ISSN: 0973-7510
E-ISSN: 2581-690X
In the human oral cavity, Streptococcus mutans is often observed and is a major contributor to tooth decay. Increased S. mutans levels may be linked to progressively more severe forms of periodontal disease because root exposure in people with periodontitis increases caries rates. Hence, a new potential antibacterial compound needs to be searched to combat this pathogenic bacterium. The butterfly pea, or Clitoria ternatea is an ornamental plant that has been reported to exhibit antibacterial properties against several bacteria. Thus, the goal of this investigation was to determine how well C. ternatea aqueous (CTA) extract inhibited S. mutans. The disk diffusion assay was performed to access the antibacterial properties of the CTA extract. The efficiency of the extract against the test bacterium was then determined through MIC/MBC determinations and a time-kill study. Meanwhile, the toxicity of the extract was tested using a brine shrimp lethality assay (BSLA). The CTA extract demonstrated substantial antibacterial activity against the test bacterium at a concentration of 200 mg/ml, with a diameter of the inhibition zone of 13.4±0.4 mm, according to the disc diffusion assay. The aqueous extract’s MIC and MBC values were found to be 100 and 400 g/mL, respectively. Time-kill analysis revealed the CTA extract exerted a strong bactericidal effect on S. mutans and this activity was dose-dependent. A scanning electron microscope (SEM) exhibited the bacterial cells experienced severe damage after being exposed to CTA extract including formation cavities, irregular shape, and crumpled cells. Thus, the present study suggested the potential of CTA extract as an antibacterial agent against oral cavity bacteria and can be used in the formulation of natural mouthwash due to no toxicity effect.
Streptococcus mutans, Clitoria ternatea, MIC/MBC Determinations, Brine Shrimp Lethality Assay (BSLA), Scanning Electron Microscope (SEM)
Streptococcus mutans is a gram-positive and facultatively anaerobic coccus with a round shape bacterium. This strain is a major contributor to tooth decay and is frequently detected in the human oral cavity. Metabolism of carbohydrates by S. mutans contributes significantly to the development of caries. This is due to lactic acid production, which is the primary byproduct of sugar metabolism in humans with high-sugar diets, and this acid can cause demineralization of dental enamel.1 One of S. mutans’ primary pathogenicity features is its ability to produce the biofilm known as dental plaque on tooth surfaces. Dental plaque, a gelatinous substance containing bacteria attached to tooth surfaces, is where demineralization occurs. By using glucosyltransferases (GTFs), the bacteria produce sticky glucan from sucrose, and the glucans help the bacteria’s cells attach firmly to tooth surfaces. Additionally, S. mutans produces a variety of glucan-binding proteins (Gbp proteins), which are thought to aid in adhesion. Furthermore, the S. mutans cell surface protein antigen c (PAc), which interacts with the salivary pellicle to facilitate bacterial attachment to tooth surfaces, is connected to the virulence of the organism in relation to the development of dental caries. Together, these bacterial surface proteins create tooth plaque, which causes cavities in the teeth.2
There is currently a lot of interest in using antimicrobial medications for the prevention and treatment of dental caries throughout the world due to the advent of antibiotic resistance. The need for rationalizing antibiotic use in the treatment of dental caries has been highlighted by the growing concern over the issue of antibiotic resistance. The incorrect prescription and usage of antibiotics have been largely blamed for the emergence of antibiotic resistance in S. mutans.3 Several drugs, including amoxicillin/clavulanic acid (A-CA), clindamycin, and moxifloxacin at concentrations of 16, 32, and 64 g/ml, were found to cause an increased frequency of antibiotic resistance in S. mutans bacterium (45.9%), according to the previous study by Loyola-Rodriguez et al.4 Besides, Jain and Pundir3 also reported that the three strains of S. mutans were resistant to commercially available antibacterial drugs including metronidazole, ciprofloxacin, and rifampicin since they did not show any inhibitory effect.
