ISSN: 0973-7510
E-ISSN: 2581-690X
Biofilm represents a potential strut in bacterial treatment failure. It has a dual action; it affords microbial resistance against antibiotics and facilitate transmission of pathogenic bacteria. Nosocomial bacteria pose a serious problem in healthcare units; it prolongs patient hospital stay and increases the mortality rates beside other awful economical effect. This study was planned for targeting nosocomial bacterial biofilm using natural and biologically safe compounds like Chitosan and/or Pluronic F127. Ninety-five isolates were recovered from 107 nosocomial clinical samples. Different bacterial and fungal species were detected, from which Klebsiella pneumonia (23%), Pseudomonas aeruginosa (19%), Acinetobacter baumannii (18%) and E.coli (17%) were the predominate organisms. Pseudomonas aeruginosa, Acinetobacter baumanni and Klebsiella pneumonia were the abundant antibiotic resistant strains with multi-resistance pattern of 72%, 65% and 59%, respectively. A significant percentage of these isolates were strong biofilm forming. Herein, we investigate the effect of Chitosan and Pluronic F127 alone and in combination with each other against biofilm production. Chitosan show variable degree of biofilm inhibition, while Pluronic F127 was able to retard biofilm formation by 80% to 90% in most strain. There is no significant difference (P< 0.05) between Pluronic F127 alone and its effect in combination with Chitosan.
Nosocomial infection, Biofilm, Chitosan, Pluronic F127
Nosocomial infection is a major health care problem. It causes a large percent of morbidity and mortality, one from ten patients is affected by this type of infection1. Nosocomial infection result in prolonged hospital stay with increased healthcare costs2. These infections are mainly caused by multi or extensive drug resistant organisms like Methicillin-resistant Staphylococcus aureus (MRSA) and extended spectrum β-lactamases (ESBL). Scarcity in discovery of a new antibiotic generation worsen the problem, so the prevention is a suitable approach for decreasing this shocking infection2.
According to National Health Institute (NIH) 65-80% of chronic microbial infections are related to biofilm forming microorganisms3. The biofilm infection may be device associated or non-device associated, upon detachment from the surface the microorganism releases hydrolytic enzymes enabling them to colonize a new area4. Colonization may occur in a sensitive and vital places in human body like endocardium, heart valves, lungs and joints resulting in dangerous and life-threatening infections5. The biofilm may be performed on or within medical devices such as venous catheter, contact lenses and prosthetic heart valves by one microorganism or mixed infections according to device type and duration. Also, biofilm down side infection treatment as it decreases antibiotic penetration and allow exchange of resistance plasmids between stains in biofilm matrix6. Resistance in biofilm forming bacteria is significantly higher than the planktonic cells7. Therefore, it is so difficult to be eradicated and removed8.
For management of these infections a new strategy should be evolved. Use of antibiofilm compounds is an interesting one, it will decrease rate of transmission by decreasing attachment and colonization or used in combination with other antibiotics to enhance its activity9. Different types of compounds are used as antibiofilm agents like sub MIC concentration of antibiotics10, antimicrobial peptides11, enzymes12,13, or quorum sensing inhibitors like N-acetyl homoserine14.
Chitosan is a natural biodegradable and biocompatible compound, has antibacterial activity in high concentration and potent antibiofilm activity at lower concentration. Molecular weight and acetylation degree affect its potency as antimicrobial agent15. Being as positively charged cations, chitosan can act in 3 different ways: by interaction with negatively charged microbial cells; interact with microbial DNA; or chelating important metals required for metalloprotein enzymes16.
Pluronic F127 is a synthetic non-ionic surfactant with amphiphilic properties. It is a copolymer of hydrophilic poly(ethylene oxide) and hydrophobic poly(propylene oxide). Due to their amphiphilic characters, Pluronic has an excellent surfactant property17. Combination of chitosan and pluronic acid were mixed together in a nanoparticle form for delivery of anticancer drugs with less side effect18,19. The combination is also used for preparation of different pharmaceutical dosage forms20,21
The aim of this study is to evaluate efficacy of chitosan and pluronic F127 alone and in combination with each other against biofilm forming nosocomial pathogens as a new tactic to retard their transmission and resistance.
a-Sample Isolation
Different biological samples (urine, sputum, endotracheal secretion and blood) were collected from 107 patients between May 2017 and June 2018. All patients admitted to hospital for at least 3 days without previous signs or symptoms of previous infection. All samples were isolated from patients in ICU and neonatal ICU following ethical consideration.
