Lurthu Reetha1, J. Johnson Rajeswar2, T.J. Harikrishnan3,Sugumar4, P. Srinivasan5and J.John Kirubaharan6
1Tamilnadu Veterinary and Animal Sciences University, Regional Research,Pudukkottai – 622004, India
2Department of Veterinary Microbiology, Veterinary College andResearch Institute, Tirunelveli-627001,India
3Directorate of Research,Tamilnadu Veterinary and Animal Sciences University Chennai -600051,India.
4Department of Veterinary Microbiology,Veterinary College and Research Institute, Namakkal -637002, India
5Veterinary University Training and Research Centre,Nagapattinam,Tamilnadu, India.
6Department of Veterinary Microbiology,Madras Veterinary College, Chennai – 600007, India.
ABSTRACT
The study was carried out at Veterinary University Training and Research Centre, Tiruchirapalli, Tamil Nadu. A total of 48 day old desi chicks obtained from a private hatchery in Namakkal, TamilNadu were maintained under cage system of rearing up to 52 weeks of age as per standard management practices. All the 48 chicks were divided into six groups having eight chicks in each group were subjected to different treatment regimes. Serum samples were collected at 21 days interval and the post vaccination antibody titre was assessed by ELISA tests. All the birds were challenged at 52 weeks of age with 0.5 ml dose of 104.0 EID50 virulent ND field virus. All the vaccinated groups in this study showed protective level of antibody titre throughout the study period of 52 weeks. The challenged birds were observed for ten days for the development of clinical symptoms, lesions and mortality. No mortality was observed in any of the vaccinated group whereas 100 percent mortality was recorded during the observation period in the unvaccinated control groups.
Keywords: Oral pellet vaccines, desi chicken, ELISA test, immune response.
INTRODUCTION
Newcastle disease (ND) is one of the most important animal diseases in the world both for the number of animals affected every year and the severe economic impact on the poultry industry1. It is an acute infectious viral disease of domestic poultry and other species of birds regardless of variation in sex and age2. Newcastle disease virus (NDV) has a wide host range, including approximately 241 species of 27 orders, out of known 50 orders of birds3. ND is fatal and still top ranked poultry disease. Annual losses caused by this disease worldwide are in millions of dollars4. Due to the severe nature of Newcastle Disease and the related consequences, NDV is included in “LISTED” agents (reportable disease) by Office International des Epizooties (OIE)5. Notification is required by OIE of any outbreak of ND6, when it meets certain criteria of virulence7. Vaccines are being used to control and prevent ND. Currently, many inactivated and live ND vaccines are available around the world8.Effective vaccination with conventional vaccines involves maintaining a cold chain, catching and handling individual birds, using skilled vaccinators and repeating the whole procedure sufficiently often to ensure that every bird receives at least two doses of vaccine at different times9. The selection of a ND vaccine for use in rural chicken will depend on the local conditions in each country. Selection criteria will include – ease of use, cost, thermo stability, immunogenicity, availability and transportability. In circumstances where the cold chain is weak or absent, the only reliable option will be the use of thermostable ND vaccines10. Clinical diagnosis based on history, signs and lesions may establish a strong index of suspicion but the laboratory confirmation must be done. Hemagglutination and hemagglutination inhibition test, virus neutralization test, Enzyme linked immune-sorbent assay, plaque neutralization test and reverse-transcriptase polymerase chain reaction (RT-PCR) can be used for confirmation of the ND virus11. Enzyme Linked Immunosorbent Assay has been extensively used for detection of ND antibodies in chicks12. There are a variety of commercial ELISA kits available and these are based on several different strategies for the detection of NDV antibodies13. Hence the present study was conducted with the objective to assess the immune response against Newcastle disease oral pellet vaccine in desi chicken by ELISA test.
Materials and Methods
All animal procedures were performed in accordance with Institutional Animal Ethical Committee regulations (Approval no.8/2012 of IAEC dated 10.08.2012). The study was carried out at Veterinary University Training and Research Centre,Tiruchirapalli, Tamil Nadu. A total of 48 day old desi chicks obtained from a private hatchery in Namakkal, TamilNadu were maintained under cage system of rearing upto 52 weeks of age under standard management practices. All the 48 chicks were divided into six groups having eight chicks in each group. First group (T1) served as unvaccinated control, second group (T2) was primed with commercially available Thermostabilised D58 vaccine followed by booster with TANUVAS oral pellet vaccine, Third group (T3) was primed as well as boosted with TANUVAS oral pellet vaccine, Fourth group (T4) was primed with RDV’F’ followed by booster with commercial vaccines (LaSota and R2B), Fifth group (T5) was primed with commercially available Thermostabilised D58 vaccine followed by booster with commercial vaccines (LaSota and R2B), Sixth group (T6) was primed with TANUVAS oral pellet vaccine followed by booster with commercial vaccines (LaSota and R2B). Serum samples were collected by following standard procedure at 21 days interval and the post vaccination antibody titre was assessed by ELISA tests14.