In tropical areas of South and Central America, the butterfly pea, or Clitoria ternatea L. (family: Fabaceae), is fairly widespread. For centuries, this plant has been utilised both as food and medicine. Syphilis, rheumatoid arthritis, chronic bronchitis, fever, indigestion, constipation, sore throats, eye, and ear issues, as well as snakebite and scorpion stings, are among the conditions for which it is traditionally recommended. Besides that, the copious anthocyanin pigments found in the flower petals of C. ternatea can be used as a source of natural colorants in various foods and beverages.5 The C. ternatea plant, which is also widely cultivated in India, the Philippines, Thailand, Malaysia, and other Asian countries, has an intense blue hue that is frequently used as a natural food colouring (for instance, in rice cakes, tea, snacks, and sweets), traditional medicine, as well as an ornamental plant.6 Phytochemical analysis of C. ternatea flower revealed that this plant possesses several compounds including alkaloids, tannins, glycosides, anthocyanin, resins, and flavonoids.7 Some of these compounds have been reported to possess antibacterial activity against several pathogenic bacteria. For instance, Dhanaksekaran et al.8 reported the efficacy of C. ternatea crude extracts against the Gram-negative bacterium, Proteus mirabilis. The anthocyanin-rich fraction of the C. ternatea flower, on the other hand, demonstrated more pronounced antibacterial activity against Bacillus cereus, B. subtilis, and Escherichia coli as compared to crude extracts, according to a prior study.6 Darsini and Shamshad, 9 reported the different crude extracts of C. ternatea showed prominent antibacterial activity against Salmonella typhimurium, Proteus vulgaris, and Shigella dysenteriae. They also revealed that flavonoids, terpenoids, and alkaloids may be potent compounds that contributed to the antibacterial properties of this plant.
Due to the plant’s well-known antibacterial characteristics, this study was designed to determine the antibacterial activity of the aqueous extract of C. ternatea against the oral cavity bacterium, S. mutans. To the best of our pieces of knowledge, this is the first report on the antibacterial activity of the aqueous extract of C. ternatea against the oral bacterium, S. mutans. Moreover, this current finding could provide insightful knowledge, especially in the formulation of wash-mouth agents.
Collection of samples
The healthy flower samples were collected from Kota Warisan, Sepang, Selangor, Malaysia (2° 49’ 22’’ North, 101° 42’ 4’’ East). The samples were placed in Ziplock plastic bags, stored in an ice box, and transported to the Microbiology Laboratory, UiTM Shah Alam within 2 hours. The flower samples were washed under running tap water to remove debris, rinsed, and dried in a 60°C oven for 24 hours.
Plant Extraction
The dried Clitoria ternatea petals were ground to a fine powder using a grinder. An amount of 100 g petal powder was then soaked in 1000 mL sterilized distilled water and incubated in an orbital shaker at 30°C for 30 minutes. The mixture was filtered using Whatman No. 1 filter paper. The filtrates were then freeze-dried for 5 days to obtain an aqueous paste.
Microorganisms and cultural maintenance
Streptococcus mutans Clarke ATCC 700610 was used as test bacteria in the present study. The Natural Approach to Oral Health (NAtOH) research initiative group, Faculty of Dentistry, Universiti Teknologi MARA, Puncak Alam Campus provided the stock culture. The strain was grown and kept alive for 24 hours at 37°C on brain heart infusion agar (BHIA). Sub-cultured was done every 3 months to ensure the viability and purity of the strain.
Inoculum preparation
Two or three colonies of S. mutans were taken from the agar plate and introduced into a Universal bottle containing 5 ml of 0.85% (w/v) physiological saline solution. The mixture was vortex and compare with 0.5 McFarland to obtain a bacterial suspension of 1 × 108 CFU/ml.
Kirby Bauer Disc Diffusion Assay
A disc diffusion assay was carried out to determine the antibacterial activity of C. ternatea aqueous (CTA) extract against S. mutans according to the CLSI method.10 The inoculum was made by carefully combining 3–4 single colonies with 5.0 mL of sterile physiological saline to produce cell suspensions that were around 1–108 CFU/mL as measured against 0.5 McFarland standards. With the aid of a spread plate technique and a sterilized cotton swab, the test microorganisms were seeded onto MHA. To make the CTA extract, 10.0 mg of freeze-dried C. ternatea flower was dissolved in 0.2 mL of 5% dimethyl sulfoxide (DMSO), and 0.8 mL of sterile distilled water was then added to achieve a concentration of 1 mg/mL. The sterile 6.0 mm antibiotic discs were air-dried after being impregnated with 20 µL of the extract and then laid out on the Mueller-Hinton seeded agar surface. As a positive control, 30 µ/mL of chloramphenicol was employed, and 1.0% of DMSO was used as a negative control. After being incubated for 24 hours, the inhibition zone that formed around the antibiotic disk was quantified. The trials were carried out in triplicate, and the results were given as the average standard deviation of the inhibitory zone generated from three separate experiments.
Determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
The efficacy of CTA extract was determined using a microdilution broth method as CLSI10 recommended and the study employed the procedures Jalil and Darah.11 described. The crude extracts were diluted to obtain a series of concentrations (4.0 mg/mL – 0.0625 mg/mL). 100 µL of the CTA extract was poured into each microtiter plate well after being diluted one-fold in a sterile Mueller-Hinton broth (MHB) medium. After that, 100 µL of test bacterial inoculum was added to each well, bringing the total amount to 200 µL. The final concentration of bacterial inoculum in each well was 1 × 107 CFU/mL. The reference drug utilized was chloramphenicol. Methanol (5%, v/v) was set as a negative control. As a growth indicator, 40 µl of p-iodonitrotetrazolium violet (INT) dissolved in 99.5% ethanol were added to each well after a 24-hour period of incubation at 37 °C. The lowest concentration of the CTA extract shown to be able to prevent the test bacteria from growing visibly after the incubation period was recognized as the MIC value. After reading the MIC values, the MBC of the CTA extract was calculated. The viable cells from wells that showed no microbial growth were counted on MHA using a traditional viable plate count after 24 hours of incubation at 37°C for an overnight timeframe. The MBC was detected and identified as the CTA crude extract concentration that reduced bacterial growth by 99.9% in comparison to the growth control.
Time-kill study
The efficacy of the CTA extract was determined via time-kill assay according to the method described by Jalil et al.12 A total of four 50 mL Erlenmeyer flasks containing 8.9 mL of brain heart infusion broth (BHIB) were prepared. Three flasks were then added with 1.0 mL of the CTA extract with a final concentration of ½ × MIC, 1 × MIC, and 1 × MBC. A volume of 1.0 mL BHIB was added to the control flask as a substitution for the aqueous extract. All four flasks received a volume of 0.1 mL bacterial inoculums (1 × 108 CFU/mL), and they were incubated at 37 °C for 48 hours in a rotary shaker with 150 rpm of agitation. Every 4 hours intervals, a volume of 1.0 mL of the mixture was withdrawn and the colony-forming per unit (CFU) was performed and the result was recorded.
Brine shrimp lethality assay (BSLA)
Toxicity testing of the CTA extract against brine shrimp was performed according to methods described by Jalil et al.12 An amount of 0.1 g of artemia salina cysts was allowed to hatch for 48 hours at 25°C in artificial seawater under constant illumination and aeration. The stock CTA extract was serially diluted into six universal bottles to obtain different extracts with final concentrations ranging from 625 to 10000 µg/mL. In each universal bottle, a total of ten 48-hour old nauplii were transferred using a Pasteur pipette. A universal bottle containing artificial seawater without the CTA extract was set as a negative control. After 6 hours in the case of acute toxicity and 24 hours in the case of chronic toxicity, the number of dead nauplii was counted, and the mortality % was computed. The curve of the LC50 value was then constructed.
Structural degeneration of bacterial cells
The morphological changes of bacterial cells after being treated with an aqueous extract of Clitoria ternatea were observed through a scanning electron microscope (SEM) according to the method described by Jalil et al.12 A volume of 8.9 mL brain heart infusion broth (BHIB) was prepared in 50 mL Erlenmeyer flasks and 1.0 mL extract with a concentration of 1 × MBC was then added. Subsequently, a volume of 0.1 mL bacterial inoculums with a concentration of 1 × 108 CFU/mL was added, and the mixture was incubated at 37°C for 48 hours in an orbital shaker with an agitation speed of 150 rpm. For control, 1.0 mL of extract was substituted with 1.0 mL of BHIB. According to Marez’s13 instructions, the bacterial samples were prepared, and the prepared samples were then examined under an SEM (Leica Cambridge, S-360, United Kingdom).
