ISSN: 0973-7510
E-ISSN: 2581-690X
The current study aimed to assess and compare the bacteriological spectrum of acute and chronic dacryocystitis and the antibiotic susceptibility and resistance of the causative pathogens to commonly used antimicrobials. This was a prospective observational study. Cases of dacryocystitis were categorized as acute or chronic, based on clinical features. Specimens were obtained by sterile cotton swabs from the lower conjunctival fornix and from the puncta by applying pressure over the lacrimal sac area or by performing lacrimal syringing. Specimens were inoculated on appropriate culture media and incubated at 37ºC for 24-48 hours. Bacterial species were identified based on colony morphology and standard biochemical tests. Antibiotic Susceptibility Testing was assessed by Kirby Bauer disc diffusion technique using Mueller Hinton agar following Clinical and Laboratory Standards Institute guidelines. Out of 50 patients, 37 (74%) had chronic dacryocystitis and 13 (26%) had acute dacryocystitis. 35 bacterial species were recovered. Gram-positive organisms were the most isolated organisms i.e., 27 out of 35 (77.2%). In chronic dacryocystitis, the predominant bacterial species were Staphylococcus epidermidis (36%). In acute dacryocystitis, the predominant bacterial species were Staphylococcus aureus (40%). Against gram-positive organisms, Vancomycin and Linezolid were most effective (100%). Against gram-negative bacterial species, Amikacin was most effective (100%). High prevalence rate of antibiotic resistance was found, with 40% of the total bacterial species resistant to 5 or more antibiotics. The alarming rate of multi-drug resistance underscores the imperative need for tailored antibiotic strategies and continuous monitoring. Evidence based antibiotic therapy may also help to prevent failures of DCR, progression to chronicity and antibiotic resistance.
Dacryocystitis, Bacterial Species, Antibiotic Sensitivity, Antibiotic Resistance
Dacryocystitis is inflammation of the lacrimal sac that occurs because of obstruction of the nasolacrimal duct (NLD).1 It presents in two forms- Acute and Chronic dacryocystitis. Acute dacryocystitis is acute onset inflammation which presents with subacute onset of pain and epiphora. Chronic dacryocystitis is commoner and presents with chronic epiphora, mucopurulent discharge and chronic or recurrent unilateral conjunctivitis.2
Bacteriological spectrum of dacryocystitis comprises of several bacterial species- staphylococci, pneumococci, streptococci – reflecting the conjunctival flora. The bacteriology of acute and chronic dacryocystitis infections may differ. The microbial spectrum might shift over time, resulting in therapy failures.3 There may also be significant geographical variation in aetiology depending on the climatic conditions.4 The pattern of antibiotic resistance may also differ across regions.5
The ever-evolving and changing trends of the already vast spectrum of bacteriology of dacryocystitis requires more and more study to ensure choice of appropriate antibiotics. Progression to chronicity, antibiotic resistance and recurrence can be reduced with specific antibiotics to which the pathogens are sensitive. This information also contributes to postoperative recovery.
This study was done to unveil the microbial landscape and antibiotic responses in acute and chronic dacryocystitis cases of Western U.P. to facilitate management of these cases. Cases of congenital dacryocystitis were not studied. So only patients aged over 20 years were included. Cases of encysted mucocele and pyocele were excluded as there would have been no regurgitation of fluid on pressure in these cases. Also, cases of maxillofacial trauma or surgery, recurrence, failed DCR and patients who had taken any topical or oral antibiotic in the last one month, wherein the bacterial profile could have been altered, were all excluded from the study sample.
Aim
To study and compare the bacteriological spectrum of acute and chronic dacryocystitis and the antibiotic susceptibility and resistance of the causative pathogens to commonly used antimicrobials.
Objectives
- To study the bacteriological spectrum of chronic dacryocystitis.
- To study the bacteriological profile of acute dacryocystitis.
- To compare the bacterial etiology of acute and chronic dacryocystitis.
- To study the antibiotic susceptibility and resistance of bacterial pathogens to commonly prescribed antimicrobial agents.