Strains were isolated and purified using different types of media (Blood agar and MacConkey agar). All isolates were stored in glycerol broth at -80°C till further analysis.
b- Bacterial Identification and Antibiotic Sensitivity
Bacterial isolates were primarily identified by Gram stain and biochemical reactions (catalase, oxidase, motility and triple sugar iron agar). Full identification was performed by VITEK2 system (BioMérieux, USA). Antibiotic sensitivity test was done by disc diffusion method according to CLSI guidelines22 using Muller-Hinton agar and Brain Heart infusion agar. The antibiotic discs used were ampicillin/sulbactam (SAM, 20µg), amoxicillin/clavulanic acid (AMC, 30µg), ceftriaxone (CRO, 30μg), piperacillin/tazobactam (TZP, 110µg), cefepime (FEP, 30μg), Ceftazidime (CAZ, 30µg), imipenem (IPM, 10μg), Colistin (CT, 10μg), gentamicin (CN, 10μg), Doxycycline (DO, 30μg), ciprofloxacin (CIP, 10μg), Chloramphenicol (C, 5µg) and Vancomycin (VA, 5µg). Sensitivity pattern of Candida isolates is not determined.
c- Biofilm Formation Assay of Isolated Pathogens
A previously isolated pure colony was resuspended in 5 mL of tryptone soya broth supplemented with 1% glucose, incubated at 37°C or 30°C for 48 hrs for bacteria and Candida, respectively. Twenty microliters of overnight culture were diluted in 180 µL of the above media in 96 well sterile microplate and incubated at 37°C or 30°C for 48 hrs. After incubation the growth was discarded, and the plates were washed three times with phosphate buffered saline pH 7.5 to remove non-adherent cells. The plates were dried in the oven at 65°C and stained with 200 µL of 1% crystal violet solution for 15 min. the plates were washed gently under running water and dried. A solution of 1% acetic acid is used to retain adsorbed C.V stain for 15 min. and measured spectrophotometrically at 600 nm by microplate reader (Tecan SunRise/USA). The strains were classified as weak, moderate or strong biofilm forming bacteria according to classification of Stepanovic et al.23 The experiments were done with six replicates in three independent experiments.
d- Effect of Chitosan and Pluronic Acid on Biofilm Formation
Chitosan was dissolved in 1% acetic acid solution to a final concentration of 10 mg/mL. The solution was stirred overnight at 50°C to ensure complete dissolution, pH was raised to 5.8-6.0 by 1 N NaOH and sterilized by 0.2µm microbial filter (Sartorius, Germany). Pluronic F127 was dissolved at a concentration of 10 mg/mL in distilled water at pH of 7±0.2. Bacterial overnight culture was diluted in TSB amended with 1% glucose to a concentration of 108 CFU/mL containing different concentrations of Chitosan and/or Pluronic F127 (5, 2.5, 1.25, 0.625 mg/ml). In a flat bottom microtiter plate, a 200 µL of the above solution was added and incubated at 37°C or 30°C for 48 hr. Extent of biofilm inhibition occurred by Chitosan and/or Pluronic F127 is detected by C.V method. Percentage of inhibition is calculated by the following equation by Pierce et al.24
Inhibition %= 100- (OD sample/OD control) x 100
e- Scanning Electron Microscope for Biofilm Inhibition Assay
Ten microliter of 0.5 MacFarland solution of diluted overnight bacterial culture was placed in the center of Sterile Millipore membrane filter (0.22 µm, 47 mm diameter, mixed cellulose esters (MCE) membrane) (Merck, USA). The filter was loaded on tryptone soya agar plates (TSA) amended with 2.5 mg/mL of Chitosan, Pluronic F127 and combination of Chitosan and Pluronic F127. The plates were incubated at 37°C for 24 hr. The membrane filters were gently removed, fixed in 3% glutaraldehyde for 30 min. The filters were washed 3 times with PBS each for 10 min. The membranes were gradually dehydrated with gradient conc of ethyl alcohol (50%, 60%, 70%, 80%, 90%, 95%, 100%), then final chemical dehydration with hexamethyldisilazane. The coupons were coated with gold, and then examined with JSM-6510 (JEOL, Japan) at a voltage of 30 kV and magnifications at x5000 to ×15000 25.
f- Statistical Analysis
Data were expressed as mean ± standard error of mean (SEM). Statistical analysis was performed using statistical package for social sciences (SPSS) computer software (version 22), IBM software, USA. One-way ANOVA test was used to evaluate significance between groups followed by tukey posthoc analysis for pairwise analysis. Differences were considered statistically significant at p<0.05.
