ISSN: 0973-7510
E-ISSN: 2581-690X
Plastic pollution is a universal problem, and microbial management of plastic waste represents a promising area of biotechnological research. This study investigated the ability of bacterial strains which were isolated from landfill soil to degrade Low-Density Polyethylene (LDPE). Strains obtained via serial dilution were screened for LDPE degradation on Minimal Essential Medium (MEM) with hexadecane. Nine isolates producing clearance zones on hexadecane-supplemented MEM were further tested for biofilm formation on LDPE sheets. High cell surface hydrophobicity isolates (>10%) were selected for detailed biodegradation studies. The C-8 bacterial isolate showed the highest LDPE weight loss (3.57%) and exhibited maximum laccase (0.0219 U/mL) and lipase activity (19 mm) among all bacterial isolates after 30 days. Weight loss was further validated by FTIR and SEM analysis. FTIR analysis revealed that in comparison to control, changes in peak were observed at 719 cm-1 (C-H bending), 875.67 cm-1 (C-C vibrations), 1307.07 cm-1 (C-O stretching), 1464.21 cm-1 (C-H bending), 2000-1650 cm-1 (C-H bending), 2849.85 cm-1 (C-H stretching) in microbial treated LDPE sheets. The treated LDPE also displayed increase in carbonyl index (upto 2.5 to 3 folds), double bond index (1 to 2-fold) and internal double bond index (2 to 2.5-fold) indicating oxidation and chain scission in the LDPE backbone. SEM analysis showed substantial micrometric surface damage on the LDPE film, with visible cracks and grooves. Using 16S rRNA gene sequencing, the C-8, C-11, C-15 and C-19 isolate were identified as Bacillus paramycoides, Micrococcus luteus, Bacillus siamensis and Lysinibacillus capsica, respectively.
LDPE, Plastic Degrading Enzymes, Bioremediation of Plastic Waste, Biodegradation of LDPE
Plastic are organic polymers which were first synthesized in 1907 by Leo Baekland.1 Plastics are extensively used in all aspects of life due to its strength, flexibility, durability and affordability.2 By the end of year 2022, around 400.3 million metric tons (MMT) of plastic was produced across the globe. Approximately in less than a month, two-third of this globally produced plastic ends up in waste.3 Plastic waste levies substantial amount of environmental burden due to its recalcitrant nature. Based on composition, plastics can take up to 500 years for decomposition. The current plastic waste disposal practices are responsible for the increment in the plastic waste deposition in the natural ecosystem.4 As per the projections, plastic waste generation will triple by 2060 if the current policies remain unchanged.5 Primary plastic use and the product lifetime are important factors which greatly influence plastic waste generation. Plastics like LDPE, which are used in packaging, have a very short “in-use” lifetime and are disposed of within 6 months to 12 months.6 LDPE waste constitutes about 18% of the total plastic waste generated.7
Landfilling has been the predominant method for management of plastic waste. However, nonbiodegradable plastic waste decomposes at a slow rate in landfill environments, leading to increasing demand for land as the volume of plastic waste rises. Furthermore, when plastic waste comes into contact with groundwater; water-soluble harmful compounds in landfills create toxic leachate which can deteriorate the surrounding soil.3,8-10 Use of conventional methods like incineration which involves burning of plastic waste are responsible for the emission of toxic pollutants into the ecosystem. Human exposure to these toxic pollutants can cause impaired lung function and respiratory distress with heightened risk of cancer development.3 Of the total plastic waste produced, only 9% is recycled globally.11 Major portion of this plastic waste is poorly managed, often being discarded unlawfully or burned openly in the environment, ultimately making its way into river or oceans. Approximately 5800 MMT of mismanaged plastic waste ended up in the environment by 2015.6 In the natural environment, decomposition of plastic can happen because of (a) physical and mechanical processes which can lead to substantial structural modifications like cracking and embrittlement. This results in oxidation of the polymer and breakdown of polymer into short-chain molecules; (b) photooxidation due to UV exposure causing polymer breakdown and generation of free radicals; (c) chemical and thermal degradation which involves the breaking and oxidation of polymer chains of plastic; (d) action of several bacteria and fungi which have the ability to degrade plastic by utilizing the polymer as a carbon source which alters the physical and chemical properties of the plastic.12 Degradation of plastics using micro-organisms can address the problem of management of plastic waste in a manner that is both cost-effective and environmental friendly.13
Several researchers in the past have reported numerous microorganisms which are able to breakdown different synthetic plastics in an eco-friendly way. Biodegradation of plastic involves four different stages. The first stage is biodeterioration, where biofilm formation takes place on the plastic surface due to microbes resulting in alterations of the physical, chemical and mechanical properties of plastic.14 In the Biofragmentation stage, the plastic polymers are converted into monomers and oligomers because of enzymatic action or generation of free radicals.15 The mixture of monomers and oligomers are capable of entering the microbial cell membrane and hence get assimilated in the microbial cell where the microbes can utilize them as source of energy and synthesize new microbial biomass. Monomers that cannot traverse the membrane remain on the outer side of the membrane and hence can never be assimilated into the cell.16 Mineralization of plastics by the microorganisms takes place due to the oxidation