This was a prospective observational study, carried out between November 2020 and April 2022. It was done after obtaining prior approval and clearance from Institutional Ethics Committee and written informed consent from all the participants.
The first 50 consecutive patients, aged 20 years or more, who presented to the Ophthalmology OPD and Casualty of our Institute during the study period, were diagnosed with acute or chronic dacryocystitis and consented to undergo bacteriological evaluation, were included in the study.
Patients were examined and cases of dacryocystitis were diagnosed. Cases were categorized as acute or chronic, based on their signs and symptoms. Pain, redness, and a tender swelling in the lacrimal sac area, as well as tears or discharge in the conjunctiva or a lacrimal abscess was labelled as Acute dacryocystitis. Patients with long-standing epiphora, mucocele, and regurgitation of mucoid or mucopurulent material on pressure on the sac area or on irrigation of the lacrimal drainage system were diagnosed with chronic dacryocystitis.1,6,7
Exclusion criteria
- Children with congenital dacryocystitis
- Adolescents <20 years of age
- Encysted mucocele and pyocele
- Patients with common canalicular blockage
- Suspected malignancy of sac
- History of maxillofacial trauma or surgery
- History of topical or systemic antibiotic use in the past 1 month
- Recurrent cases and cases of failed Dacryocystorhinostomy
The first 50 consecutive patients diagnosed with acute or chronic dacryocystitis and fulfilling the inclusion/exclusion criteria were enrolled. The subjects underwent a thorough ophthalmic assessment.
Specimens were obtained by wiping a sterile cotton swab across the lower conjunctival fornix of the diseased eye, making sure the swab did not touch the eyelids. Samples were also taken from the puncta by applying pressure to the lacrimal sac area, or by lacrimal irrigation with saline and collecting the sample from the refluxing material. Along with swabs, pus discharge following spontaneous abscess bursting or incision and drainage were obtained in cases of acute dacryocystitis.1,8
Samples of every patient were collected in two sterile swabs. One swab was inoculated immediately on 5% Sheep blood agar, Chocolate agar and MacConkey agar. The other swab was used for Gram staining for direct microscopy. After inoculation, the culture plates were incubated overnight at 37°C. Then the plates were examined for any growth of organisms. Organisms grown on the culture plates were identified by gram staining and standard biochemical tests using standard laboratory criteria.9 Bacterial species identification was followed by Antibiotic Susceptibility Testing (AST). AST was done by Kirby Bauer disc diffusion technique on Mueller Hinton Agar (MHA) following Clinical and Laboratory Standards Institute (CLSI) guidelines.10 The results were declared as sensitive or resistant. Methicillin-Resistant Staphylococcus aureus (MRSA) and Methicillin-Resistant Staphylococcus epidermidis (MRSE) were tested by Cefoxitin (30µg) disc diffusion test. Staphylococci demonstrating resistance to Cefoxitin were considered Methicillin-resistant. All culture media and antibiotic discs were procured from Hi-media Laboratories Pvt Ltd, Mumbai, India.
The data was tabulated, and IBM SPSS Version 20.0 software was utilised to analyse it. P value of <0.05 was considered statistically significant.
Dacryocystitis can become life-threatening, and can progress to orbital cellulitis, orbital abscess, meningitis, and cavernous sinus thrombosis.11 It requires special attention to initiate appropriate treatment at the earliest.
Out of the 50 patients, 37 or 74% were suffering from chronic dacryocystitis. 13 patients or 26% of the patients were diagnosed with acute dacryocystitis. Chronic dacryocystitis is more frequently encountered than acute dacryocystitis. This has also been observed by Bharathi et al.,12 Prakash et al.,13 Mills et al.,14 Rizvi et al.15 and Ray et al.16 in their study.
Majority of the patients (80% of the total) were >30 years of age. In the acute group 30.8% patients were ≤ 30 years of age and in the chronic group 16.2% patients were ≤30 years old. This difference in distribution of age between the two groups was statistically significant (p= 0.031). This means that there was significantly higher number of people aged less than 30 years of age in the acute group as compared to the chronic group (Table 1). In the study conducted by Bahram et al.,17 similar distribution of patients aged ≤ 30 years was seen (35% in acute vs 2.5% in chronic). Similar results were also reported in Bharathi et al.12 study (23.6% vs 10% respectively).