Bacterial Isolation and Identification
Out of 107 clinical samples taken, 95 samples were positive for bacterial or fungal culture. Large number of isolated strains were found in sputum (30 strains) and endotracheal intubation (28 strains). Urine samples were positive for 27 specimens while blood gives only 10 isolates (Table 1).
Table (1):
Distribution of Nosocomial bacteria isolated from different clinical samples.
| Organism | Number | Isolation specimen | |||
|---|---|---|---|---|---|
| Urine | Sputum | endotracheal secretion | Blood | ||
| Staphylococcus aureus | 8 | 1 | 3 | 3 | 1 |
| Staphylococcus haemolyticus | 4 | 1 | 2 | 1 | – |
| E.coli | 16 | 13 | 1 | 1 | 1 |
| Enerobacter spp. | 2 | 2 | – | – | – |
| Klebsiella pneumonia | 22 | 2 | 10 | 7 | 3 |
| Acinetobacter baumannii | 17 | – | 6 | 9 | 2 |
| Pseudomonas aeruginosa | 18 | 4 | 6 | 7 | 1 |
| Proteus spp. | 3 | 2 | 1 | – | – |
| Candida albicans | 3 | 1 | 1 | – | 1 |
| Candida tropicalis | 2 | 1 | – | – | 1 |
| Total No. | 95 | 27 | 30 | 28 | 10 |
The top four species were Klebsiella pneumoniae (23%), Pseudomonas aeruginosa (19%), Acinetobacter baumannii (18%) and E.coli (17%). Gram +ve bacteria also present in a significant percentage represented by Staphylococcus aureus and Staphylococcus haemolyticus with 8% and 4% respectively. Other Gram-negative bacteria like Proteus spp. and Enterobacter spp. were found in a small frequency (3% and 2% consequently). Fungal infection with Candida albicans and Candida tropicalis were also reported with a lower incidence at 3 and 2% respectively. (Table 1)
Antibiotic Sensitivity Testing
All Gram-positive strains were sensitive to Vancomycin, while all Gram-negative strains were sensitive to Colistin. Ten isolates (45%) of Klebsiella, seven isolates (41%) of Acinetobacter and six isolates (33%) of Pseudomonas show resistance against all tested antibiotics. Four strains of E.coli and one isolate of Staphylococcus haemolyticus, Enterobacter spp., and Proteus spp. were resistant to nine of tested antibiotics. Overall, resistance to the Piperacillin/tazobactam and Imipenem were found to be much lower. (Table 2)
Table (2):
Sensitivity testing of recovered clinical isolates: Number and Percentage of resistance of each pathogen to different antibiotics.
| Organism | Resistance pattern | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SAM | AMC | CRO | CAZ | FEP | DO | C | VA | CIP | IPM | CN | CT | TZP | |
| Staphylococcus aureus | 2 (25%) |
3 (37.5%) | 1 (12.5%) | 4 (50%) | 0 (0%) |
2 (25%) |
1 (12.5%) | 0 (0%) | 1 (12.5%) | 0 (0%) |
2 (25%) |
N.T | 0 (0%) |
| Staphylococcus haemolyticus | 2 (50%) |
4 (100%) |
3 (75%) |
4 (100%) | 2 (50%) |
3 (75%) |
3 (75%) |
0 (0%) | 2 (50%) |
0 (0 %) |
2 (50%) |
N.T | 0 (0%) |
| E.coli | 12 (75%) | 14 (87.5%) | 5 (31.25%) | 4 (25%) | 2 (12.5%) | 7 (43.75%) | 7 (43.75%) | N.T | 4 (25%) |
2 (12.5%) |
5 (31.25%) | 0 (0%) | 0 (0%) |
| Enerobacter spp. | 2 (100%) | 2 (100%) | 1 (50%) |
1 (50%) |
0 (0%) |
1 (50%) |
1 (50%) |
N.T | 0 (0%) |
0 (0%) |
1 (50%) |
0 (0%) | 0 (0%) |
| Klebsiella pneumonia | 19 (86%) | 22 (100%) | 12 (54%) |
14 (63%) | 10 (45%) | 15 (68%) | 13 (59%) |
N.T | 10 (45%) | 10 (45%) | 14 (63%) |
0 (0%) | 10 (45%) |
| Acinetobacter baumannii | 17 (100%) | 17 (100%) | 13 (76%) |
14 (82%) | 11 (64%) | 12 (70.5%) | 14 (82%) |
N.T | 11 (64%) | 9 (53%) | 13 (76%) | 0 (0%) | 7 (41%) |
| Pseudomonas aeruginosa | 16 (88.8%) | 15 (83%) | 13 (72%) |
11 (61%) | 9 (50%) | 14 (77.7%) | 15 (83%) | N.T | 9 (50%) | 8 (44%) | 11 (61%) | 0 (0%) | 6 (33%) |
| Proteus spp. | 3 (100%) | 3 (100%) | 3 (100%) | 1 (33%) | 1 (33%) | 1 (33%) | 2 (66%) | N.T | 1 (33%) | 0 (0%) |
1 (33%) | N.T | 0 (0%) |
*Number of resistant samples/numbers of tested samples (%).