The test was conducted to find out the antibody titre of the serum collected from the desi birds using Enzyme-linked immunosorbent assay (IDEXX NDV Ab test, Liebefeld-Bern, Switzerland). Virulent field virus maintained at Department of Veterinary Microbiology, Veterinary College and Research Institute, Namakkal was used as challenge virus in this study. All the desi birds were challenged at 52 weeks of age with 0.5 ml dose of 104.0 EID50 virulent ND field virus . The challenged birds were observed for ten days for the development of clinical symptoms, lesions and mortality.
An analysis of variance (ANOVA) with one factor was used to assess the immunity effects of different types of vaccine . The values presented were expressed by assigned average standard error of the mean. In the case of significant difference, Tukey’s HSD test was used to separate homogeneous groups at a significant level of 1%.
Table :1 Humoral immune response in desi chicken – Mean ± SE of ELISA log10titre in different treatment groups (n=8)
| Age at serum
collection (Weeks) |
Treatment groups | ||||||
| T 1 | T 2 | T 3 | T 4 | T 5 | T 6 | F Value | |
| Day old | 3.38 ± 0.02 | – | |||||
| 4 | 2.49 ± 0.01a | 3.17 ± 0.01b | 3.20 ± 0.02b | 3.44 ± 0.01c | 3.18 ± 0.02b | 3.20 ± 0.01b | 556.77** |
| 7 | 2.50 ± 0.02a | 3.37 ± 0.01b | 3.41 ± 0.02b | 3.60 ± 0.01c | 3.57 ± 0.01c | 3.58 ± 0.01c | 923.37** |
| 10 | 2.52 ± 0.02a | 3.79 ± 0.02b | 3.81 ± 0.02b | 3.98 ± 0.01c | 3.93 ± 0.02c | 3.94± 0.02 c | 945.26** |
| 13 | 2.54 ± 0.03a | 3.89 ± 0.02b | 3.92 ± 0.02b | 4.04 ± 0.01c | 4.01 ± 0.02c | 4.02 ± 0.02c | 979.45** |
| 16 | 2.55 ± 0.03 a | 3.83 ± 0.02 b | 3.84 ± 0.02b | 3.95 ± 0.01c | 3.92 ± 0.01c | 3.93 ± 0.01c | 766.61** |
| 19 | 2.60 ± 0.03 a | 3.95 ± 0.03 b | 3.96 ± 0.02b | 4.09 ± 0.01c | 4.07 ± 0.01c | 4.08 ± 0.01c | 867.45** |
| 22 | 2.59 ± 0.03 a | 4.02± 0.02 b | 4.03 ± 0.02b | 4.13 ± 0.01c | 4.10 ± 0.01c | 4.11 ± 0.01c | 1220.80** |
| 25 | 2.62 ± 0.03 a | 3.98 ± 0.03 b | 4.00 ± 0.02b | 4.10 ± 0.0 c | 4.08 ± 0.01c | 4.09 ± 0.01c | 937.35** |
| 28 | 2.63 ± 0.02 a | 3.97 ± 0.02 b | 3.98 ± 0.02b | 4.07 ± 0.01c | 4.05 ± 0.01c | 4.06 ± 0.01 c | 1091.61** |
| 31 | 2.67 ± 0.04 a | 4.05 ± 0.01b | 4.06 ± 0.01b | 4.15 ± 0.01c | 4.14 ± 0.02 c | 4.14 ± 0.01 c | 890.13 |
| 34 | 2.66 ± 0.04 a | 4.04 ± 0.01b | 4.05 ± 0.01b | 4.14 ± 0.01c | 4.12 ± 0.01c | 4.13 ± 0.01 c | 1097.26** |
| 37 | 2.69 ± 0.04 a | 4.03 ± 0.01b | 4.04 ± 0.01b | 4.13 ± 0.01c | 4.11 ± 0.01c | 4.12 ± 0.01 c | 985.13** |