Table (1):
Comparing age with type (Acute/ Chronic) of Dacryocystitis
| Age (Years) | Dacryocystitis | Total | P value | |
|---|---|---|---|---|
| Acute | Chronic | |||
| ≤30 | 4 (30.8%) | 6 (16.2%) | 10 (20.0%) | 0.031 |
| >30 | 9 (69.2%) | 31 (83.8%) | 40 (80.0%) | |
| Total | 13 (100%) | 37 (100%) | 50 (100%) | |
Females comprised of majority of the patients in the chronic group (26 patients or 70.3%) as well as overall (32 patients or 64%). Chaudhry et al.18 reported a female majority of 65.4%. Sun et al.19 reported 85.7% females. The smaller nasolacrimal canal diameter and hormonal variables may explain the female predilection.20
Most patients (37 patients or 74%) were residents of rural areas. Most of the patients belonged to the upper lower class (23 patients or 46%). Similar findings were reported by Prakash et al.13 These patients were likely to be working in smoky kitchens and engaging in agricultural work where hygiene may have been poor.
Overall, 35 out of 50 (70%) samples showed bacterial growth on culture, while 15 (30%) samples showed no growth (sterile sample). 35 bacterial species were recovered from the 35 samples that were culture positive. No sample revealed mixed growth. However, it should be mentioned that only aerobic culture testing was done in the current study. The 15 samples that were sterile may have had the presence of anaerobes, fungal or viral pathogens, which were not assessed.
Within the acute dacryocystitis group the bacterial culture was positive in 77% samples and in 67.6% samples in the chronic group. Hence, the culture positivity rate was more in acute dacryocystitis as compared to chronic dacryocystitis and this was statistically significant (p=0.028) (Table 2). In a study conducted by Rizvi et al.,15 bacterial growth was found in 76.47% of acute cases compared to only 37.84% of chronic cases. Hence, infectious etiology was significantly associated with acute dacryocystitis. Contrary to these findings, Mills et al.14 found there was greater growth positivity rate in chronic cases compared to acute.
Table (2):
Comparing culture positivity rate in Acute and Chronic Dacryocystitis
| Growth | Dacryocystitis | Total | P value | |
|---|---|---|---|---|
| Acute | Chronic | |||
| Absent | 3 (23.0%) | 12 (32.4%) | 15 (30.0%) | 0.028 |
| Present | 10 (77.0%) | 25 (67.6%) | 35 (70.0%) | |
| Total | 13 (100%) | 37 (100%) | 50 (100%) | |
Gram-positive organisms were most isolated i.e., 27 out of 35 (77.2%). The gram-negative bacterial species were seen in fewer samples, i.e., 8 out of 35 (22.8%) (Table 3). The predominance of gram-positive bacterial species was also seen when analyzing acute and chronic dacryocystitis separately. The predominance of gram-positive organisms in both acute and chronic dacryocystitis is the general trend in several previous studies, like those of Bharati et al.,12 Hartikainen et al.,6 Mills et al.,14 Chaudhry et al.18 and Brook et al.21 and the commonest of them are Staphylococcus species (Staphylococcus aureus and Staphylococcus epidermidis) and Streptococcus pneumoniae.
Table (3):
Comparing bacterial species isolated in acute and chronic dacryocystitis
| Bacterial species Type | Dacryocystitis | Total | P value | |
|---|---|---|---|---|
| Acute | Chronic | |||
| Gram Negative | 3 (30.0%) | 5 (20.0%) | 8 (22.8%) | 0.660 |
| Gram Positive | 7 (70.0%) | 20 (80.0%) | 27 (77.2%) | |
| Total | 10 (100%) | 25 (100%) | 35 (100%) | |
In the chronic dacryocystitis group, gram-positive organisms were found in 80% samples, whereas gram-negative were found in 20% samples. In acute dacryocystitis, gram-positive organisms were recovered in 70% and gram-negative in 30% samples. Thus, gram-negative bacterial species were more prevalent within the acute group as compared to the chronic group (30% vs. 20%). However, the difference was not statistically significant (p=0.660) (Table 3). Some recent studies, like that of Bahram et al.,17 have suggested an increasing isolation of gram-negative organisms, especially in cases of acute dacryocystitis. There are other recent articles too, that have hinted on the changing trends in the microbiologic spectrum of dacryocystitis, including increased prevalence of Gram-negative and MRSA infections.22-25
Most common gram-positive bacterial species found overall was Staphylococcus epidermidis, found in 28.6% of all samples. Pseudomonas was the most frequently seen gram-negative organism and was isolated in 14.3% samples (Table 4). Ray et al.16 and Briscoe et al.22 also found Pseudomonas aeruginosa to be the most common Gram-negative organism in their studies.
