broad spectrum antimicrobial peptide has significant implications in regenerative dentistry. A plethora of studies have reported the antimicrobial properties of Nisin specifically against multi-drug resistant organisms.6 Owing to its prominent role in immunomodulation, Nisin loaded hydrogels can function as potent drug delivery agents.7 Injectable polysaccharide hydrogels loaded with Nisin yielded biocompatible drug delivery systems for periodontal infections. Several research studies highlight the role of tuneable hydrogel templates for encapsulating Nisin yielding an efficient drug delivery system.8 The present study specifies the potential of using hydrogels as drug delivery devices to deliver the antimicrobial peptide, Nisin to an oral multi-species biofilm and reported fast Nisin release with a sustained delivery to increase the long-term effect of Nisin released from hydrogels, thus, maintaining the healthy microbiome of the oral cavity, ultimately improving the life-quality of many patients worldwide. The study aims to determine the biological efficacy of Nisin loaded PVA-Alginate network as a biomaterial based drug delivery system in controlling the growth and proliferation of major oro-dental pathogens.
Nisin was procured from Sigma Aldrich. The test organisms used for the present study include: Streptococcus mutans (ATCC 25175), Enterococcus faecalis (ATCC 29212) and the fungal strain used is Candida albicans (ATCC 10231). The organisms were sub cultured in Nutrient Agar, Nutrient broth and Muller Hinton Agar and stored at 37°C for 24 to 48 hours for further studies.
Evaluation of antimicrobial properties of visin
Agar well diffusion assay was performed to confirm the antimicrobial activity of Nisin. Bacterial cultures of S. mutans and E. faecalis were seeded in Muller Hinton Agar medium and different concentrations of Nisin (250µg/ml, 500µg/ml and 1000µg/ml) were added to 10 mm diameter wells. After incubation at 37°C for 24 hours, the diameter of the zone of inhibition was estimated in comparison to positive control Streptomycin.9
The antifungal properties of nisin was determined by measuring zone of inhibition in the culture of C. albicans grown in Potato Dextrose Agar Medium. Clotrimazole was used as positive control.9
A two-fold serial dilution method was used to detect the Minimum Inhibitory Concentration of Nisin. The inoculum suspensions were diluted twice and 100µl of each is added to 96 well microtiter plates. Nisin was dissolved in 0.05% acetic acid to obtain a concentration of 10mg/ml and different concentrations of the sample (6.25µg, 12.5µg, 25µg, 50µg, 100µg) were added to the wells. Control wells with inoculum alone were also maintained. The plates were monitored and the optical density of the solution was measured at 630 nm using an ELISA plate reader at the 0th and 24th hour and the percentage of inhibition were calculated using the following formula.10
Percentage of inhibition = (OD of control – OD of test)/ (OD of control) ׳ 100
Evaluation of anti biofilm activity of Nisin
The Crystal violet assay was performed by rinsing the incubated plates were rinsed with distilled water, air dried and stained with 100µl of 1% aqueous solution of crystal violet for 20 minutes. The plates were then washed to remove the excess stain and dried. Stained cells were washed with 100µl of Di methyl Sulfoxide (DMSO). The percentage inhibition was determined from the absorbance measured at 630nm.11
The biofilm containing plates were treated with Nisin and the control plates were stained with Ethidium bromide (EtBr) followed by washing with Phosphate Buffer Saline (PBS). The plates were incubated at room temperature in the dark for 15 minutes. The plates were photographed using fluorescent microscope.12
Anti-adherence assay was conducted by adding the Nisin sample of different concentrations and control to the biofilm plates kept for overnight incubation. Then media was removed and washed with PBS.100µl of PBS was added and the biofilm was scrapped and plated to Muller Hinton Agar and kept for incubation overnight. The results were observed by counting colony forming unit.13
Synthesis of Nisin loaded Poly Vinyl Alcohol (PVA)-alginate hydrogel
Poly Vinyl Alcohol was dissolved in distilled water under constant stirring at 80°C. The solution was cooled down to room temperature and 3% Alginate solution was added to it followed by stirring for 15 minutes. 0.1% Nisin was added dropwise to the solution and subjected to continuous stirring for 24 hours. The mixture was further poured into petri dish and dried in an oven.