process, which results in generation of carbon dioxide, methane, nitrogen and water.17 Several bacterial genera, including Pseudomonas, Rhodococcus, Micrococcus, Acinetobacter and Brevibacillus, isolated from various sources, have demonstrated the potential to degrade LDPE.1,18 Bacterial isolates Methylobacterium radiotolerans, Methylobacterium fujisawaense and Lysinibacillus fusiformis isolated from the Koshe Municipal solid waste dumping site in Addis Ababa, Ethiopia achieved significant loss in weight of LDPE sheets by 42%, 37% and 23.87%, respectively, after incubation for 60 days.19 Furthermore, Stenotrophomonas sp. and Achromobacter sp., were isolated from garbage dumpsite and drilling fluids, have been shown to degrade LDPE, with AFM and SEM revealing alterations such as cracks and grooves on the polymer surface.2 Plastic-degrading Pseudomonas aeruginosa strains have been identified and sequenced from Zophobas atratus larvae frass.20
Extracellular enzymes secreted by microorganisms are essential for LDPE degradation. Their involvement in LDPE degradation can be confirmed by observing the change in the weight and functional groups present in LDPE films.2 Lipase and esterase belonging to the class of ‘Hydrolases’, convert long carbon chains to smaller monomers and dimers which accumulate inside the microbial cell and utilized as source of carbon.21,22 Laccases function as oxido-reductases, facilitating the oxidation of diverse aromatic compounds and concurrently driving reduction reactions that convert oxygen into water. Use of oxidizing and hydrolyzing enzyme like laccase and esterase for LDPE degradation is crucial for development of effective bioremediation strategies to address the global issue of plastic pollution. Several studies have reported the role of laccases and lipases in degradation of PE in an effective manner. Johnnie et al.23 isolated laccase from Trichoderma viride and used it for LDPE degradation. After treatment of LDPE sheets with laccase, the density of the LDPE sheets decreased by 0.15 g/cm3. Kim et al.24 analyzed twenty distinct hydrolases for degradation of oxidized polyethylene. They reported weight reduction in LDPE sheet on treatment with Pelosinus fermentans lipase 1 by 44.6% in a period of 5 days due to hydrolysis of ester bonds. In this study, bacterial strains isolated from waste dumpsite soil of Ahmedabad were evaluated for their ability to degrade LDPE. Most of the studies done so far have worked with pre-treated LDPE where the LDPE is modified by exposure to UV causing photo-oxidation or by physical and mechanical processes but biodegradation of untreated LDPE is not well studied.2 So, the bacterial isolates were screened on minimal essential medium (MEM) supplemented with hexadecane to evaluate their ability for LDPE degradation. Furthermore, involvement of laccase and lipase enzyme in LDPE degradation was examined.
Collection of soil samples and bacterial strain isolation
Collection of soil samples was done from two different garbage dumpsites across Ahmedabad. The areas were dumpsite near (a) Gotagam 23° 09’ 80.39” N and 72° 53’ 79.05” E (b) Gyaspur dumpsite (22° 96’ 37.17” N and 72° 54’ 61.83” E). A clean shovel was used to collect the soil samples. For the isolation of bacterial isolates, 1 gram of soil was added to 20 mL of Nutrient Broth medium (Himedia) and was kept under shaking conditions overnight. Serial dilutions were prepared with 0.9% NaCl (Himedia) solution. 10-6, 10-8 and 10-10 dilutions were spread on nutrient agar plates (Himedia) with overnight incubation at 37 °C. Pure isolates were obtained by streaking on nutrient agar medium using four flame method.25
Screening of bacterial isolates
The potential of isolates to degrade LDPE was assessed by culturing the isolates in MEM Agar plates supplemented with hexadecane using bath sonication. The minimal essential media contained 0.4 g/L of sodium chloride (Himedia), 3.0 g/L potassium dihydrogen phosphate (Himedia), 0.12 g/L magnesium sulfate (Himedia), 0.1 g/L calcium chloride (Himedia), 6.0 g/L disodium hydrogen phosphate, 0.1 g/L Tween-20 and 3% Agar with supplementation of 1.0 g/L of n-hexadecane (Himedia). Loopful culture of pure bacterial isolate was smeared on MEM agar plates containing hexadecane with incubation for a week at 37 °C. After one week, zone of clearance was observed by staining with Coomassie Brilliant Blue R-250 (CBB) (Himedia).26
BATH assay
Bacterial strains were cultivated in Nutrient Broth till the mid-logarithmic growth phase was attained. Furthermore, centrifugation was carried out at 8000 rpm for 5-7 min to harvest the bacterial cells at room temperature. The pelleted cells were washed twice using phosphate buffer saline (PBS) having pH of 7.1 followed by resuspension in 2 mL of PBS buffer. The optical density (OD) of the bacterial cell suspension was measured at 600 nm. Subsequently, the bacterial cell suspension was supplemented with 750 µL of hexadecane, followed by vortexing for 2 min and incubation of 15 min for proper phase separation. After incubation, the OD of the aqueous phase was recorded at 600 nm. Cell-free buffer served as the blank. Calculation of bacterial adhesion to hydrophobic surface was carried out as follows:
Cell surface hydrophobicity = {(initial OD- final OD) / initial OD} x 100
Biodegradation assay for LDPE degradation
To evaluate the biodegradation potential of bacterial isolates, 3.0 mL of bacterial culture (4 McFarland turbidity) was added to 20 mL MEM broth medium. Surface sterilized LDPE sheet having 2 cm × 2 cm dimension (weighing 0.05 g) were inoculated in each culture flask and incubated under shaking conditions for 30 days.25 The assay was performed in duplicates. To carry out the assay, LDPE sheets were procured from Smita Polycon from Ahmedabad, Gujarat, India. Transparent green colored LDPE sheet with a thickness of 51 micron were used in the study.