Statistical analysis
The gathered duplicate data were subjected to an analysis of a completely randomised design using SPSS software version 12.0 (Chicago, IL, USA), and the results were judged statistically significant if p ≤ 0.05.
Antibacterial activity of the extract on disc diffusion assay
The disk diffusion was employed to determine the susceptibility of Streptococcus mutans Clarke ATCC 700610 to different concentrations of the CTA extract. Table 1 exhibits the antibacterial activity of different concentrations of the CTA extract against S. mutans Clarke ATCC 700610. The finding showed the inhibitory effect of the CTA extract against test bacteria can be observed after being treated at a concentration of more than 50 mg/mL. The inhibitory zone was 8.1 ± 0.6 mm in diameter at an extract concentration of 50 mg/mL. The diameter of the inhibitory zone rose to 11.2 ± 0.2 and 13.4 ± 0.4 mm, respectively, when the extract concentration was increased to 100 and 200 mg/mL. However, at a lower extract concentration (25 mg/mL), the CTA extract exerted no inhibitory effect on the test bacterium, S. mutans Clarke ATCC 700610. This phenomenon may be due to the induction effect of the extract on bacterial growth or biofilm formation which is a virulence factor for S. mutans. Kaplan14 reported a low concentration of antibiotics could induce biofilm formation and the subminimum inhibitory dose of antibiotics could have an agonistic effect on bacterial biofilm formation.
Table (1):
Antibacterial activity of different concentrations of Clitoria ternatea aqueous extract against Streptococcus mutans on disc diffusion assay.
Clitoria ternatea aqueous extract (mg/ml) |
Diameter of inhibition zone (mm) |
|---|---|
25 |
– |
50 |
8.1 ± 0.6 |
100 |
11.2 ± 0.2 |
200 |
13.4 ± 0.4 |
Control (Chloramphenicol, 30 µg/mL) |
28.1 ± 0.2 |
Evaluation of MIC and MBC
To assess the MIC and MBC values of the CTA extract against the oral bacterium S. mutans, a broth microdilution assay was carried out. Table 2 displays the results of the susceptibility test for the CTA extract against S. mutans. The MBC value was found to be 400 µg/ml while the MIC value was 100 µg/ml. The MBC/MIC ratio was found to be 4, indicating that the CTA extract significantly reduced the growth of S. mutans with bactericidal activity. According to Mogana et al.15 the effect of the antimicrobial compound was considered bactericidal if the MBC/MIC ratio was less or equal to 4. Meanwhile, if the MBC/MIC was found to be more than 4, the antimicrobial compound was defined to possess a bacteriostatic effect against test microorganisms. Additionally, it was discovered that the MBC value was greater than the MIC value, indicating that in order to kill bacterial cells rather than just prevent their development, a higher concentration of the extract would need to be used. A similar observation has been reported by Satria et al.16 who revealed the inhibitory effect of butterfly pea flower ethanolic extract on S. mutans ATCC 25175 was increased with the increment of extract concentration. The outcomes demonstrated the antibacterial capability of the CTA extract against oral cavity bacterium.