Table (4):
Comparing bacterial species in Acute and Chronic Dacryocystitis
| Bacterial species | Dacryocystitis | Total | P value | ||
|---|---|---|---|---|---|
| Acute | Chronic | ||||
| Gram Negative | Escherichia coli | 1 (10%) | 0 (0) | 1 (2.8%) | 0.128 |
| Klebsiella pneumoniae | 0 (0) | 1(4%) | 1 (2.8%) | ||
| Pseudomonas aeruginosa | 2 (20%) | 3 (12%) | 5 (14.3) | ||
| Haemophilus influenzae | 0 (0) | 1 (4%) | 1 (2.8%) | ||
| Gram Positive | Staphylococcus epidermidis | 1 (10%) | 9 (36%) | 10(28.6%) | 0.081 |
| Staphylococcus aureus | 4 (40%) | 4 (16%) | 8(22.8%) | ||
| Streptococcus pneumoniae | 2 (20%) | 7 (28%) | 9(25.7%) | ||
| TOTAL | 10(100%) | 25(100%) | 35(100%) | ||
Within the chronic dacryocystitis group, the predominant bacterial species were Staphylococcus epidermidis (36%), followed by Streptococcus pneumoniae (28%) and Staphylococcus aureus (16%). Within the acute dacryocystitis group, the predominant bacterial species were Staphylococcus aureus (40%), followed by Pseudomonas aeruginosa and Streptococcus pneumoniae (20%) (Table 4). Correlating strongly with these findings are the findings of Bahram et al.,17 wherein Staphylococcus aureus and Pseudomonas species were more prevalent in acute dacryocystitis and Staphylococcus epidermidis was more prevalent in chronic dacryocystitis. As per Bharathi et al.,12 Staphylococcus aureus (22.3%) and Pseudomonas species (21.1%) were isolated as the predominant bacterial species in acute dacryocystitis, whereas in chronic dacryocystitis, Coagulase-negative Staphylococcus aureus (Staphylococcus epidermidis) was the predominant bacterial pathogen (44.2%). It can be concluded that the more virulent bacterial species (like Staphylococcus aureus) are responsible for acute dacryocystitis and the indolent bacterial species (Staphylococcus epidermidis) are more likely to result in dacryocystitis of chronic onset.
A total of 6 methicillin-resistant bacterial species were isolated in the study sample, 3 in the acute group and 3 in the chronic group. The prevalence of methicillin-resistant strains was more in acute dacryocystitis as compared to chronic (30% vs. 12%). However, the difference was not statistically significant (Table 5). Such higher frequency of MRSA infections in the acute group may be because they are more aggressive and present with acute onset of symptoms and are unlikely to be chronic, low-grade infections.
Table (5):
Types and Distribution of Methicillin-resistant Staphylococci in Acute and Chronic dacryocystitis
| Bacterial culture isolates | Dacryocystitis | Total | P value | |
|---|---|---|---|---|
| Acute | Chronic | |||
| STAPH. EPIDERMIDIS-MRSE | 1 | 2 | 3 | 0.261 |
| STAPH.AUREUS -MRSA | 2 | 1 | 3 | |
MRSE: Methicillin Resistant Staphylococcus Epidermidis, MRSA: Methicillin Resistant Staphylococcus Aureus
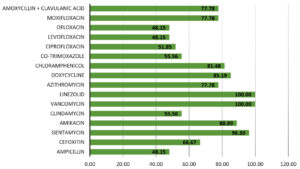
Figure 1 shows the overall sensitivity of gram-positive bacterial species. Vancomycin and Linezolid were the most effective antibiotics with 100% efficacy, i.e., all gram-positive bacterial species recovered in this study were sensitive to them. Least sensitivity was exhibited to Ofloxacin, Levofloxacin, and Ampicillin (48.15%). Similarly, in a study conducted in India by Prakash et al.,13 the gram-positive bacterial species were most sensitive to vancomycin (100%), tobramycin and linezolid (99.36%).