Assessment of degradation of LDPE
Evaluation of LDPE polymer degradation was performed by measuring the change in weight of LDPE followed by FTIR and SEM analyses. The weight loss of LDPE sheet was calculated as follows:
Weight loss (%) = [(Initial weight – Final weight) / Initial weight ] × 100
To assess the chemical modifications of LDPE sheets because of action of bacterial isolates FTIR was performed. Treated and control LDPE sheets were treated with 2% SDS followed by overnight drying after 30 days. Furthermore, scanning of LDPE film in transmission mode was carried out in IR spectrophotometer (IR Spirit-Shimadzu).2
Analysis using SEM was done to observe the alterations on the LDPE surface because of microbial action. Washing with alcohol followed by overnight drying was carried out to observe the alterations in morphology of LDPE polymer.2
Enzyme assays
To carry out the laccase and the lipase activity, the bacterial strains were cultured in MEM supplemented with LDPE sheets which served as the only source of carbon. The enzyme activity was evaluated on the 15th and the 30th day.
Laccase assay
To evaluate the laccase activity, a reaction mixture containing 1 mL of 2 mM Guaiacol (Himedia) prepared in CH3COONa buffer, 3 mL of 10 mM CH3COONa (Himedia) buffer and 1 mL of enzyme [cell free supernatant] was prepared. In the blank, instead of the enzyme, water (1 mL) was used. The enzyme activity was evaluated at 450 nm after incubation of 15 min using microplate reader (Bio-Rad iMark).27 Assay was performed in triplicates. Laccase activity calculation was done as given below:
Enzyme actvity: = A x V / t x e x v
Where A is absorbance, V represents the volume of the total mixture in mL, t is the incubation time, e represents the extinction coefficient of guaiacol which is 0.6740 1 M/cm and the enzyme volume in mL.
Lipase assay
To evaluate lipase production, media containing 0.5% peptone (Himedia), 3% yeast extract (Himedia), 10 mM MgSO4.7H2O (Himedia), 2.5 mM CaCl2 (Himedia), 10 mL Tributyrin (Himedia) and 3% agar (Himedia) was prepared. The bacterial isolates were then spotted on the plates followed by incubation for 48 hours at 37 °C. The medium was observed for zone of clearance around each isolate.18 The assay was performed in triplicates.
Bacterial strain identification and Phylogenetic analysis
Bacterial genomic DNA isolation was carried out using HiPurA® Bacterial Genomic DNA Purification Kit. Bacterial universal primers (27F: 5’-AGAGTTTGATCCTGGCTCAG-3’ and 1492R: 5’-TACCTTGTTACGACTT-3′) were used for the amplification of 16S rRNA gene. PCR amplification process included initial denaturation step at 95 °C for 5 min, 35 cycles of 95 °C for 45 s, 58 °C for 45 s, 72 °C for 1.5 min, and with a final extension of 72 °C for 7 min.2 The sequencing was carried out on Genetic Analyzer 3500XL (ThermoFisher Scientific). The forward and the reverse gene sequence of 16S rRNA gene was assembled to obtain ~1400 bp gene sequence using the BioEdit CAP contig assembly program. EzTaxon server and BlastN of NCBI database tool were used to carry out database similarity search of the 16S rRNA gene of bacterial isolates with type strains. In the final dataset, all the ambiguous positions were removed by Pairwise deletion. Multiple sequence alignments were performed using MUSCLE program in MEGA 11. For the construction and validation of Phylogenetic tree, NJ algorithm with 1000 bootstrap replicates, employing Maximum Composite Likelihood model. The reliability of the phylogenetic tree was evaluated by carrying out additional analysis by maximum likelihood (ML) and minimum-evolution (ME) methods.2
Statistical analysis
All statistical analysis was carried out using MS Excel and GraphPad Prism Software 8. The graphs were prepared using GraphPad Prism Software 8.
Bacterial strain isolation and screening
Collection of soil samples was carried out from two different garbage dumpsites across Ahmedabad (Figure 1). From these samples, eighteen isolates which displayed different morphological characteristics in terms of their color, type, elevation and margin were screened from Gyaspur dumpsite. The bacterial isolates were given a specific identification number from C-1 to C-18. On the other hand, twelve different isolates were obtained from Gota gam dumpsite which were given a specific code from C-19 to C-30.
Figure 1. Garbage dumpsites used for collection of soil samples (a) Gota gam road (b) Gyaspur dumpsite. (Source: Google Maps)
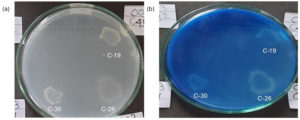
To carry out the primary screening, bacterial isolates were cultured on a MEM including hexadecane as the chief carbon source. The bacterial culture was smeared on MEM plate containing hexadecane and incubated for a week. CBB R-250 dye was used to stain the plates to observe the zone of clearance (Figure 2). Six bacterial strains namely C-5, C-6, C-7, C-8, C-11 and C-15 (Supplementary Figure 1) from Gyaspur soil sample and three isolates (C-19, C-26 and C-30) from Gota gam soil sample gave zone of clearance on MEM plates containing (hexadecane) (Figure 2).