*N.T: not tested
SAM: Ampicillin/Sulbactam; AMC: Amoxicillin/Clavulanic; CRO: Ceftriaxone; CAZ: Ceftazidime; FEP: Cefepime; DO: Doxycycline; C: Chloramphenicol; VA: Vancomycin CIP: Ciprofloxacin; IPM: Imipenem; CN: Gentamicin; CT: Colistin; TZP: Piperacillin/Tazobactam
Biofilm Formation Assay of Isolated Pathogen
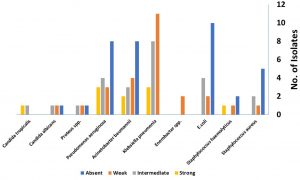
Thirty-five isolates show no biofilm activity, while 26 and 24 isolates show weak and moderate biofilm forming ability, respectively. Only 10 isolates were strong biofilm producer and they were distributed as one strain of St. haemolyticus, one strain Candid tropicalis, three strains of Klebsiella pneumonia, two strains of Acinetobacter baumannii and three strains of Pseudomonas aeruginosa. (Fig. 1).
Fig. 1. Biofilm forming ability of clinical isolates recovered from different nosocomial clinical samples.
Effect of Chitosan and Pluronic F127 on Biofilm Formation
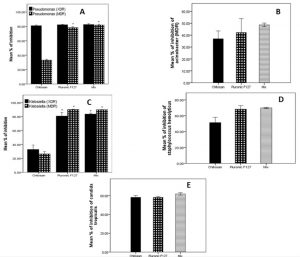
All isolated strains were significantly reduced to different levels by chitosan at the concentration used 2.5 mg/mL. The most affected strain was the extensively drug resistant Pseudomonas aeruginosa (XDR) strain, it was decreased by 81% of initial biofilm formed in planktonic cells. Multi- and extensive drug resistant Klebsiella pneumonia strains (MDR and XDR) and Acinetobacter baumannii were inhibited by 25-35%. Also, chitosan diminished biofilm of Pseudomonas aeruginosa (MDR), Staphylococcus haemolyticus and Candida albicans by 33%, 51% and 58% respectively. (Fig. 2)
Fig. 2. Effect of Chitosan and Pluronic F127 on biofilm formation on (a) multi drug resistant Pseudomonas aeruginosa (MDR) and extensive drug resistant Pseudomonas aeruginosa (XDR) (b) Staphylococcus haemolyticus (c) multi drug resistant Klebsiella pneumoniae (MDR) and extensive drug resistant Klebsiella pneumoniae (XDR) (d) extensive drug resistant Acinetobacter baumannii (e) Candida tropicalis.
Pluronic F127 has a more potent effect than Chitosan in prevention of biofilm creation at concentration used 1.25 mg/mL. It inhibits all Pseudomonas and Klebsiella spp. by more than 80%. It also prohibits biofilm production in other strains of Staphylococcus haemolyticus, Acinetobacter baumannii and Candida albicans by 69%, 43% and 59% respectively. Statistical analysis shows significant difference (P<0.05) in biofilm inhibition between Pluronic F127 and Chitosan in Klebsiella pneumonia (XDR, MDR) and Pseudomonas aeruginosa (MDR). Other strains show no significant difference between Pluronic F127 and Chitosan. overall, all strains show no significant difference between Pluronic F127 and Mixture of both materials. (Fig. 2)
Table (3):
Multi-drug resistance pattern of isolated nosocomial bacterial strains.