Table (2):
Evaluation of MIC and MBC values of the extract through broth microdilution assay
Test bacteria |
MIC (µg/mL) |
MBC (µg/mL) |
Ratio (MBC/MIC) |
|---|---|---|---|
S. mutans Clarke ATCC 700610 |
100 |
400 |
4 |
Time-kill curve
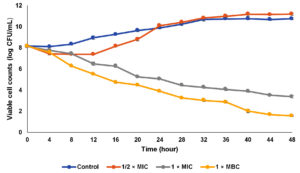
The efficacy of the CTA extract on bacterial cells was determined via time-kill analysis at different extract concentrations. Three different extract concentrations were tested in the present study including ½ × MIC (50 µg/mL), 1 × MIC (100 µg/mL), and 1 × MBC (400 µg/mL) as shown in Figure 1. For control, bacterial cells were grown exponentially until the maximum growth was achieved at stationary phase. When the bacterial cells were exposed to ½ × MIC extract concentration, the bacterial cell was slightly reduced especially after 4 hours of the incubation period. However, after 16 hours of exposure, the bacterial cells resumed their growth until reached viable cell counts higher than the control. A similar observation which is the regrowth of bacterial cells has been reported by Nunart et al.17 According to Taufiq and Darah,18 the post-antibiotic effect, where the surviving bacteria start to multiply again, might happen at low extract concentrations. A biphasic killing curve with a decline in killing rate over time was seen when bacterial cells were exposed to decreasing concentrations of extract, pointing to the development of persister cells that are more resistant to antimicrobial substances. The occurrence of regrowth was linked to two discrete subgroups with varying susceptibilities, in which the deliberate expansion of a resistant subpopulation replaces the killing of the susceptible population in a preferred manner at a designated time interaction duration Tam et al.19 After being exposed to the CTA extract at the MIC level, the number of viable cells gradually declined throughout the 48 hours incubation period. The viable cell counts recorded were 1.1 × 105 and 1.1 × 105 CFU/mL at incubation hours of 24 and 48, respectively. The present finding showed a higher concentration of extract (extract at MBC level) could rapidly kill the bacterial cells in which the viable cell counts were reduced to 3.2 × 105 CFU/mL (99.9%) after 12 hours exposed to the extract. This result revealed the CTA extract exerted a strong bactericidal effect on S. mutans and this activity was dose-dependent whereby higher concentration leads to lower viable cell counts. Ibrahim et al.20 reported a similar observation in which MRSA and Klebsiella pneumonia cells required a higher dose of the extract to have inhibitory or lethal effects on them. Table 3 displays the killing effectiveness of the extract on S. mutans, with no viable cell decrease seen for the growth control. Regarding the half-MIC concentration, 50% of the starting inoculum was reduced after 12–16 hours of incubation period following treatment with the extract. However, the viable cell counts were not reached 90, 95, and 99.9% of growth reduction for sub-MIC value due to the re-growth of persister cells which was induced by lower extract concentration. After an incubation time of between 16 and 20 and 8 to 12 hours, respectively, the MIC and MBC values of the extract showed growth reduction up to 99.9% of the initial inoculum, showing the MBC value is capable of swiftly eliminating all the bacterial cells.
Table (3):
The amount of time needed to reduce growth by 25, 50, 90, 99, and 99.9% in the original inoculum of Streptococcus mutans Clarke 700610
| Percentage of reduction (%) | Time (h) | |||
|---|---|---|---|---|
| Control | ½ × MIC | 1 × MIC | 1 × MBC | |
| 50 | NR | 8 – 12 | 4 – 8 | 0 – 4 |
| 90 | NR | NR | 8 – 12 | 4 – 8 |
| 95 | NR | NR | 8 – 12 | 4 – 8 |
| 99.9 | NR | NR | 16 – 20 | 8 – 12 |
Key: NR: not reached
Figure 1. Time-kill curve of aqueous extract of Clitoria ternatea against Streptococcus mutans. (The data were recorded in triplicate, mean ± SD)
Toxicity test
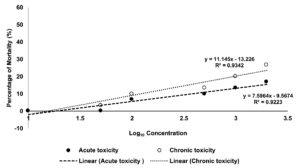
The process of determining the degree to which a substance of interest negatively impacts an organism’s regular biological functions, given a specific exposure duration, route of exposure, and substance concentration, is known as toxicology testing, also known as safety assessment or toxicity testing. Figure 2 shows the toxicity test of the CTA extract towards brine shrimp. The LC50 values after 6 hours (acute toxicity) and 24 hours (chronic toxicity) of incubation showed that the aqueous extract was not harmful to the shrimps, according to the results. The outcome was consistent with Meyer’s toxicity index, according to which crude extracts with LC50 values larger than 1000 µg/mL were deemed non-toxic, and those with LC50 values less than 1000 µg/mL were deemed harmful.21 The non-toxic CTA extract could benefit cosmeceutical industries, especially in the production of wash mouth as well as a handwash.