Figure 2. Zone of clearance observed in bacterial isolates cultured on MEM medium supplemented with hexadecane (a) before staining with Coomassie Brilliant Blue (b) after staining with Coomassie Brilliant Blue
BATH assay
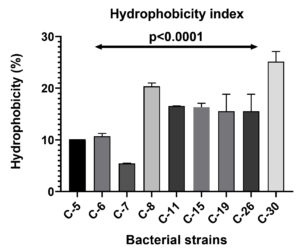
BATH assay was performed to assess the adhesion of bacterial cells to hydrophobic surfaces. The assay revealed that the C-30 strain exhibited the highest hydrophobicity at 25% among the eight isolates (Figure 3). Bacterial strains with hydrophobicity exceeding 10% were subsequently chosen for a biodegradation assay. This assay demonstrated the bacterial cells affinity for organic hydrocarbons, indicating the potential of these microbes to adhere to polyethylene surfaces. Such adherence could enhance biofilm formation and the degradation process.2
Biodegradation assay of LDPE
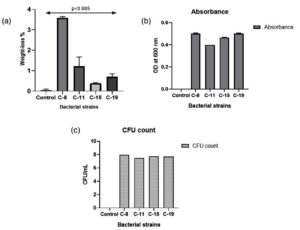
To check the ability of bacterial isolates to carry out LDPE degradation, pre-washed LDPE film was incubated in MEM as the only carbon source for a period of 30 days (Supplementary Figure 2). After 30 days, change in weight of LDPE film was measured to determine the biodegradation capabilities of the bacterial isolates. The weight loss of LDPE sheets for the four bacterial isolates is illustrated in Figure 4. In the control sample, the weight of LDPE sheet remains unchanged. Of the four bacterial isolates, C-8 demonstrated highest weight-loss of LDPE sheet at 3.57%. For isolates C-11, C-15 and C-19, weight losses were 1.22%, 0.378%, and 0.7%, respectively. Yao et al.28 demonstrated in their study that Bacillus subtilis ATCC6051 and Bacillus licheniformis ATCC14580 were able to degrade LDPE by 3.49% and 2.83%, respectively, after incubating with bacterial culture for 30 days. This result is comparable to alteration observed in weight of LDPE in the present study by C-8 isolate. C-8 also demonstrated good hydrophobicity percentage as per the BATH assay in Figure 4.
Figure 4. The graph indicates (a) weight-loss observed in LDPE films after treatment with bacterial isolates (b) Absorbance and (c) CFU count of four bacterial isolates after 30 days of incubation. The data represents the average value in triplicates. Bars show the standard deviation
FTIR analysis
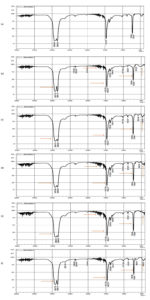
Extracellular enzymes secreted by the microorganisms during the biodegradation process, cause a number of chemical reactions which are responsible for the changes observed in the functional groups present in LDPE. Modifications in spectral peaks were reported at 719 cm-1, 875.57 cm-1, 1307 cm-1, 1462.21 cm-1, 2000-1650 cm-1, 2849.85 cm-1 and 2924.13 cm-1 (Figure 5) in the LDPE films of all the four isolates. Compared to the control, the C-8 isolate exhibited notable changes in the infrared spectrum, with changes in peak observed at 875.67 cm-1 (C-H bending), 1017.09 cm-1 and 1308 cm-1 (O-H bending), 1378 cm-1 (O-H bending), 2345 cm-1 (C=O stretching) and 2375 cm-1, 2854 cm-1, 2912 cm-1 (C-H stretching). Similarly, the C-11 isolate showed distinct spectral changes with peaks at 1085 cm-1, 1307 cm-1, 1378 cm-1, 1646 cm-1 and 2300 cm-1, corresponding to C-O stretching, C-H bending, C=C stretching and O=C=O stretching, respectively. In the C-15 isolate, alterations were detected at 1378.50 cm-1 relative to the control, indicative of alkane C-H bending, and in the range of 1710-1685 cm-1, suggesting C=O stretching. Additionally, shifts in peaks were observed at 875.67 cm-1, 1307.07 cm-1, and in the 2000-1650 cm-1 region, corresponding to C-C vibrations, C-O stretching, and C-H bending, respectively. C-19 isolate exhibited distinct spectral changes with peaks at 719.96 cm-1 (C-H bending), 729.96 cm-1 and 875.67 cm-1 (C=C bending), 1018.52 cm-1, 1079.94 cm-1 and 1307.07 cm-1 (C-O stretching), 1378 cm-1 and 1438 cm-1 (C-H bending) and 2378 cm-1 (O=C=O stretching).
Figure 5. FTIR spectra of LDPE sheets (a) control (b) C-8 isolate (c) C-11 isolate (d) C-15 isolate (e) C-19 isolate
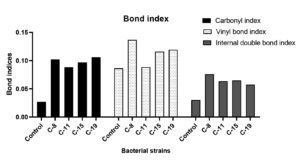
Apart from spectral analysis, calculation of different indices including Carbonyl Index (CI), Double Bond Index (DBI), and Internal Double Bond Index (IDBI) was performed to assess the changes in LDPE chains due to functional bond formation and events chain scission, oxidation and vinylene group formation. The treated LDPE samples exhibited increase in CI, up to 2.5 to 3 folds, along with a 1 to 2 fold increase in DBI and a 2 to 2.5 fold increase in IDBI (Figure 6). These substantial increases in the indices for the treated samples indicate effective biodegradation as bacterial isolates used LDPE sheets as carbon source and incorporated functional groups and transformed the material into simpler molecules like alcohols, ketones and fatty acids.