| Strain | Degree of resistance (No of antibiotic) | ||||
|---|---|---|---|---|---|
| ≥ 11 | 8-10 | 5-7 | 2-4 | ≤ 1 | |
| Staphylococcus aureus (n=8) | 0 (0%) | 1 (12.5%) | 1 (12.5%) | 2 (25%) | 4 (50%) |
| Staphylococcus haemolyticus (n=4) | 0 (0%) | 2 (50%) | 0 (0%) | 2 (50%) | 0 (0%) |
| E.coli (n=16) | 0 (0%) | 2 (12.5%) | 3 (19%) | 9 (56%) | 2 (12.5%) |
| Enerobacter spp. (n=2) | 0 (0%) | 0 (0%) | 1 (50%) | 1 (50%) | 0 (0%) |
| Klebsiella pneumonia (n=22) | 10 (45%) | 0 (0%) | 3 (14%) | 6 (27%) | 3 (14%) |
| Acinetobacter baumannii (n=17) | 7 (41%) | 3 (18%) | 1 (6%) | 1 (6%) | 5 (29%) |
| Pseudomonas aeruginosa (n=18) | 6 (33%) | 3 (17%) | 4 (22%) | 1 (6%) | 4 (22%) |
| Proteus spp. (n=3) | 0 (0%) | 1 (33%) | 0 (0%) | 2 (66%) | 0 (0%) |
Electron Microscopic Image of Bacterial Strains
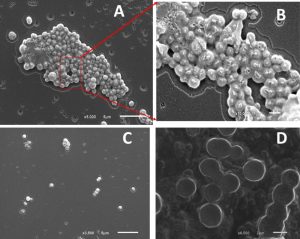
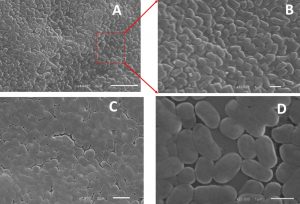
Biofilm inhibition by chitosan and/or pluronic acid were also confirmed by imaging with scanning electron microscope (SEM). Untreated strains show a high percentage of exopolysaccharide matrix with increased aggregation of cells in thick multicellular pattern. While, treated cells with chitosan and/or pluronic acid show a well isolated microcolonies with limited or no exopolysaccharide materials formed. (Fig. 3,4)
Fig. 3. Biofilm formation in Staphylococcus haemolyticus (a) Planktonic cell (b) enlargement of red box view of planktonic cell (c) in presence of Pluronic F127 (d) in presence of Chitosan.
Most nosocomial infections are attributed to organisms with considerable degree of antibiotic resistance. This leads to increased demand on discovering new types of antibiotics with decreased resistance. Targeting bacterial biofilm is another strategy to reduce their transmission and increase efficacy of antibiotics.
The present study shows the high incidence of nosocomial infection among clinical samples, it is approximately 88%. This remarkable higher level may be attributed to misuse of prescribed antibiotics and absence of disinfection policy in these hospitals This finding is in accordance with Mama et al who found a similar result (87%) at Jimma hospital26. Also, Dessie and his colleagues reported a high incidence level (84%) in Addis Ababa hospital27. Other researchers recorded a lower level 66%28 at Gondor University hospital and 70% at Ethiopia29.
Klebsiella pneumonia, Pseudomonas aeruginosa, Acinetobacter baumannii and E.coli were the predominate isolates with a low incidence of Staphylococcus aureus infection. This results is in agreement with Matta et al. who reported that Pseudomonas aeruginosa (12%), Klebsiella pneumoniae (6.2%) and Acinetobacter baumannii (3.1%) were the paramount isolates recovered from 258 patients in Lebanon30. Another study by Peters et al. accounted for eighty-five percent of hospital-acquired infection were Acinetobacter baumanii (28%), Klebsiella pneumoniae (25%), Pseudomonas aeruginosa (21%), Escherichia coli (9%) and Serratia marcescens (3%)31. On contrary, Sserwadda and his colleagues reported that Klebsiella pneumonia and Staphylococcus aureus were the most frequently isolated strains in Kawolo general hospital, Uganda32. Other researchers in a Tertiary Hospital of northern Tanzania mentioned that Staphylococcus aureus was the most common isolated microorganism followed by Enterococcus and other coliform33.