Morphological changes of bacterial cells exposed to the extract
Scanning electron microscopy (SEM) analysis of the CTA extract’s impact on Streptococcus mutans Clarke ATCC 700610 indicated that the structural degeneration of the extract-treated cells was irreparable (Figure 3). Figure 3a shows the untreated cells which exhibited the presence of regular Gram-positive cocci-shaped bacteria with an undamaged and smooth surface. The SEM photomicrographs also revealed that the cells were growing actively (indicated by the arrow). However, after 48 hours treated with the CTA extract, the cells experienced severe damage on the surface and become irregular in shape (Figure 3b). Some bacterial cells were cavitized, crumpled, and no longer had the characteristic coccal shape. These damages and the uneven phenomenon could lead to the death of bacterial cells. Similar findings were made by Taufiq and Darah18 who showed that S. mutans cells were disrupted after being treated (for 36 hours) with fungal extract, leading to the appearance of more cavities and cell debris. Subsequently, the cell was lysed and completely collapsed after the treatment. Additionally, they stated that fungal extract treatment created anomalies in bacterial cells, and that these changes may have been brought on by the invasions and morphological chaos.
The current study’s findings demonstrate the potential of CTA extract as an antibacterial agent against the bacterium that causes caries. Kirby Bauer disc diffusion assay revealed that CTA extract exerted significant antibacterial activity against Streptococcus mutans and the activity was increased with the concentration of the extract. On the other hand, the broth microdilution assay exhibited that the CTA extract possesses a bactericidal effect on the test bacterium due to the MBC/MIC value ratio being 4. The effectiveness of the CTA extract was assessed using a time-kill study, and the results revealed that the extract’s antibacterial activity is dose-dependent, with high concentrations of the extract being able to inhibit and kill bacterial cells. However, low extract concentration could induce the regrowth of bacterial cells. The toxicity test revealed that the CTA extract was non-toxic to humans due to a higher LC50 value (> 1000 µg/mL). Structural degeneration of S. mutans cells exposed to CTA extract was observed via scanning electron microscope and the microphotographs revealed the test bacterium loses their original shape and underwent severe damage including crumpled, cavitized, and some of them becoming irregular in shape leading to cell death. The use of water as a solvent to extract the bioactive compounds from the C. ternatea flower could be beneficial especially in formulating a commercial mouthwash with natural colorant in a safe manner. Additional research should be recommended, particularly in gaining access to the phytochemical characteristics of the CTA extract that result in antibacterial activity through an examination using gas chromatography-mass spectroscopy (GC-MS) and liquid chromatography-mass spectroscopy (LCMS) techniques.
ACKNOWLEDGMENTS
The authors would like to thank Universiti Sains Malaysia for our access to their lab equipment in conducting this research work and the Natural Approach to Oral Health (NAtOH) Research Initiative Group, Faculty of Dentistry, Universiti Teknologi MARA, Puncak Alam Campus for providing bacterial culture.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
LMZ and MAHAZ performed the experiments including extraction of Clitoria ternatea flower, antibacterial activity, time-kill study, toxicity test, and structural degeneration (SEM). FA, NAZ, DI, and FZMY analyzed the data and performed the statistical analysis. MTMJ was the principal investigator, and supervised the entire work. All authors wrote, reviewed, edited and approved the manuscript for publication.
FUNDING
This study was supported by Geran Penyelidikan MyRA Lepasan PhD (LPhD), Universiti Teknologi MARA (UiTM), account number [600-RMC/GPM LPHD 5/3 (127/2021)].
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Vujanac M, Iyer VS, Sengupta M, Ajdic D. Regulation of Streptococcus mutans PTS Bio by the transcriptional repressor NigR. Mol Oral Microbiol. 2015;30(4):280-294.
Crossref - Matsumoto-Nakano M. Role of Streptococcus mutans surface proteins for biofilm formation. Jpn Dent Sci Rev. 2018;54(1):22-29.
Crossref - Jain P, Pundir RK. Antibiotic sensitivity pattern of Streptococcus mutans against commercially available drugs. J Pharm Res.2009;2(7):1250-1255.
- Loyola-Rodriguez JP, Ponce-Diaz ME, Loyola-Leyva A, et al. Determination and identification of antibiotic-resistant oral streptococci isolated from active dental infections in adults. Acta Odontol Scand. 2018;76(4):229-235.
Crossref - Chayaratanasin P, Caobi A, Suparpprom C, et al. Clitoria ternatea Flower Petal Extract Inhibits Adipogenesis and Lipid Accumulation in 3T3-L1 Preadipocytes by Downregulating Adipogenic Gene Expression. Molecules. 2019;24(10):1894.