Physicochemical characterization of Nisin loaded PVA-Alginate Hydrogel
Swelling properties of Nisin incorporated hydrogel
The dried hydrogels were cut into 1׳1 cm discs and initial weight was noted. The discs were incubated in 3ml Phosphate Buffer Saline for 24 hours at room temperature. After incubation, the hydrogel discs were removed from the buffer, blotted dry and the weight was recorded.14
The swelling ratio was calculated using the formula:
[ Weight after swelling / Initial weight ] × 100
Release kinetics of Nisin loaded hydrogel
The prepared hydrogel was further assayed for studying the release kinetics. The hydrogel was cut into square with dimension of 1cm x 1cm. The hydrogel was dipped into a beaker containing 100ml of phosphate buffer with pH 7.4. The beaker containing hydrogel was suspended in phosphate buffer which was kept in a shaker for facilitating stable and continuous shaking. The buffer was collected periodically within a time interval of 1hr upto 6hr and further after 24 hours and 48hr for protein estimation. The buffer collected from the beaker was replaced with an equal quantity of phosphate buffer each time. The collected buffer was then used for protein for studying release kinetics.
Estimation of protein in Nisin loaded hydrogel by Bradford method
The protein in solution can be measured quantitatively by different methods. This method uses the capacity of the protein to bind a dye, quantitatively. Each collected protein fraction (10µl) was mixed with 200µl of diluted Coomassie Brilliant blue dye solution. The solution was mixed well and the calibration graph was plotted using Bovine serum albumin as the standard.
Fourier Transform Infra-Red Spectroscopy (FTIR)
The sample was placed in FTIR spectrometer and measured how much of the beam and at which frequencies the sample absorbs the infrared light and the qualitative analysis of the sample was done.15 The IR spectrum of hydrogel was recorded using a Fourier transmission infrared spectrometer, model Nicolet 7700 with a spectral range of 4000cm-1 – 350cm-1
Cytotoxicity assay
Cytotoxicity test of Nisin loaded hydrogel was determined in mouse fibroblast cell line Obtained from NCCS, Pune. The cytotoxicity assay was performed according to ISO 10993-5 standard. After 24 hours of incubation at 37°C the cell viability was determined for both control and Nisin loaded hydrogel treated in L929 cell line.16
Cell viability was determined using a formula,
Cell viability = [Nt / Nc] ×100
Nt = absorbance of the cells treated with LDPE degraded byproduct
Nc = absorbance of the control
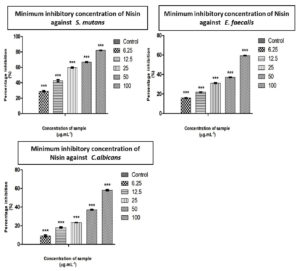
Nisin is well evidenced as a potent antimicrobial peptide and has been reported to have extensive applications in the pharmaceutical industry.15 The antibacterial and anti-fungal activity of Nisin against E. faecalis, S. mutans, C. albicans were performed by agar well diffusion method and recorded by measuring the zone of inhibition (Table 1). Studies conducted using organisms isolated from root canal systems indicated that nisin was more effective than calcium hydroxide in inhibiting the growth of E. faecalis and S. gordonii cells.17 The minimum inhibitory concentration for S. mutans, C. albicans, and E. faecalis were found to be 30.16 µg/ml, 83.63 µg/ml and 77.97 µg/ml, respectively (Table 2). The differences in inhibition diameters represents the bacteriostatic activities of Nisin against test pathogens. The inhibitory effect of Nisin on common pathogens involved in dental infections has been reported by Tong et al.18 Khan et al. conducted a comparative study on the antimicrobial efficacy of chlorhexidine gluconate (CHX) with Nisin, Nisin demonstrated the highest bacteriostatic effect and proved its potential as an effective intracanal irrigant.19 A recent study reported that Nisin was found to be effective against inhibition of dental plaque formation,. The highest sensitivity to nisin was exhibited by S. mutans, a major cariogenic Gram-positive bacteria.20 The results from the study showed that Streptococcus mutans was the most sensitive strain requiring only 30.16 µg/ml to inhibit the growth, followed by Enterococcus faecalis and Candida albicans (Figure 1).