| 40 | 2.72 ± 0.03 a | 4.03 ± 0.02b | 4.03 ± 0.02b | 4.12 ± 0.01c | 4.10 ± 0.01c | 4.11 ± 0.01c | 991.24** |
| 43 | 2.71 ± 0.04 a | 4.06 ± 0.01b | 4.07 ± 0.01b | 4.16 ± 0.01c | 4.14 ± 0.02c | 4.15 ± 0.01c | 849.40** |
| 46 | 2.75 ± 0.04 a | 4.06 ± 0.01b | 4.07 ± 0.01b | 4.15 ± 0.01c | 4.14 ± 0.01c | 4.14 ± 0.01c | 1120.15** |
| 49 | 2.71 ± 0.03 a | 4.05 ± 0.01b | 4.06 ± 0.01b | 4.15 ± 0.01c | 4.13 ± 0.01c | 4.14 ± 0.01c | 1707.87** |
| 52 | 2.72 ± 0.03 a | 4.04 ± 0.01b | 4.05 ± 0.01b | 4.13 ± 0.01c | 4.11 ± 0.01c | 4.12 ± 0.01c | 1766.30** |
Means ± S.E having same super script in a row do not differ significantly.** Highly significant (P<0.01)
Results and discussion
The ELISA titre value of the different treatment regimes during the study period were presented in Table-1 and Figure-1. The ELISA log10 titre value in T4 group was higher (3.44± 0.01) followed by T6 (3.20±0.01), T3 (3.20±0.02), T5 (3.18±0.02) and T2 (3.17± 0.01) on 4th week of age. In this study , all the vaccines produced protective level of immune response till the next vaccination was done on 28th day. In earlier study similar finding was reported that three weeks after first vaccination a protective titre was recorded and stated that a single oral vaccination is insufficient and that the immune response needs to be boosted by at least one further oral vaccination[15] and administered LaSota on 28th day after the chicks were primed with RDV’F’ on 7th day [16]. However, highly significant (P<0.01) increase was observed steadily from seventh week onwards after booster vaccination.
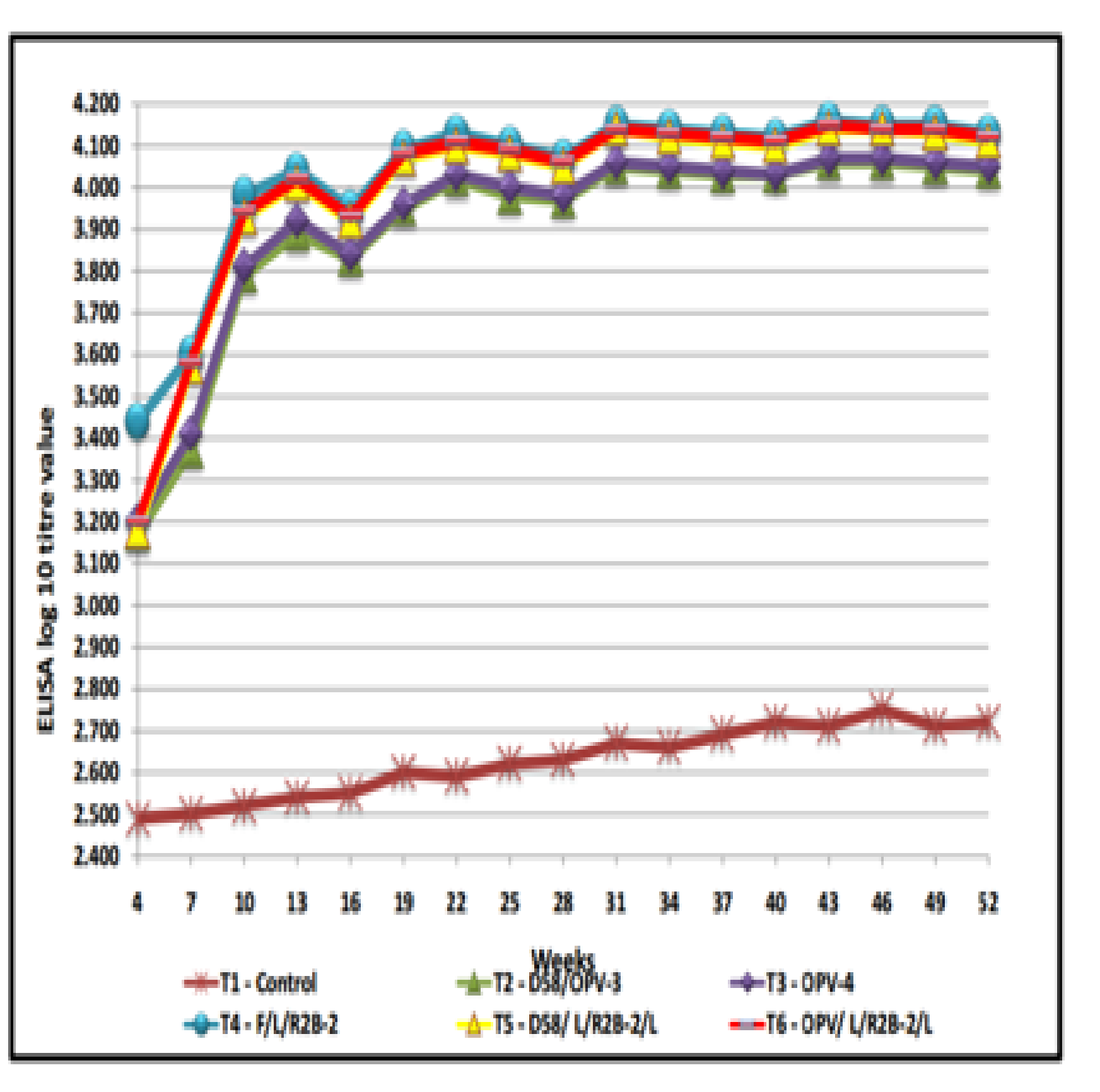
Fig. 1. Humoral Immune response in desi chicken – ELISA log 10 litr value in different treatment groups
On 8th week, mesogenic strain R2B was given in T4, T5 and T6 groups and TANUVAS oral pellet vaccine was given to T2 and T3 group as booster vaccination. The increase in titre was observed at 13th week of age and the titre started declining slowly . In the present study, the ELISA log10 titre at 13th week of age in T4 group was found to be higher (4.04±0.01) followed by T6 (4.02±0.02), T5 (4.01 ± 0.02), T3 (3.92±0.02) and T2 (3.89±0.02). Similar pattern of results was observed in chicken with thermostable pelleted vaccine comparable to D58 in which about two third of birds produced detectable antibody following booster administration of I-2 vaccine17 and there were increased number of plasma cells and secretion of immunoglobulin A which is referred as secretory antibody on the mucosal surfaces of avian intestine, bronchi and oviduct when thermo stable vaccines are administered orally18.