Vancomycin was the most sensitive antibiotic against all isolated Staphylococcus strains. Also, there is no detectable resistance to Colistin in isolated Gram-negative bacteria. The antibiotic with least resistance was found to be against Piperacillin/Tazobactam, Imipenem and Meropenem. Large percentage of Klebsiella pneumonia, Acinetobacter baumannii and Pseudomonas aeruginosa were extensive drug resistant strains, they were resistant to twelve antibiotics from different classes. A similar finding was presented by Mauldin et al. who reported that fifty percent of hospital acquired infections were multi-drug resistant (MDR)34.
In our study Klebsiella pneumonia, Pseudomonas aeruginosa and Acinetobacter baumannii were potent biofilm producer while other types like Staphylococcus and Candida spp show weak to moderate biofilm forming ability. These results coincide with Mulla et al. who demonstrated that Acinetobacter, Pseudomonas, Klebsiella, Staphylococcus spp. were the main cause of infection in indwelling devices due to biofilm formation35. Also, Singhai et al. show that Klebsiella pneumonia represents the most abundant biofilm forming nosocomial microbe with multi-resistant pattern and extended β-lactamase producer36. Other researchers show relationship between microbial resistance and biofilm formation37,38.
For this work, Chitosan has a variable inhibitory effect on biofilm formation regarding different bacterial species. These findings are in agreement with Polyudova et al. who found that Chitosan has a fourfold inhibition of Mycobacterium smegmatis biofilm while has a minimal effect on E.coli strains, he refer his foundation to the increase in the hydrophobicity of attachment surfaces that will decrease the effect of chitosan as a biofilm inhibitor39. Puga and his colleagues suggest that environmental stress leads to increased tolerance to Chitosan effect40. Also, molecular weight and acetylation degree may control the effectiveness of Chitosan41.
Pluronic F127 shows potent antibiofilm activity at low concentration (1.25 mg/mL), its activity reaches up to 80% inhibition for strong biofilm producing strains. Treter et al. show similar effect of Pluronic F127 on Staphylococcus epidermidis, it inhibits 90% of biofilm formation42. Although, combination of Pluronic with Chitosan has a similar effect like Pluronic alone, but Pluronic can increase release, solubility and bioavailability of Chitosan. Alvarado-Gomez et al. reported synergistic activity of Pluronic F127 and silver nanoparticles in hydrogel form against biofilm forming Pseudomonas spp and Staphylococcus spp43. Another study by Manaspon et al. reported increased cytotoxic activity of doxorubicin against breast cancer cells using folate-conjugated pluronic F127/chitosan core-shell nanoparticles18. From our work and previous studies, we conclude that Pluronic F 127 can increase effect of Chitosan suggesting their use in combination at low concentration level with high efficacy against biofilm formation.
Nosocomial infection represents a substantial health problem. A significant number of nosocomial isolates were moderate to strong biofilm producers. Despite of finding a new effective antibiotic for treatment of highly resistant organisms, inhibition of transmission can represent a new effective approach. Chitosan and Pluronic F127 are safe biocompatible agents used in different medical formulation. They show a potential degree of biofilm inhibition. They could be used as an alternative source for inhibition of bacterial resistance and transmission.
ACKNOWLEDGMENTS
We acknowledge Dr. Mohammed Ragab (Pharmacology Department, Faculty of Pharmacy, Beni-Suef University) for performing statistical analysis using SPSS software.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
AFA and WGH design the experiment; DE, AFA performed the experiments; WGH, AFA, OMS analyzed the data; AFA, OMS write the original draft. All authors read and approved the manuscript.
FUNDING
None.
ETHICS STATEMENT
The study was approved by Ethics Committee, Faculty of Medicine, Beni-Suef University.
AVAILABILITY OF DATA
All datasets generated or analyzed during this study are included in the manuscript.
- Edwardson S, Cairns C. Nosocomial infections in the ICU. Anaesthesia & Intensive Care Medicine. 2019;20(1):14-18.
Crossref - Jenkins DR. Nosocomial infections and infection control. Medicine. 2017;45(10):629-633.
Crossref - Jamal M, Ahmad W, Andleeb S, et al. Bacterial biofilm and associated infections. Journal of the Chinese Medical Association. 2018;81(1):7-11.
Crossref - Sutherland IW. Polysaccharases for microbial exopolysaccharides. Carbohydrate Polymers. 1999;38(4):319-328.
Crossref - Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15(2):167-193.
Crossref - Donlan RM. Biofilms: microbial life on surfaces. Emerg Infect Dis. 2002;8(9):881-890.
Crossref - Hoyle BD, Costerton JW. Bacterial resistance to antibiotics: the role of biofilms. Progress in drug Research Fortschritte der Arzneimittelforschung Progres des Recherches Pharmaceutiques. 1991;37:91-105.