Crossref - Jeyaraj EJ, Lim YY, Choo WS. Antioxidant, cytotoxic, and antibacterial activities of Clitoria ternatea flower extracts and anthocyanin-rich fraction. Sci Rep. 2022;12:14890.
Crossref - Manjula P, Mohan CH, Sreekanth D, Keerthi B, Devi BP. Phytochemical Analysis of Clitoria Ternatea Linn., A Valuable Medicinal Plant. J Indian Bot Soc. 2013;92(3&4):173-178.
- Dhanasekaran S, Rajesh A, Mathimani T, Samuel SM, Shanmuganathan R, Brindhadevi K. Efficacy of crude extracts of Clitoria ternatea for antibacterial activity against gram negative bacterium (Proteus mirabilis). Biocatal Agric Biotechnol. 2019;21:101328.
Crossref - Darsini IP, Shamshad S. Antimicrobial Activity and Phytochemical Evaluation of Clitoria Ternatea. Int J Sci Res. 2013;4(5):823-825.
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. 11th ed. CLSI Standard M07. Wayne, PA. Clinical and Laboratory Standard Institute. 2018.
- Jalil MTM, Ibrahim D. Effect ethyl acetate extract of Lasiodiplodia pseudotheobromae Industrial Biotechnology Research Laboratory OS-64 on growth and cell morphology of a foodborne bacterium, Bacillus cereus. Thai J Pharm Sci. 2021;17(1):1-12.
- Jalil MTM, Zakaria NA, Suhaimi NSM, Ibrahim D. Crude extracts of an endophytic fungus attenuate the growth of pathogenic bacteria in aquaculture. Iran J Microbiol. 2022;14(3):383-394.
- Marez D. Electron microscopy of Microsporum cookie after in vitro treatment with protoanemonin: A combined SEM and TEM study. Mycopathol. 1989;108:37-46.
Crossref - Kaplan JB. Antibiotic-induced biofilm formation. Int J Artif Organs. 2011;34(9):737-51.
Crossref - Mogana R, Adhikari A, Tzar MN, Ramliza R, Wiart C. Antibacterial activities of the extracts, fractions and isolated compounds from Canarium patentinervium Miq. against bacterial clinical isolates. BMC Complement Med Ther. 2020;20:55.
Crossref - Satria D, Sofyanti E, Wulandari P, Fajarini, Pakpahan SD, Limbong SA. Antibacterial activity of Medan Butterfly pea (Clitoria ternatea L.) corolla extract against Streptococcus mutans ATCC®25175™ and Staphylococcus aureus ATCC®6538™. Pharmacia. 2022;69(1):195-202.
Crossref - Nunart T, Chatsuwan T, Treyaprasert W. Time-kill study of in vitro antimicrobial activity of tedizolid against methicillin-resistant Staphylococcus aureus. Thai J Pharm Sci. 2017;41:25-29.
- Taufiq MMJ, Darah I. Effect of ethyl acetyl extract of Lasiodiplodia pseudotheobromae IBRL OS-64 against oral cavity bacteria with emphasis on Streptococcus mutans. J Appl Pham Sci. 2019;9(11):78-85.
- Tam VH, Schilling AN, Nikolaou M. Modelling time-kill studies to discern the pharmacodynamics of meropenem. J Antimicrob Chemother. 2005;55(5):699-706.
Crossref - Ibrahim D, Lee CC, Yenn TW, Zakaria L, Sheh-Hong L. Effect of the Extract of Endophytic fungus, Nigrospora sphaerica CL-OP 30, Against the Growth of Methicillin-Resistant Staphylococcus aureus (MRSA) and Klebsiella pneumonia cells. Trop J Pharm Res. 2015;14(11):2091-2097.
Crossref - Ocal S, Ferrer RB, Namoc K, Guzman SCSD. Phytochemical Analysis and Cytotoxicity Potential of Pittosporum pentandrum (Mamalis) Crude Leaf Extract Using Brine Shrimp Lethality Assay. Asian J Multidiscip. Stud. 2018;1(1):50-60.
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.