SEM analysis
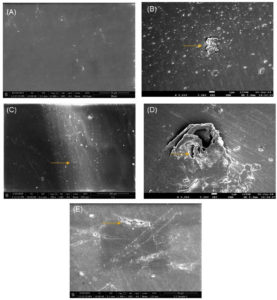
Scanning Electron Microscopy was used to observe the alterations on LDPE surface. Compared to the untreated (control) film, the LDPE sheet treated with bacterial isolates exhibited cracks and grooves on the surface, likely attributed to the enzymes released by the isolates (Figure 7). The control LDPE film, on the other hand, displayed a smooth surface. The presence of biofilm on the LDPE surface substantiated the degradation of LDPE.29
Figure 7. Alterations on LDPE surface (a) Control and treated with (b) C-8 isolate (c) C-11 isolate (d) C-15 isolate and (e) C-19 isolate observed under SEM at 5000 X amplification. Surface alterations, including corrosion and crack formation, were exclusively observed in the treated samples after incubation with bacterial isolates as indicated by the arrows
Enzyme assays
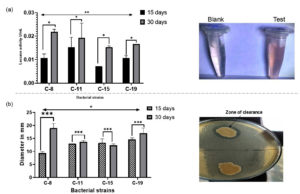
The enzymatic activities of laccase and lipase are illustrated in Figure 8. Estimation of laccase activity was carried out using Guaiacol as the substrate27 and zone clearance method was used to check the lipase activity.18 Highest laccase (0.0219 U/mL) and lipase (19 mm) was exhibited by the C-8 isolate, after 30 days of incubation. The other bacterial isolates demonstrated laccase activity ranging from 0.0148 to 0.0192 U/mL. Statistically significant difference was observed in laccase enzyme activity among the four bacterial isolates. Furthermore, difference in laccase enzyme activity was also observed with increase in time interval from 15th day to 30th day. In comparison to 15th day, higher laccase activity was observed in all the bacterial isolates on 30th day. Except for C-15 isolate, all the other bacterial isolate reported higher lipase activity on 30th day and exhibited zone of clearance ranging from 12 mm to 17 mm. Increment in lipase activity on 30th day compared to 15th day could be attributed to formation of biofilms on LDPE sheet which enhanced enzyme secretion and substrate interaction.30 Additionally, intermediate degradation products generated during LDPE degradation may have further stimulated lipase production.24 The difference in lipase activity among the bacterial isolates and on 15th and 30th day was statistically significant.
Figure 8. Extracellular enzyme production by bacterial isolates (a) Laccase activity observed by bacterial isolates. The microcentrifuge tube labelled test indicates the color development due to laccase production and (b) Lipase activity of the bacterial isolates. Laccase and Lipase assay were carried out in triplicates. The error bar in the graph indicates standard deviation
Identification of bacteria using 16S rRNA sequencing and Phylogenetic analysis
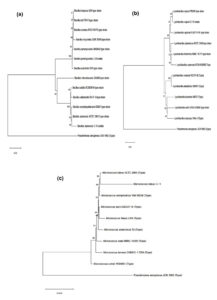
Using Ez taxon and BLAST, the C-8 isolate was identified as Bacillus paramycoides (Accession number – PQ578758), C-11 isolate as Micrococcus luteus (Accession number – PQ578756), C-15 isolate as Bacillus siamensis (Accession number – PQ578759) and C-19 isolate as Lysinibacillus capsici (Accession number – PQ578757). Isolate C-8 showed the highest identity of 99.79% with the type strain of Bacillus paramycoides NH24A2; while C-11, C-15 and C-19 isolate exhibited identity of 90.86% with type strain of Micrococcus luteus NCTC 2665, 98.88% with type strain of Bacillus siamensis KCTC 13613 and 99.78% with type strain of Lysinibacillus capsici PB300, respectively. Phylogenetic tree constructed using Neighbor-joining method displayed that C-8 isolate formed coherent cluster with Bacillus paramycoides NH24A2 (type strain), C-11 isolate with Micrococcus luteus NCTC 2665 (type strain), C-15 isolate with Bacillus siamensis KCTC 13613 (type strain) and C-19 isolate with Lysinibacillus capsici PB300 (type strain) (Figure 9). Bootstrap reiterations in combination with ML and ME-based phylogenetic analysis, revealed a related tree topology, which suggests a strong association between C-8 isolate and Bacillus paramycoides NH24A2 (type strain), C-11 isolate and Micrococcus luteus NCTC 2665 (type strain), C-15 isolate and Bacillus siamensis KCTC 13613 (type strain) and C-19 isolate and Lysinibacillus capsici PB300 (type strain).