The titre values declined in 16th week of age, later started increasing due to booster vaccination of mesogenic strain R2 B given to T4, T5 and T6 groups and oral pellet vaccine given to T2 and T3 groups. In earlier study similar finding was reported that in which booster vaccination with mesogenic strain elicited revival of egg production and also rise in HI titre. The increase in titre was observed up to 22nd week of age and then the titre declined slightly19. The ELISA log10 titre at 22nd week of age in T4 group was higher (4.13±0.01) followed by T6 (4.11±0.01), T5 (4.10± 0.01), T3 (4.03±0.02) and T2 (4.02±0.02). It has been reported that [20] where the circulating antibodies induced by the primary vaccination given during first two weeks of life did not interfere with subsequent vaccination with more virulent K/ R2B strain, infact it tended to give best response to revaccination by providing solid immunity for a longer period and in countries with a high prevalence of virulent ND, mesogenic strains of NDV are generally preferred for booster vaccinations21.
To maintain the protective level of antibody titre, 28th week booster vaccination was carried out in all the vaccinated groups. The increase in titre was observed up to 31st week of age and then declined slightly. Higher titre was observed in 31st week in T4 group (4.15±0.01) followed by T6 (4.14±0.01), T5 (4.14± 0.02), T3 (4.06±0.01) and T2 (4.05±0.01) . In earlier study similar finding was reported that22 the ND booster vaccination is the best, essential and most cost effective means of ND control and has been used successfully in village settings all over the world to reduce the clinical disease associated with outbreaks of ND.
To maintain the protective level of the titre , next vaccination was done on 40th week. Later, the peak titre was seen in 43rd week and the titre in T4 group was higher (4.16±0.01) followed by T6 (4.15±0.01), T5 (4.14± 0.02), T3 (4.07±0.01) and T2 (4.06±0.01) groups and then the titre started declining. However protective level of antibody titre was maintained throughout the study in all the vaccinated groups. It has been reported that23 high levels of HI antibodies within two weeks after booster vaccination and also observed that a high percentage of chickens vaccinated only once died from the challenge24.