Table (1):
The antimicrobial activity of Nisin against test Pathogens
| Sample | Organism | Concentration (μg/mL) | Zone of inhibition (mm) |
|---|---|---|---|
|
NISIN |
Enterococcus faecalis | Streptomycin (100µg) | 19 |
| 150 | 12 | ||
| 200 | 13 | ||
| 250 | 16 | ||
| Streptococcus mutans | Streptomycin (100µg) | 25 | |
| 150 | 12 | ||
| 200 | 18 | ||
| 250 | 20 | ||
| Candida albicans | Clotrimazole ( 100µg) | 19 | |
| 150 | 15 | ||
| 200 | 17 | ||
| 250 | 19 |
Table (2):
Percentage inhibition of S. mutans, C. albicans and E. faecalis against different concentrations of Nisin
| Organism: Streptococcus mutans (ATCC 25175) | ||
|---|---|---|
| Concentration (µg/mL) | Absorbance | Percentage of Inhibition |
| Control | 0.4724 | 0.00 |
| 6.25 | 0.3422 | 27 |
| 12.5 | 0.2822 | 40 |
| 25 | 0.1960 | 58 |
| 50 | 0.1599 | 66 |
| 100 | 0.0866 | 81 |
| Organism: Enterococcus faecalis (ATCC 29212) | ||
| Concentration (µg/mL) | Absorbance | Percentage of Inhibition |
| Control | 0.4876 | 0.00 |
| 6.25 | 0.4082 | 16 |
| 12.5 | 0.3791 | 22 |
| 25 | 0.3322 | 31 |
| 50 | 0.3082 | 36 |
| 100 | 0.1992 | 59 |
| Organism: Candida albicans (ATCC 10231) | ||
| Concentration (µg/mL) | Absorbance | Percentage of Inhibition |
| Control | 0.8883 | 0.00 |
| 6.25 | 0.8166 | 8 |
| 12.5 | 0.7307 | 17 |
| 25 | 0.6803 | 23 |
| 50 | 0.5545 | 37 |
| 100 | 0.3849 | 56 |
Figure 1. Determination of Minimum inhibitory concentration of Nisin using ED50 PLUS V1.0 software against S. mutans, E. faecalis and C. albicans
Graphical representation depicting the inhibitory action of sample on S. mutans, E. feacalis, C. albicans by MIC method. Along Y axis Percentage inhibition, Along X axis varied concentration of sample. All experiments were done in triplicates and results represented as Mean+/- SE. One-way ANOVA and Dunnets test were performed to analyse data. ***p < 0.001 compared to control groups.
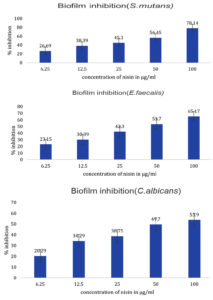
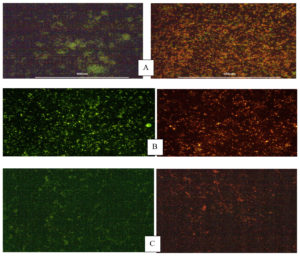
The potency of Nisin in arresting the development of biofilms containing common Cariogenic bacteria was evaluated by crystal violet assay and biofilm inhibition studies confirming the bioactivity of the Nisin. Evaluation of biofilm inhibition potential and anti-adhesion assay was performed to confirm the antimicrobial effect of peptides in preventing orodental infections.21 The minimum biofilm inhibitory concentration (MBIC) of Nisin against Streptococcus mutans, Enterococcus faecalis, Candida albicans was observed to be 78.14%, 65.17 %, and 53.90%, respectively (Figure 2). A recent study observed that the addition of Nisin in dental adhesives considerably improved their ability to inhibit biofilm formation in a concentration dependent manner.22 The bacteriostatic properties of Nisin were further demonstrated by Ethidium bromide /Acridine orange staining on biofilm treated with Nisin. The Nisin treated biofilm showed a significantly higher rate of cell death compared to the untreated control, as depicted in Figure 3. The anti-adhesion studies manifested the efficacy of Nisin in retarding the attachment of Cariogenic bacteria. The results obtained manifested that Nisin could significantly reduce the bacterial count in the treated group compared to the control (Table 3). Shin et al. reported that the treatment with Nisin caused disruption of biofilm integrity with considerable reduction in biomass and thickness.20
Table (3):
Anti adherence activity of Nisin against biofilm. The adherence power test of Nisin treated hydrogels was performed to assess the comparability of bacterial adherence rates on each treated surface
Concentration of Nisin |
CFU |
|---|---|
Control |
595 |
150 |
429 |
250 |
172 |
Figure 2. Quantitative estimation of percentage biofilm inhibition of different concentrations of Nisin against S. mutans, E. faecalis and C. albicans. All experiments were done in triplicates and results represented as Mean+/- SE. One-way ANOVA and Dunnets test were performed to analyse data. ***p < 0.001 compared to control group
Figure 3. Fluorescent microscopy of biofilm inhibition – A) S.mutans, B) E.faecalis and C) C.albicans after a 24 hr treatment of a preformed biofilm with Nisin. Cells were treated with Ethidiumbromide/acridine orange, the live cells are shown in green and the dead cells in orange to red colour.