For soil sample collection, garbage dumpsites containing large amount of plastic waste were used. Indigenous microorganisms were isolated from soil samples using the serial dilution method.25 Numerous microorganisms have the ability to utilize linear paraffin molecules within a period of 20 days. Many species capable of degrading polyethylene (PE) can also metabolize linear n-alkanes such as paraffin. The process of n-alkanes oxidation by the microorganisms is well documented. As hexadecane and PE share a common chemical structure, in the current study hexadecane was used as a model compound to investigate LDPE biodegradation.31 The screening for hexadecane-degrading bacteria was conducted using the zone clearance method. Similar screening method using hexadecane as the sole carbon source was employed by Yoon et al.26 to check the ability of Pseudomonas sp. E4 isolated from beach soil having high contamination of crude oil to degrade Low-Molecular-Weight Polyethylene (LMWPE). Upon staining with Coomassie Brilliant Blue, distinct clearance zones around most colonies indicated the potential of bacterial isolates to metabolize hexadecane as the only source of carbon.26 Skariyachan et al.32 also used Coomassie Brilliant Blue to stain the plates after screening on MEM plates containing polyethylene glycol (PEG) to check the potential of microbial consortia isolated from garbage dumpsite from Bangalore, India, having the ability to degrade LDPE. Bacterial adhesion and biofilm formation on hydrophobic surface of LDPE is crucial for the degradation of the polymer by bacterial isolates. The biofilm formation takes place due to secretion of exopolysaccharides by the microbial cells, which aid not only in the attachment of the cells to the hydrophobic surfaces found in plastic33 but also enables the microorganisms to use insoluble substrates via series of enzyme catalyzed reactions. In this study, all bacterial isolates displayed high cell surface hydrophobicity indicating their ability to form biofilm bind to hydrophobic surfaces such as polyethylene.2 Bacillus paramycoides (C-8 isolate) exhibited significant cell surface hydrophobicity (~20%) and robust biofilm-forming ability, contributing to the maximal observed weight reduction of LDPE film. Dey et al.2 aimed to isolate bacteria capable of degrading unpretreated LDPE from waste dumpsites and drilling fluid. Cell-surface hydrophobicity was employed as a key parameter to assess the potential of the isolates for LDPE degradation. Notably, isolates exhibiting high cell-surface hydrophobicity demonstrated the greatest reduction in LDPE sheet weight. The study also tested LDPE degradation potential of bacterial isolates by observing the alteration in weight of LDPE sheet. Bacillus paramycoides (C-8 isolate) reported the maximum amount of weight decrement of the LDPE sheet, indicating its higher efficiency to degrade LDPE within thirty days of incubation. Similar findings were reported by Khampratueng et al.,34 who observed that Bacillus sp. AS3 and Sphingobacterium sp. AS8, isolated from a dumping site, achieved LDPE plastic weight reductions of 3.06% and 2.01% (w/w), respectively, following a 30-day incubation period. The weight reduction can be contributed majorly to extracellular enzymes secreted by bacterial isolates attached to the LDPE surface. The four bacterial isolates were cultured in MEM containing LDPE as the chief carbon source for a period of 30 days in aerobic conditions. After incubation, the LDPE sheets were analyzed using SEM and FTIR, to observe the changes in surface topology and chemical structure. SEM analysis showed the formation of fracture and depressions on LDPE surface in combination with reduction in the structural integrity of LDPE films caused by microbial activity.2,25,32 Similar results were observed by Munir et al.25 where they reported structural changes on the surface of LDPE film due to bacterial isolates. The results obtained indicate that LDPE degradation was initiated by bacterial isolates through secretion of extracellular enzymes like laccase, esterase, peroxidases and lipases playing crucial roles.35,36 Sowmya et al.37 isolated Trichoderma harzianum from garbage dumpsite Shivamogga District for polyethylene degradation. Their study revealed that laccase and manganese peroxidase play a vital role in polyethylene degradation since incubation of polyethylene sheets with crude extract of the enzymes lead to significant amount of weight-loss which was further confirmed by FITR analysis. Skariyachan et al.32 identified lipase as a key extracellular enzyme contributing to LDPE degradation. Their study demonstrated significant lipase activity in Pseudomonas sp. strains isolated from garbage dumpsites, suggesting a potential role in plastic breakdown. Similarly, Muhonja et al.18 investigated the involvement of laccases in LDPE degradation and observed the highest plastic weight loss of 35.2% in Bacillus cereus. The strain exhibiting the maximum degradation efficiency also displayed elevated laccase activity, indicating a strong correlation between laccase production and the biodegradation of LDPE. To support the results of weight-loss and SEM analysis and to study the chemical modifications of the LDPE; FTIR analysis was conducted. Alterations of the carbon backbone and modifications of functional groups on LDPE were observed using FTIR. It is a powerful analytical method for characterization of organic, polymeric and inorganic substances. It has been widely employed in the field of plastic degradation to analyze the chemical modifications in plastics.38,39 The analysis revealed the emergence of new functional groups, including carbonyl and alkoxy groups, along with structural changes such as chain cleavage, O-H stretching, and the formation of double bonds. Nadeem et al.39 reported spectral shifts at 2915.12, 2846.42, 1456.84, and 874.39 cm–¹, corresponding to -C-H stretching, -C-H bending, CH‚ bending, and skeletal C-C vibrations, respectively, upon exposure of plastic fragments to the AK-2 (Serratia sp.) strain. Spectral data and bond indices (CI, DBI, and IDBI) suggest that the degradation of LDPE may have been triggered by radical formation, promoting oxidative reactions with oxygen and water. Dey et al.2 also observed significant increase in CI, DBI and TDBI indices due to degradation of LDPE using Stenotrophomonas sp. and Achromobacter sp. obtained using enrichment culture technique.