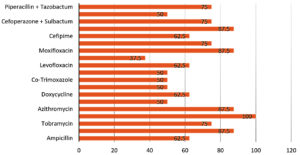
Figure 2 shows the overall sensitivity of gram-negative bacterial species. Amikacin was the most effective antibiotic with 100% sensitivity. Least effective against gram-negative organisms was Ofloxacin (37.5%).
Like the findings of Bharathi et al.,12 Amikacin was highly effective against both Gram-positive and Gram-negative isolates (100% against gram-negative, 88.89% against gram-positive). A possible explanation is that Amikacin is not frequently used in our region.
A trend of increase in resistance of ocular pathogens against fluoroquinolones has been observed. Moxifloxacin, which is a fourth-generation quinolone, has been reported to have a broader efficacy against Gram-positive pathogens as compared to ciprofloxacin.26 This was also true in the current study, with susceptibility of ciprofloxacin and moxifloxacin to gram-positive bacterial species being 51.85% and 77.78%, respectively, and to gram-negative bacterial species being 50% and 87.5%, respectively. Ofloxacin had even lower efficacy than ciprofloxacin. Widespread use of ciprofloxacin for all bacterial eye infections in our population has probably led to the decrease in susceptibility.
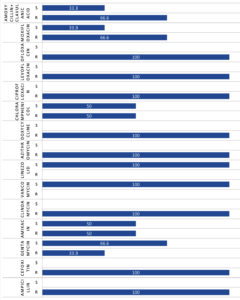
MRSA infections of the eye and its adnexa are a new and increasing problem. We should consider the possible involvement of MRSA while treating dacryocystitis.27 Figure 3 shows the percentage of Methicillin-resistant Staphylococci that were sensitive/ resistant to the corresponding antibiotics. Methicillin-resistant Staphylococci were those showing resistance to Cefoxitin. All the MRSA and MRSE isolated in this study sample were resistant to Ampicillin, Clindamycin, Azithromycin, Doxycycline, Ciprofloxacin, Levofloxacin and Ofloxacin. However, 100% Methicillin-resistant Staphylococci were sensitive to Vancomycin and Linezolid. Likewise, in a study conducted by Gajapati et al.,28 the Methicillin-resistant CONS showed 100% sensitivity to Linezolid and Vancomycin.
As seen in Table 6, not a single bacterium was found to be sensitive to all antibiotics (R0=0). Most of the bacterial species exhibited resistance to 2 antibiotics i.e., 25.7% of the total bacterial culture isolates. 22.9% bacterial species showed resistance to 3 antibiotics. 40% of the total bacterial species were found to be resistant to 5 or more antibiotics. Methicillin-resistant Staphylococci exhibited resistance to maximum number of drugs. Broad spectrum antibiotics are widely prescribed empirically, without definite diagnosis. This enhances and escalates the natural antibiotic resistance mechanisms of bacterial species. This may be responsible for the relatively higher prevalence rate of resistance in the current study.