Our results suggest that the test samples used in this study have the potential to be an excellent antimicrobial alternative with anti-adherence properties that might facilitate the prevention and control of dental infections. Alginated-based nanocarriers have been implicated as ideal delivery vehicles for the controlled release of pharmacologically active compounds.23
Polyvinyl alcohol and Alginate were successfully polymerized under controlled conditions and Nisin was incorporated into the hydrogel network as per standard protocols. Such a strategy combines the bio functionality of hydrogels as efficient templates for targeted drug delivery in orodental infections.20 The gel weight was found to be 3.308g.
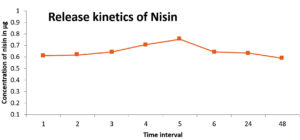
The equilibrium swelling ratio of the hydrogel was found to be 71.05%, confirming its water holding capacity. The water imbibing capacity of hydrogel templates is crucial in modulating the cell-biomaterial interface.24 PVA –Alginate hydrogel facilitated adequate and sustained release of Nisin, as shown in the release kinetics data. Dissolution medium: Phosphate Buffer pH 7.4 Solution, temperature: 37 ± 0.5°C, rotation speed: 150 rpm. It was observed that the maximum release occurred at the 5th hour (Figure 4). The results indicated that the Nisin loaded PVA-alginate hydrogel can function as a controlled release matrix for the delivery of Nisin.25 Many studies proved that the more controlled release profiles that offer more adaptability are essential to next generation wound healing technologies as such systems can augment wound healing through the entire regeneration process. NisinZ-loaded nanosponges exhibited significant anti-cancer activity owing to controlled and sustained release of nisin from the polymeric matrix.26
Such biomaterials as polyvinyl alcohol and alginate have already been utilized to load silver, which can swell to form hydrogels in aqueous settings. Also, silver cross-linked nanocrystalline cellulose (CNC) has shown the controlled release formula of silver to prolong the antimicrobial activity in dental practice.
The antimicrobial peptides have shown remarkable developments recently, among which the nisin has gained great attraction as a viable biomolecular alternative owing to their great broad-spectrum anti-bactericidal properties. AMPs are applied in dental applications for implants-coating and adhesive materials doping to overcome the drug resistant pathogens.
Apart from directly killing or inhibiting bacteria, the hydrogel loaded with nisin could resist the adhesion of cariogenic bacteria to prevent bacterial proliferation on the enamel. The clinical potential of this prepared nisin loaded hydrogel in effectively stopping caries causing process and protecting the individual from dental caries.
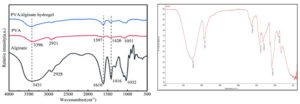
ATR-IR spectral analysis confirmed the presence of surface functional groups corresponding to polyvinyl alcohol, Alginate and Nisin in the hydrogel network when compared with reference spectrum.27 (Figure 5). As shown in the spectra of Nisin loaded hydrogel sample, the strong absorption peaks around 3263, 2941, 1642, 1416 and 1088 cm are related to the stretching vibration of –OH, –C–H, –COO–(asymmetric), –COO–(symmetric), and C–O–C, respectively. The PVA mainly showed bands at 3396 cm (–OH stretching), 2918 cm (–CH stretching) and 1045 cm (–C–O–stretching). The peak corresponding to –OH groups shifted to a higher wave number in the inter penetrating network where Nisin was loaded with PVA/alginate hydrogel due to the hydrogen bonding interactions between PVA/alginate and Nisin. The formation of these ionic bonds and hydrogen bonds in the Nisin loaded PVA/alginate hydrogel shows the presence of nisin in the sample hydrogel.