Crossref - Cerqueira GM, Peleg AY. Insights into Acinetobacter baumannii pathogenicity. IUBMB Life. 2011;63(12):1055-1060.
Crossref - Molina-Manso D, Del-Prado G, Gomez-Barrena E, Cordero-Ampuero J, Fernandez-Roblas R, Esteban J. Effect of different agents with potential antibiofilm activity on antimicrobial susceptibility of biofilms formed by Staphylococcus spp. isolated from implant-related infections. The Journal Of Antibiotics. 2016;69:686-688.
Crossref - Algburi A, Comito N, Kashtanov D, Dicks LMT, Chikindas ML. Control of Biofilm Formation: Antibiotics and Beyond. Appl Environ Microbiol. 2017;83(3):e02508-02516.
Crossref - Rossi LM, Rangasamy P, Zhang J, Qiu XQ, Wu GY. Research advances in the development of peptide antibiotics. J Pharm Sci. 2008;97(3):1060-1070.
Crossref - Eckhart L, Fischer H, Barken KB, Tolker-Nielsen T, Tschachler E. DNase1L2 suppresses biofilm formation by Pseudomonas aeruginosa and Staphylococcus aureus. Br J Dermatol. 2007;156(6):1342-1345.
Crossref - Kalpana BJ, Aarthy S, Pandian SK. Antibiofilm activity of alpha-amylase from Bacillus subtilis S8-18 against biofilm forming human bacterial pathogens. Appl Biochem Biotechnol. 2012;167(6):1778-1794.
Crossref - Uroz S, Dessaux Y, Oger P. Quorum Sensing and Quorum Quenching: The Yin and Yang of Bacterial Communication. Chem BioChem. 2009;10(2):205-216.
Crossref - Huh AJ, Kwon YJ. “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J Control Release. 2011;156(2):128-145.
Crossref - Goy RC, de Britto D, Assis OBG. A review of the antimicrobial activity of chitosan. Polimeros. 2009;19(3):241-247.
Crossref - Romic MD, Klaric MS, Lovric J, et al. Melatonin-loaded chitosan/Pluronic® F127 microspheres as in situ forming hydrogel: An innovative antimicrobial wound dressing. Eur J Pharm Biopharm. 2016;107:67-79.
Crossref - Manaspon C, Viravaidya-Pasuwat K, Pimpha N. Preparation of Folate-Conjugated Pluronic F127/Chitosan Core-Shell Nanoparticles Encapsulating Doxorubicin for Breast Cancer Treatment. Journal of Nanomaterials. 2012;2012:1-11.
Crossref - Hosseinzadeh H, Atyabi F, Dinarvand R, Ostad SN. Chitosan-Pluronic nanoparticles as oral delivery of anticancer gemcitabine: preparation and in vitro study. Int J Nanomedicine. 2012;7:1851-1863.
Crossref - Escalona-Rayo CF, Serrano-Castaneda P, Lopez-Cervantes M, Escobar-Chavez JJ. Optimization of Unidirectional Mucoadhesive Buccal Patches Based on Chitosan and Pluronic® F-127 for Metoprolol Controlled Release: In Vitro and Ex Vivo Evaluations. Journal of Pharmaceutical Innovation. 2019.
Crossref - Pepic I, Hafner A, Lovric J, Pirkic B, Filipovic-Grccic J. A Nonionic Surfactant/Chitosan Micelle System in an Innovative Eye Drop Formulation. Journal of Pharmaceutical Sciences. 2010;99(10):4317-4325.
Crossref - CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 26th ed. CLSI supplement M100S ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2016.
- Stepanovic S, Vukovic D, Dakic I, Savic B, Svabic-Vlahovic M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods. 2000;40(2):175-179.
Crossref - Pierce CG, Uppuluri P, Tristan AR, et al. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nature Protocols. 2008;3(9):1494-1500.
Crossref - Gomes LC, Mergulhao FJ. SEM Analysis of Surface Impact on Biofilm Antibiotic Treatment. Scanning. 2017;2017:2960194.
Crossref - Mama M, Abdissa A, Sewunet T. Antimicrobial susceptibility pattern of bacterial isolates from wound infection and their sensitivity to alternative topical agents at Jimma University Specialized Hospital, South-West Ethiopia. Ann Clin Microbiol Antimicrob. 2014;13(14).