Table (6):
Multiple antibiotic resistance pattern
Bacterial species |
Total |
R0 |
R1 |
R2 |
R3 |
R4 |
≥R5 |
|---|---|---|---|---|---|---|---|
E. COLI |
1 |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
1/1(100%) |
H. INFLUENZAE |
1 |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
1/1 (100%) |
K. PNEUMONIAE |
1 |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
1/1 (100%) |
P. AERUGINOSA |
5 |
0 (0) |
0 (0) |
0 (0) |
1/5 (20) |
0 (0) |
4/5 (80.0%) |
STAPH. EPIDERMIDIS |
7 |
0 (0) |
2/7 (28.6%) |
2/7(28.6%) |
3/7 (42.9%) |
0 (0) |
0 (0) |
STAPH. EPIDERMIDIS-MRSE |
3 |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
3/3(100%) |
STAPH. AUREUS |
5 |
0 (0) |
0 (0) |
4/5 (80.0%) |
1/5 (20.0%) |
0 (0) |
0 (0) |
STAPH. AUREUS- MRSA |
3 |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
3/3 (100%) |
S. PNEUMONIAE |
9 |
0 (0) |
1/9 (11.1%) |
3/9 (33.3%) |
3/9 (33.3%) |
1/9 (11.1%) |
1/9(11.1%) |
TOTAL |
35 |
0 |
3 (8.6%) |
9 (25.7%) |
8
(22.9%) |
1 (2.9%) |
14(40.0% |
R0- sensitive to all antibiotics, R1 – resistant to 1 antibiotic. R2- resistant to 2 antibiotics, R3 – resistant to 3 antibiotics, etc.
Limitations
This study was carried out in a single centre with a relatively small sample size. Additionally, only aerobic culture testing was performed, and the presence of anaerobic bacterial species was not studied. Hence, the current study demonstrates the role of only aerobic bacterial pathogens and their susceptibility to commonly used antimicrobials.
Knowledge of the underlying etiology of dacryocystitis facilitates the choice of antimicrobial agent. The findings of this study contribute to the existing literature and offer guidance for clinicians in the region, with implications for global antibiotic stewardship. The authors recommend conducting bacterial culture and sensitivity testing before prescribing antibiotics in these cases. This will help provide efficient treatment and avoid unnecessary load of antibiotics, progression to chronicity and microbial resistance.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
SR, NM, ABM, NG conceptualized and designed the study. SS, SR and RB data acquisition. SR, NM, ABM, NG performed clinical studies. SR performed literature search, data and statistical analysis. SR, NM, ABM, NG wrote the manuscript. NM, SR, NG, ABM, SS and RB reviewed the manuscript. NM, SR, NG and ABM edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Institutional Ethics Committee, Shri Ram Murti Smarak Institute of Medical Sciences, Bareilly with reference number SRMSIMS/ECC/2019-20/147
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- Iliff NT. Infections of the lacrimal drainage system. In: Peopse JS, Holland GN, Wilhelmus KR (eds). Ocular Infection and Immunity. St Louis, MO: Mosby. 1996:1346-1355.
- Bowling B. Kanski’s Clinical Ophthalmology. 8th ed. Great Britain: Elsevier. 2016.
- Chung SY, Rafailov L, Turbin RE, Langer PD. The microbiologic profile of dacryocystitis. Orbit. 2018; 38(1):72-78.
Crossref - Burd EM. Bacterial keratitis and conjunctivitis: bacteriology. In: Smolin G, Thoft RA (eds). The Cornea: Scientific Foundations and Clinical Practices. 3rd ed. Boston: Little, Brown & Co. 1994:115-124.
- Brook I. Ocular infections due to anaerobic bacterial species in children. J Pediatr Ophthalmol Strabismus. 2008;45(2):78-84.
Crossref - Hartikainen J, Lehtonen OP, Saari KM. Bacteriology of lacrimal duct obstruction in adults. Br J Ophthalmol. 1997;81(1):37-40.
Crossref - Korn BS, Burkat CN, Carter KD, et al. Oculofacial Plastic and Orbital Surgery. Basic and Clinical Science Course Section 7. China: American Academy of Ophthalmology. 2019.
- Wilhelmus KR, Liesegang TJ, Osato MS, Jones DB. Cumitech 13 A, Laboratory Diagnosis of Ocular Infections. Washington, DC: American Society for Microbiology Press. 1994.
- Carroll KC, Pfaller MA. Manual of Clinical Microbiology.12th Edition. Washington DC: American Society for Microbiology Press. 2019.