Cytotoxicity test
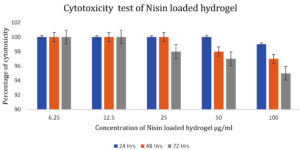
Figure 6 shows the viability of mouse fibroblast cells exposed to the nisin loaded hydrogel along with the control after 1 to 3 days of incubation at 37°C. The cell viability after 3 days of exposure with nisin loaded hydrogel was about 95 %. The results demonstrated that the nisin loaded hydrogel was not cytotoxic to the mouse fibroblast cells during the investigation. Increased cellular viability in wells treated with nisin loaded hydrogel confirmed the non-cytotoxic nature of the hydrogel. The in vitro cyto compatibility of fucoidan-loaded gellan gum-alginate hybrid hydrogels was confirmed by MTT assay in L929 fibroblast cells. Such assays can confirm the non-toxic nature of drug loaded hydrogels.28
The investigation of the effect of nisin incorporated hydrogel showed greater biofilm activity. Cariogenic pathogen remains in the cavity after the removal of carious tooth tissue in treatment. This antibacterial Nisin loaded hydrogel will inhibit bacterial adhesion and subsequently prevent the occurrence of infections. Despite of the advance mentsin hydrogel based materials in dental treatment therapies and restoration, it still remains a challenge to design and fabricate stable drug delivery systems. The moist dental micro environment warrants the use of drug delivery systems with enhanced adhesion properties. Developing robust matrices for sustained and controlled release of drug is integral in determining its bio functionalities and long term applications. Hydrogel based drug delivery matrix offers bio compatibility, bioactivity, mechanical integrity and long term stability facilitating repair and regeneration of target sites.
In the present pilot study, the anti-bacterial, anti-fungal and anti-biofilm efficacy of Nisin against cariogenic bacteria was examined and was found that Nisin can be used as a potential therapeutic agent in the treatment of cariogenic infections. New strategies comprise novel formulations of lantibiotics mainly Nisin including their loading into drug delivery systems and deposition/binding to biologically applied surfaces which enhance their stability, biocompatibility and proper drug release. This therapeutic biomaterial could be an appropriate choice to treat the dental infections. Nisin is a potential alternative agent for drug-resistant infections, thereby we could decrease the use of antibiotics.
ACKNOWLEDGMENTS
The Authors would like to thank Chairman of PMS College of Dental Science and Research, Trivandrum, Kerala and Management of Karpagam Academy of Higher Education, Coimbatore, Tamil Nadu, for their constant support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
Both authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Griffith A, Mateen A, Markowitz K, et al. Alternative Antibiotics in Dentistry: Antimicrobial Peptides. Pharmaceutics. 2022;14(8):1679.
Crossref - Makhlynets OV, Caputo GA. Characteristics and therapeutic applications of antimicrobial peptides. Biophysics Reviews. 2021;2(1):011301.
Crossref - Haugen HJ, Basu P, Sukul M, Mano JF, Reseland JE. Injectable Biomaterials for Dental Tissue Regeneration. Int J Mol Sci. 2020;21(10):3442.
Crossref - Avila JD, Stenberg K, Bose S, Bandyopadhyay A. Hydroxyapatite reinforced Ti6Al4V composites for load-bearing implants. Acta Biomater. 2021;123:379-392.
Crossref - Makvandi P, Josic U, Delfi M, et al. Drug Delivery (Nano)Platforms for Oral and Dental Applications: Tissue Regeneration, Infection Control, and Cancer Management. AdvSci (Weinh). 2021;8(8):2004014.
Crossref - Yehia HM, Alkhuriji AF, Savvaidis I, Al-MASOUD AH. Bactericidal effect of nisin and reuterin on methicillin-resistant Staphylococcus aureus (MRSA) and S. aureus ATCC 25937. Food Sci Technol. 2022;42(11):e105321.
Crossref - Goudarzi F, Esmaeilzadeh M, Yaghoubi H. The Mechanisms of Anticancer Activity of Nisin Peptide on Myelogenous Leukemia Cell Line (K562) As a New Treatment: Inducing Apoptosis by Changing in the Expression of Bax and Bcl-2 Genes. Int J Pept Res Ther. 2021;27(3):2661-2670.
Crossref - Flynn J, Durack E, Collins MN, Hudson SP. Tuning the strength and swelling of an injectable polysaccharide hydrogel and the subsequent release of a broad spectrum bacteriocin, nisin A. J Mater Chem B. 2020;8(18):4029-4038.
Crossref - Nyasulu P, Kasubi M, Boniface R, Murray J. Understanding Laboratory Methods and Their Impact on Antimicrobial Resistance Surveillance, at Muhimbili National Hospital, Dar es Salaam, Tanzania. AiM. 2014;4(1):33-38.
Crossref - Cunha E, Trovao T, Pinheiro A, et al. Potential of two delivery systems for nisin topical application to dental plaque biofilms in dogs. BMC Vet Res. 2018;14(1):375.