In this study No significant differences were noticed between T2, T3, T5 and T6 groups at 4th week of age. Between T2 and T3, no significant differences (P<0.01) were noticed from 4th week to 52 week of age. On 4th week of age significant differences were noticed between T4 and other (T2, T3, T5, T6) groups. Later from 7th week onwards no significant differences (P<0.01) were noticed between T4, T5 and T6 up to 52nd week of age. But from 7th to 52 weeks significant differences (P<0.01) were noticed between T2, and T4, T5, T6 groups and between T3 and T4, T5, T6 groups.
All the desi birds were challenged with 0.5 ml dose of 104.0 EID50 virulent ND field virus. Before challenge the ELISA log10 titre in T1, T2, T3, T4, T5 and T6 groups were 2.72±0.03, 4.04±0.01, 4.05±0.01, 4.13±0.01, 4.11±0.01 and 4.12±0.01 respectively. In the present study, the challenged birds were observed for ten days for the development of clinical signs, mortality and lesions. No mortality was observed in any of the vaccinated group whereas 100 per cent mortality was recorded during the observation period in the unvaccinated control group. The birds exhibited clinical signs like greenish white diarrhoea and torticollis. Post mortem examination revealed pin point petechial haemorrhages at the tip of the proventriculus papillae. In another recent study [25] reported that clinical symptoms of torticollis and lateral movement of the head and also few birds developed paralysis of both legs. The gross pathological lesions consisted of extensive involvement of proventriculus submucosa and intestinal follicles, severe haemorrhagic necrotic lesions adjacent to lymphoid plaques were also common.
Conclusion
From this study, it is concluded that the administration of Newcastle disease oral pellet vaccine to desi chicken showed protective level of antibody titre throughout the study period of 52 weeks. No mortality was observed in any of the vaccinated group whereas 100 percent mortality was recorded in the unvaccinated control groups .The thermostable TANUVAS oral pellet vaccine had found to be more advantageous and the vaccine can support poultry farmers to get rid from Newcastle disease globally.
Acknowledgements
The authors express their gratitude to Tamil Nadu Veterinary and Animal Sciences University for granting permission to carry out this doctoral research on part time basis.
References
- Thompson, R. Newcastle disease; a review of field recognition and current methods of laboratory detection. J Vet Diagn Invest., 2015; 23(4): 637-656.
- Iram, N., Shah, M.S., Ismat, F., Habib,M., Iqbal, M., Hasnain, S.S., Rahman. M. Heterologous expression, characterization and evaluation of the matrix protein from Newcastle disease virus as a target for antiviral therapies. Appl. Microbiol. Biotechnol., [Epub ahead of print]., 2013.
- Madadgar , O., Karimi, V., Nazaktabar, A., Kazemimanesh, M., Ghafari, M.M., Dezfouli, SM. A., Hojjati, P. A study of Newcastle disease virus obtained from exotic caged birds in Tehran between 2009 and 2010. Avian Pathol., 2013; 42(1) :27-31.
- Waheed, U., Siddique, M., Arshad, M., Ali, M., Saeed, A. Preparation of newcastle disease vaccine from VG/GA strain and its evaluation in commercial broiler chicks. Pak. J. Zool., 2013; 45(2):339-344.
- Boynukara, B., Gulhan, T., Coven, .F., Kiziroglu, I., Durmus, A. Determination of Newcastle disease virus among wild bird populations in Lake Van basin, Turkey. Turkish J. Vet. Anim. Sci., 2013; 37:01-09.
- Cao, Y., Gu, M., Zhang, X., Liu, W., Liu ,X .Complete Genome Sequences of Two Newcastle Disease Virus Strains of Genotype VIII. J. Genome Announcements., 2013; 1(1): 01.
- Munir, M., Shabbir, MZ, Yaqub, T., Shabbir, MAB., Mukhtar, N., Khan, MR., Berga, M. Complete Genome Sequence of a Velogenic Neurotropic Avian Paramyxovirus 1 Isolated from Peacocks (Pavo cristatus) in a Wildlife Park in Pakistan. J. Virol. 2012; 86(23): 13113-13114.