Extracellular enzyme production is vital for polymer degradation. Enzyme catalyze breakdown of the polymer into smaller subunits which can traverse the cell membrane and assimilate in microbial cells to be metabolized as a carbon substrate, resulting in generation of carbon dioxide, water and energy. This study investigated the production of extracellular enzymes laccase and lipase. Both enzyme activities increased with longer incubation periods, and production increased when microbes were in close proximity to polyethylene.18 Among all the bacterial isolates, the highest reduction in weight loss was observed in Bacillus paramycoides (C-8 isolate) which also displayed highest activity of laccase and lipase enzymes, suggesting a strong correlation between the production of these enzymes and LDPE degradation.
Plastic waste poses a significant environmental challenge, with polyethylene being a major contributor. Traditional disposal methods like landfills and incineration result in toxic by-products, and much of this waste is poorly managed in Indian Subcontinent. This study was designed with the aim to assess the potential of bacterial isolates from garbage dumpsite soil of Ahmedabad to degrade LDPE. Among the isolates, Bacillus paramycoides demonstrated the highest biodegradation efficiency over a 30-day period. Prolonged incubation is expected to further enhance degradation levels. FTIR and SEM analyses confirmed LDPE degradation, with FTIR revealing change in spectral peaks due to C=O stretching, C=C vibrations and C-H bending, and SEM showing fractures and depressions on LDPE surface. The high levels of laccase and lipase activity in B. paramycoides, which corresponded with its superior biodegradation performance, suggest these enzymes play a key role. The findings from this study could aid in understanding the importance of different enzymes in LDPE degradation. Designing the pathway of LDPE degradation could further help in developing more effective microbial consortia with greater potential for LDPE degradation worldwide.
Additional file: Additional Figure S1-S2.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
This research work was funded by Gujarat State Biotechnology Mission (GSBTM, DST, GOG), vide grant number – GSBTM/JD/R&D/618/21-22/3672.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Thakur S, Mathur S, Patel S, Paital B. Microplastic Accumulation and Degradation in Environment via Biotechnological Approaches. Water. 2022;14(24):4053.
Crossref - Dey AS, Bose H, Mohapatra B, Sar P. Biodegradation of Unpretreated Low-Density Polyethylene (LDPE) by Stenotrophomonas sp. and Achromobacter sp., Isolated From Waste Dumpsite and Drilling Fluid. Front Microbiol. 2020;11:603210.
Crossref - Huang S, Wang H, Ahmad W, et al. Plastic Waste Management Strategies and Their Environmental Aspects: A Scientometric Analysis and Comprehensive Review. Int J Environ Res Public Health. 2022;19(8):4556.
Crossref - Andrady AL, Neal MA. Applications and societal benefits of plastics. Philos Trans R Soc Lond B Biol Sci. 2009;364(1526):1977-1984.
Crossref - Lebreton L, Andrady A. Future scenarios of global plastic waste generation and disposal. Palgrave Commun. 2019;5(1):6.
Crossref - Geyer R, Jambeck JR, Law KL. Production, use, and fate of all plastics ever made. Sci Adv. 2017;3(7):e1700782.
Crossref - Wojnowska-Baryla I, Bernat K, Zaborowska M. Plastic Waste Degradation in Landfill Conditions: The Problem with Microplastics, and Their Direct and Indirect Environmental Effects. Int J Environ Res Public Health. 2022;19(20):13223.
Crossref - Alyousef R, Ahmad W, Ahmad A, Aslam F, Joyklad P, Alabduljabbar H. Potential use of recycled plastic and rubber aggregate in cementitious materials for sustainable construction: A review. J Clean Prod. 2021;329:129736.
Crossref - Teuten EL, Saquing JM, Knappe DRU, et al. Transport and release of chemicals from plastics to the environment and to wildlife. Philos Trans R Soc B Biol Sci. 2009;364(1526):2027-2045.
Crossref - Zahra S, Abbas SS, Mahsa MT, Mohsen N. Biodegradation of low-density polyethylene (LDPE) by isolated fungi in solid waste medium. Waste Manag. 2010;30(3):396-401.
Crossref - Liu X, Lei T, Bore A, Lou Z, Abdouraman B, Ma W. Evolution of global plastic waste trade flows from 2000 to 2020 and its predicted trade sinks in 2030. J Clean Prod. 2022;376:134373.
Crossref - Pilapitiya PGCNT, Ratnayake AS. The world of plastic waste: A review. Cleaner Materials. 2024;11:100220.
Crossref - Shilpa, Basak N, Meena SS. Microbial biodegradation of plastics: Challenges, opportunities, and a critical perspective. Front Environ Sci Eng. 2022;16(12):161.
Crossref - Gu JD. Microbiological deterioration and degradation of synthetic polymeric materials: recent research advances. Int Biodeterior Biodegradation. 2003;52(2):69-91.
Crossref - Mateo C, Palomo JM, Fernandez-Lorente G, Guisan JM, Fernandez-Lafuente R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb Technol. 2007;40(6):1451-1463.
Crossref - Kale SK, Deshmukh AG, Dudhare MS, Patil VB. Microbial degradation of plastic: a review. J Biochem Tech 2015;6(2):952-961.