Crossref - Karimaei S, Mashhadi R, Mirzaei A, Deyhimfar R, Shabestari AN, Rahimnia R. Antibacterial and Antibiofilm Activities of Nisin from Lactococcuslactis and Alteration of the Bacteria-Induced Pro-Inflammatory Responses on kidney and bladder tumor Cell Lines. Transl Res Urol. 2022;4(1):47-53.
Crossref - Goudarzi F, Asadi A, Afsharpour M, Jamadi RH. In Vitro Characterization and Evaluation of the Cytotoxicity Effects of Nisin and Nisin-Loaded PLA-PEG-PLA Nanoparticles on Gastrointestinal (AGS and KYSE-30), Hepatic (HepG2) and Blood (K562) Cancer Cell Lines. AAPS Pharm Sci Tech. 2018;19(4):1554-1566.
Crossref - Hage M, Chihib NE, Abdallah M, et al. Nisin-based coatings for the prevention of biofilm formation: Surface characterization and antimicrobial assessments. Surfaces and Interfaces. 2021;27:101564.
Crossref - Hamidi M, Azadi A, Rafiei P. Hydrogel nanoparticles in drug delivery. Adv Drug Deliv Rev. 2008;60(15):1638-1649.
Crossref - An Yumin, Dong Liguo, Yang Yi, JiaYongna . Mechanical properties of an interpenetrating network poly (vinyl alcohol)/alginate hydrogel with hierarchical fibrous structures. RSC Adv. 2022;12(19): 11632-11639.
Crossref - Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1-2):55-63.
Crossref - Turner SR, Love RM, Lyons KM. An in-vitro investigation of the antibacterial effect of nisin in root canals and canal wall radicular dentine. Int Endod J. 2004;37(10):664-671.
Crossref - Tong Z, Ni L, Ling J. Antibacterial peptide nisin: a potential role in the inhibition of oral pathogenic bacteria. Peptides. 2014;60:32-40.
Crossref - Khan M, Samant PS, Chauhan R, Khan R, Siddiqui S, Sachan S. Comparative Evaluation of Effectiveness of Nisin, Amoxicillin/Clavulanic Acid (Augmentin) and Chlorhexidine on E. Faecalis as an IntracanalIrrigant: An In-vitro Study. J Pharm Bioallied Sci. 2023;15(Suppl 2):S1317-S1320.
Crossref - Shin JM, Ateia I, Paulus JR, et al. Antimicrobial nisin acts against saliva derived multi-species biofilms without cytotoxicity to human oral cells. Front Microbiol. 2015;6:617.
Crossref - Tong Z, Dong L, Zhou L, Tao R, Ni L. Nisin inhibits dental caries-associated microorganism in vitro. Peptides. 2010;31(11):2003-2008.
Crossref - Zhao M, Qu Y, Liu J, Mai S, Gu L. A universal adhesive incorporating antimicrobial peptide nisin: effects on Streptococcus mutans and saliva-derived multispecies biofilms. Odontology. 2020;108(3):376-385.
Crossref - Karim A, Rehman A, Feng J, et al. Alginate-based nanocarriers for the delivery and controlled-release of bioactive compounds. Adv Colloid Interface Sci. 2022;307:102744.
Crossref - Li Y, Na R, Wang X, et al. Fabrication of Antimicrobial Peptide-Loaded PLGA/Chitosan Composite Microspheres for Long-Acting Bacterial Resistance. Molecules. 2017;22(10):1637.
Crossref - Liang JY, Li Q, Feng LB, et al. Injectable antimicrobial hydrogels with antimicrobial peptide and sanguinarine controlled release ability for preventing bacterial infections. Am J Transl Res. 2021;13(11):12614-12625.
- KhazaeiMonfared Y, Mahmoudian M, Cecone C, et al. Stabilization and Anticancer Enhancing Activity of the Peptide Nisin by Cyclodextrin-Based Nanosponges against Colon and Breast Cancer Cells. Polymers. 2022;14(3):594.
Crossref - Ghalia MA, Dahman Y. Radiation crosslinking polymerization of poly (vinyl alcohol) and poly (ethylene glycol) with controlled drug release. J Polym Res. 2015;11(22):1-9.
Crossref - Shanmugapriya K, Kim H, Kang HW. Fucoidan-loaded hydrogels facilitates wound healing using photodynamic therapy by in vitro and in vivo evaluation. Carbohydr Polym. 2020;247:116624.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.