- Xiao, S., Paldurai, A., Nayak, B., Mirande, A., Collins, P.L, Samal, SK. Complete genome sequence of a highly virulent Newcastle disease virus currently circulating in Mexico. J. Genome Announcements ., 2013; 1(1):01-02.
- Moreki, K., Dikeme and Poroga, B. The role of village poultry in food security and HIV/AIDS mitigation in chobe district of Botswana. Livest. Res. Rural. Dev., 2010; 22.
- Desalew Tadesse., Harpal Singh., Ashenafi Mengistu., Wondmeneh Esatu., and Tadelle Dessie. Study on productive performances and egg quality traits of exotic chickens under village production system in East Shewa, Ethiopia. Afr. J. Agric. Res., 2013; 8(13): 1123-1128.
- Chaka, H., Goutard. F., Gil, P., Abolnik,C., Almeida,R., Bisschop, SPR.,Thompson,PN . Serological and molecular investigation of Newcastle disease in household chicken flocks and associated markets in Eastern Shewa zone, Ethiopia. Trop. Anim. Health Prod., 2013; 45: 705-714.
- Marquardt, W.W., Snyder, D.B., Savage, P.K., Kadavil, S.K., and F.S. Yancy. Antibody respose to Newcastle disease virus by two different routes as measured by ELISA and haemagglutination inhibition test and associated tracheal immunity. Avian Dis., 1984; 29: 71-79.
- OIE. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals:Mammals, Birds and Bees, Biological Standards Commission. World Organization for Animal Health, Paris., 2012; pp 1–19.
- OIE. Manual of diagnostic tests and vaccines for terrestrial animals. OIE publications. 2004.
- Ideris, A., Ibrahim, A.L., and Spradbrow P.B. Vaccination of chickens against Newcastle disease with a food pellet vaccine. Avian Pathol, 1990; 19: 371-384.
- Krishnanmohan Reddy, Y., Dorairajan, N., Mohan, B., and Ramesh Babu, N.G. Experimental Evaluation of inactivated RD viral vaccine containing local velogenic strain. Indian Vet. J., 1994; 71: 964-967.
- Echeonwu, G.O.N., Iroegbu, C.U., Echeonwu, B.C., Ngene, A., Nwosuh, C.I., Joannis, T.M., and Ndako. Immune response and protection of free range chickens vaccinated orally with feeding of Newcastle disease vaccine coated cassava granules. African J. Microbiol. Res., 2008;2: 120-125.
- Jayawardane, G.W.L and Spradbrow, P.B. Cell mediated immunity in chickens vaccinated with the V4 strain of Newcastle disease virus. Vet. Microbiol., 1995; 46: 37-41.
- Ganesan, P.l., Appaji Rao, V.N., and Venugopal, K. Effect of immune status to Ranikhet disease on production performance in layer poultry. Indian Vet. J., .1992; 69:199-202.
- Verma, K.C., Panisup, A.S., Kateria, J.M., Singh, S.D., and Mohanty, G.C. A modified vaccination regimen for the control of Ranikhet (Newcastle) disease in chicken. Indian. J. Poult. Sci., 1985; 20(4): 231-235.
- Nasser, M., Lohr, J.E., Mebratu, G.Y., Zessin, K.H., Baumann, M.P.O., and Ademe, Z. Oral Newcastle disease vaccination trials in Ethiopia. Avian Pathol., 2000; 29: 27-34.
- Msoffe, P.L.M., Bunn, D., Muhairwa, A.P., Mtambo, M.M.A., Mwamhehe, H., Msago, A., Mlozi, M.R. , and Cardona, C.J. Implementing poultry vaccination and biosecurity at the village level in Tanzania: a social strategy to promote health in free-range poultry populations. Trop. Anim. Health and Prod., 2010; 42: 253–263.
- Spradbrow, P.B and Samuel, J.L. Oral New castle disease vaccine in experimental chickens in Australia. In: J.W.Copland (Ed.) Newcastle disease in poultry. A new feed pellet vaccine. Canberra: Australian centre for International Agricultural Research.1987; 5: 44-49.
- Ibrahim, A.L., Chulan, U., and Babjee, A.M. An assessment of the Australian V4 strain of Newcastle disease virus as a vaccine by spray, aerosol and drinking water administration. Aust. Vet. J. , 1981; 57: 277– 280.
- Singh, K.V., Najla Saad and Zein., A. El. Preliminary observations on the pathogenesis of a virulent strain of Newcastle disease virus in chickens. Appl. Microbiol., 1971; 21: 946-948