- Nakajima-Kambe T, Onuma F, Kimpara N, Nakahara T. Isolation and characterization of a bacterium which utilizes polyester polyurethane as a sole carbon and nitrogen source. FEMS Microbiol Lett. 1995;129(1):39-42.
Crossref - Muhonja CN, Magoma G, Imbuga M, Makonde HM. Molecular Characterization of Low-Density Polyethene (LDPE) Degrading Bacteria and Fungi from Dandora Dumpsite, Nairobi, Kenya. Int J Microbiol. 2018;2018(1):1-10.
Crossref - Nademo ZM, Shibeshi NT, Gemeda MT. Isolation and screening of low-density polyethylene (LDPE) bags degrading bacteria from Addis Ababa municipal solid waste disposal site “Koshe.” Ann Microbiol. 2023;73(1):6.
Crossref - Zaman I, Turjya RR, Shakil MS, et al. Biodegradation of polyethylene and polystyrene by Zophobas atratus larvae from Bangladeshi source and isolation of two plastic-degrading gut bacteria. Environmental Pollution. 2024;345:123446.
Crossref - El Salamony DH, El Gayar DA, El Mahdy AR, Zaghloul TI. Preparation and characterization of silica nanoparticles as an efficient carrier for two bio-detergents based enzymes. J Surfactants Deterg. 2023;26(4):577-592.
Crossref - Kaushal J, Khatri M, Arya SK. Recent insight into enzymatic degradation of plastics prevalent in the environment: A mini – review. Clean Eng Technol. 2021;2:100083.
Crossref - Johnnie DA, Issac R, Prabha ML. Bio Efficacy Assay of Laccase Isolated and Characterized from Trichoderma viride in Biodegradation of Low Density Polyethylene (LDPE) and Textile Industrial Effluent Dyes. J Pure Appl Microbiol. 2021;15(1):410-420.
Crossref - Kim DW, Lim ES, Lee GH, et al. Biodegradation of oxidized low density polyethylene by Pelosinus fermentans lipase. Bioresour Technol. 2024;403:130871.
Crossref - Munir E, Sipayung FC, Priyani N, Suryanto D. Potential of bacteria isolated from landfill soil in degrading low density polyethylene plastic. IOP Conf Ser: Earth Environ Sci. 2018;126(1):012144.
Crossref - Yoon MG, Jeon HJ, Kim MN. Biodegradation of Polyethylene by a Soil Bacterium and AlkB Cloned Recombinant Cell. J Bioremed Biodegrad. 2012;03(04):1000145.
Crossref - El-Monssef RAA, Hassan EA, Ramadan EM. Production of laccase enzyme for their potential application to decolorize fungal pigments on aging paper and parchment. Ann Agric Sci. 2016;61(1):145-154.
Crossref - Yao Z, Seong HJ, Jang YS. Degradation of low density polyethylene by Bacillus species. Appl Biol Chem. 2022;65(1):84.
Crossref - Khruengsai S, Sripahco T, Pripdeevech P. Low-Density Polyethylene Film Biodegradation Potential by Fungal Species from Thailand. J Fungi. 2021;7(8):594.
Crossref - Mohanan N, Wong CH, Budisa N, Levin DB. Characterization of Polymer Degrading Lipases, LIP1 and LIP2 From Pseudomonas chlororaphis PA23. Front Bioeng Biotechnol. 2022;10:854298.
Crossref - Montazer Z, Najafi MBH, Levin DB. Challenges with Verifying Microbial Degradation of Polyethylene. Polymers. 2020;12(1):123.
Crossref - Skariyachan S, Megha M, Kini MN, Mukund KM, Rizvi A, Vasist K. Selection and screening of microbial consortia for efficient and ecofriendly degradation of plastic garbage collected from urban and rural areas of Bangalore, India. Environ Monit Assess. 2015;187(1):4174.
Crossref - Wilkes RA, Aristilde L. Degradation and metabolism of synthetic plastics and associated products by Pseudomonas sp.: capabilities and challenges. J Appl Microbiol. 2017;123(3):582-593.
Crossref - Khampratueng P, Rice D, Anal AK. Biodegradation of low-density polyethylene by the bacterial strains isolated from the dumping site community. Discov Appl Sci. 2024;6(7):348.
Crossref - Ahmed T, Shahid M, Azeem F, et al. Biodegradation of plastics: current scenario and future prospects for environmental safety. Environ Sci Pollut Res. 2018;25(8):7287-7298.
Crossref - Ru J, Huo Y, Yang Y. Microbial Degradation and Valorization of Plastic Wastes. Front Microbiol. 2020;11.
Crossref - Sowmya HV, Ramalingappa, Krishnappa M, Basaiah T. Degradation of polyethylene by Trichoderma harzianum-SEM, FTIR, and NMR analyses. Environ Monit Assess. 2014;186(10):6577-6786.
Crossref - Montazer Z, Habibi-Najafi MB, Mohebbi M, Oromiehei A. Microbial Degradation of UV-Pretreated Low-Density Polyethylene Films by Novel Polyethylene-Degrading Bacteria Isolated from Plastic-Dump Soil. J Polym Environ. 2018;26(9):3613-3625.
Crossref - Nadeem H, Alia KB, Muneer F, et al. Isolation and identification of low-density polyethylene degrading novel bacterial strains. Arch Microbiol. 2021;203(9):5417-5423.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.