Crossref - Performance Standards for Antimicrobial Susceptibility Testing. 30th ed. CLSI supplement M100.Wayne PA: Clinical and Laboratory Standards Institute; 2020
- Gupta AK, Raina UK, Gupta A. The lacrimal apparatus. In: Textbook of Ophthalmology. First edition. New Delhi: BI Churchill Livingstone; 1999.
- Bharathi MJ, Ramakrishnan R, Maneksha V, Shivakumar C, Nithya V, Mittal S. Comparative bacteriology of acute and chronic dacryocystitis. Eye. 2008;22(7):953-960.
Crossref - Prakash R, Girish Babu RJ, Nagaraj ER, Prashanth HV, Jayashree S. Shah A Bacteriological Study of Dacryocystitis. J Clin Diagn Res. 2012;4(Suppl-2), 6(4):652-655.
- Mills DM, Bodman MG, Meyer DR, Morton AD., 3rd ASOPRS dacryocystitis study group. The microbiologic spectrum of dacryocystitis: a national study of acute versus chronic infection. Ophthal Plast Reconstr Surg. 2007;23(4):302-306.
Crossref - Rizvi SAR, Rizvi M, Raut SD, Gupta Y, Maheshwari P. Etiology and Antimicrobial Sensitivity Pattern in Acute and Chronic Dacryocystitis. Int J Curr Microbiol Appl Sci. 2015;Spl. Issue-1:269-280.
- Ray S, Islam MN, Saha M. Bacteriological Study and Antimicrobial Sensitivity Pattern of Dacryocystitis. Int J Sci Study. 2018;6(9):42-45.
- Eshraghi B, Abdi P, Akbari M, Fard MA. Microbiologic spectrum of acute and chronic dacryocystitis. Int J Ophthalmol. 2014;7(5):864-867.
- Chaudhry IA, Shamsi FA, Al-Rashed W. Bacteriology of chronic dacryocystitis in a tertiary eye care center. Ophthal Plast Reconstr Surg. 2005;21(3):207-210.
Crossref - Sun X, Liang Q, Luo S, et al. Microbiological analysis of chronic dacryocystitis. Ophthalmic Physiol Opt. 2005;25(3):261-263.
Crossref - Gilliland G. Dacryocystitis. In: Agarwal S, Agarwal A, Apple DJ, Buratto L, Alio JL, Pandey SK, Agarwal A, editors. Textbook of Ophthalmology. 1st ed. New Delhi: Jaypee brothers Medical Publishers (P) Ltd. 2002:705-712.
- Brook I, Frazier EH. Aerobic and anaerobic microbiology of dacryocystitis. Am J Ophthalmol. 1998;125(4):552-554.
Crossref - Briscoe D, Rubowitz A, Assia EI. Changing bacterial speciesl isolates and antibiotic sensitivities of purulent dacryocystitis. Orbit. 2005;24(2):95-98.
Crossref - Cahill KV, Burns JA. Management of acute dacryocystitis in adults. Ophthal Plast Reconstr Surg. 1993;9(1):38-42.
- Kotlus BS, Rodgers IR, Udell IJ. Dacryocystitis caused by community-onset methicillin-resistant Staphylococcus aureus. Ophthal Plast Reconstr Surg. 2005;21(5):371-375.
Crossref - Kubo M, Sakuraba T, Arai Y, Nakazawa M. Dacryocystorhinostomy for dacryocystitis caused by methicillin- resistant Staphylococcus aureus: report of four cases. Jpn J Ophthalmol. 2002;46(2):177-182.
Crossref - Fynn-Thompson N, Pineda R. Antibiotic advances in Ophthalmology. Int Ophthalmol Clin. 2004;44(3):91-102.
Crossref - Rutar T, Chambers HF, Crawford JB, et al. Ophthalmic manifestations of infections caused by the USA300 clone of community-associated methicillin-resistant Staphylococcus aureus. Ophthalmology. 2006;113(8):1455-1462.
Crossref - Gajapati CV, Venkataramana PA, Kulkarni RD, Patil SH, Nishant N. Bacteriology and antibiotic susceptibility of chronic dacryocystitis. International Journal of Ocular Oncology and Oculoplasty. 2017;3(2):122-125.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.