Crossref - Dessie W, Mulugeta G, Fentaw S, Mihret A, Hassen M, Abebe E. Pattern of Bacterial Pathogens and Their Susceptibility Isolated from Surgical Site Infections at Selected Referral Hospitals, Addis Ababa, Ethiopia. Int J Microbiol. 2016;2016:2418902-2418902.
Crossref - Feleke T, Eshetie S, Dagnew M, et al. Multidrug-resistant bacterial isolates from patients suspected of nosocomial infections at the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. BMC Research Notes. 2018;11(1):602-602.
Crossref - Azene MK, Beyene BA. Bacteriology and antibiogram of pathogens from wound infections at Dessie Laboratory, North-east Ethiopia. Tanzania Journal of Health Research. 2011;13(4):68-74.
Crossref - Matta R, Hallit S, Hallit R, Bawab W, Rogues A-M, Salameh P. Epidemiology and microbiological profile comparison between community and hospital acquired infections: A multicenter retrospective study in Lebanon. Journal of Infection and Public Health. 2018;11(3):405-411.
Crossref - Peters L, Olson L, Khu DTK, et al. Multiple antibiotic resistance as a risk factor for mortality and prolonged hospital stay: A cohort study among neonatal intensive care patients with hospital-acquired infections caused by gram-negative bacteria in Vietnam. PLoS ONE. 2019;14(5):e0215666.
Crossref - Sserwadda I, Lukenge M, Mwambi B, Mboowa G, Walusimbi A, Segujja F. Microbial contaminants isolated from items and work surfaces in the post- operative ward at Kawolo general hospital, Uganda. BMC Infectious Diseases. 2018;18(1):68.
Crossref - Kassam NA, Damian DJ, Kajeguka D, Nyombi B, Kibiki GS. Spectrum and antibiogram of bacteria isolated from patients presenting with infected wounds in a Tertiary Hospital, northern Tanzania. BMC Research Notes. 2017;10(1):757.
Crossref - Mauldin PD, Salgado CD, Hansen IS, Durup DT, Bosso JA. Attributable Hospital Cost and Length of Stay Associated with Health Care-Associated Infections Caused by Antibiotic-Resistant Gram-Negative Bacteria. Antimicrob Agents Chemother. 2010;54(1):109.
Crossref - Mulla S, Revdiwala S. Assessment of biofilm formation in device-associated clinical bacterial isolates in a tertiary level hospital. Indian J Pathol Microbiol. 2011;54(3):561-564.
Crossref - Singhai M, Malik A, Shahid M, Malik MA, Goyal R. A study on device-related infections with special reference to biofilm production and antibiotic resistance. J Glob Infect Dis. 2012;4(4):193-198.
Crossref - Yang D, Zhang Z. Biofilm-forming Klebsiella pneumoniae strains have greater likelihood of producing extended-spectrum β-lactamases. J Hosp Infect. 2008;68(4):369-371.
Crossref - Vuotto C, Longo F, Balice MP, Donelli G, Varaldo PE. Antibiotic Resistance Related to Biofilm Formation in Klebsiella pneumoniae. Pathogens. 2014;3(3):743-758.
Crossref - Polyudova TV, Shagdarova BT, Korobov VP, Varlamov VP. Bacterial Adhesion and Biofilm Formation in the Presence of Chitosan and Its Derivatives. Microbiology. 2019;88(2):125-131.
Crossref - Puga CH, SanJose C, Orgaz B. Biofilm development at low temperatures enhances Listeria monocytogenes resistance to chitosan. Food Control. 2016;65:143-151.
Crossref - Mu H, Zhang A, Zhang L, Niu H, Duan J. Inhibitory effects of chitosan in combination with antibiotics on Listeria monocytogenes biofilm. Food Control. 2014;38:215-220.
Crossref - Treter J, Bonatto F, Krug C, Soares GV, Baumvol IJR, Macedo AJ. Washing-resistant surfactant coated surface is able to inhibit pathogenic bacteria adhesion. Appl Surf Sci. 2014;303:147-154.
Crossref - Alvarado-Gomez E, Martinez-Castanon G, Sanchez-Sanchez R, Ganem-Rondero A, Yacaman MJ, Martinez-Gutierrez F. Evaluation of anti-biofilm and cytotoxic effect of a gel formulation with Pluronic F-127 and silver nanoparticles as a potential treatment for skin wounds. Materials Science and Engineering: C. 2018;92:621-630.
Crossref
© The Author(s) 2020. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.