ISSN: 0973-7510
E-ISSN: 2581-690X
Influenza viruses are major communicable pathogens responsible for respiratory diseases affecting millions worldwide and denoted by increased morbidity and significant mortality. Antiviral drugs and periodical vaccination are used to control Influenza infections. The utility of currently available drugs is of major concern due to emergence of drug resistance. This necessitates the development of novel antiviral drugs from natural resources. Broad arsenal of highly effective novel anti-influenza drugs can be developed from actinomycetes which have been explored for development of an array of antimicrobials. Fractions of methanol, ethanol, ethyl acetate and aqueous of the Saccharopolyspora jiangxiensis IMA1 were employed to assess the antiviral activity against Oseltamivir resistant influenza A/(H1N1)pdm09 virus. MTT, Plaque Reduction, Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) and DAPI staining assays were performed to validate the study findings. Selectivity Index value of 18.38µg/ml concentration of the fraction was found to be effective to inhibit the growth of influenza viruses employing the Madin-Darby Canine Kidney cell line. Fraction produced a visually noticeable reduction in cytopathic effect as well as a reduction in viral titre as determined by the reduction in plaque formation. qRT-PCR assay clearly showed a linear relationship between the fraction concentration and the Ct values, demonstrating the virus growth inhibitory activity of the fraction. S.jiangxiensis IMA1 ethyl acetate fraction showed promising antiviral activity as revealed by inhibiting the amplification of influenza virus type A/(H1N1)pdm09. The research findings will be useful for the development of new antiviral drug from the bioactive actinomycetes extractions.
Influenza A/(H1N1) pdm09, Actinomycetes, Antiviral Activity, DAPI Staining, Plaque Assay, qRT-PCR
Globally, acute lower respiratory infections (ALRI) are the foremost cause of illness in all age group.1 Though ALRI is associated with high morbidity and mortality, the magnitude of illness varies widely among various age and socioeconomic groups. Both children and elderly are affected by viral etiological agents like influenza virus, Respiratory Syncytial Virus (RSV) and are associated with the most episodes of ALRI.2 Antiviral resistance developed by influenza viruses is one of the most important global health concerns.3 Reports emphasise on the rapid emergence of drug-resistance in type A influenza virus as it led to three major pandemics in the twentieth century and is predicted that more pandemic events can occur in future mainly due to the said concern. Antigenic drift associated with the core surface glycoprotein neuraminidase (NA) and mutations in M-protein enable the influenza viruses to build resistance to the available antivirals. Frequent antigenic variations lead to new influenza outbreaks that may be more severe than the previous epidemics.4 Influenza virus genome lacks proof reading mechanism like all other RNA viruses genome and thus it mutates persistently. Mutations may offer the virus varied selective advantages such as resistance to antiviral drugs and immune escape features. Even a single amino acid change in viral Hemaglutinin (HA) and NA antigen sequences results in antigenic drift which causes the virus to escape from the host’s adaptive immunity, increase in the virulence, infectivity rate, parallel spread (transmission occurs in the same species) and perpendicular spread (transmission to other species, like pig to human and avian to human). Further, genetic re-assortment events magnify the above challenges due to generation of diverse variants.5-7
Natural products are the organic molecules derived from primary or secondary metabolism of living organisms such as plants and microorganisms. Around 50 to 60 percent are produced by plants (alkaloids, flavonoids, terpenoids, steroids, and carbohydrates), and 5% are of microbial origin. Among the microorganisms, actinomycetes are reported to serve as vital resources for the development of major anti-infectious, anticancer, antibacterial, antiviral, anti-inflammatory, antimalarial, and antidiabetic drugs.8
The marine environment constitutes approximately half of the global biodiversity and provides immense biological assets for the production of curative drugs. In the past few decades marine organisms (e.g. bacteria, algae, sponges, corals, etc.) have been explored for their bioactive natural products.9-11 Ecological pressures, such as struggle for space, predation, symbiosis and tide variations, throughout thousands of years, originated the biosynthesis of complex secondary metabolites by these organisms, which in turn, allowed their adaptation to a competitive and combative environment.12 It is clear that the marine environment will play a crucial role in the future development and trials of anti-infective drugs. The purpose of this study was to establish an in-vitro template to screen marine concentrates for antiviral activity with a long-term aim of exploring new marine composites to be used as prospective antiviral drug candidates.13
Extraction of extracellular secondary metabolites
S.jiangxiensis IMA1 was isolated from the coral reef environment of Munaikkadu, Palk bay (9 38’58″ N- 79 57’38″ E). Extracellular secondary metabolite fractions from S. jiangxiensis was extracted with water, methanol, ethanol and ethyl acetate as described by Krishnamoorthy et al.14
Cell line
Madin-Darby Canine Kidney (MDCK) cells were cultured in Dulbecco’s Modified Eagle’s Medium (MEM; Wako, Osaka, Japan), supplemented with 10% fetal bovine serum (FBS; Life Technologies, CA, USA), 50 units/mL penicillin and 50 µg/mL streptomycin (P/S; Life Technologies), and 4 mM/L-glutamine, at 37°C in the presence of 5% CO2.15
Cell Culture infectious dose (CCID50) assay
In this study, A/India/Che-1851811/2018(H1N1) influenza strain was used. Viral titres were determined by CCID50 assay. About 1.5×104 MDCK cells/well were seeded into 96-well plate and incubated at 37°C with 5% CO2 till the complete monolayer was formed. Ten-fold serial dilutions of virus stock of Influenza A/(H1N1)pdm09 was prepared and inoculated into the seeded 96-well plates (10 wells for each dilution). CCID50 value was determined by Karber formula L-d (S-0.5), where L=log of lowest dilution used in the test, d=difference between log dilution steps, S=sum of proportion of “positive” tests (i.e., wells showing CPE).16
Determination of Maximum Non Cytotoxic Concentration (MNCC)
The extracts of known concentrations were dissolved in Dimethyl sulfoxide (DMSO – Sigma–Aldrich, St. Louis, MO) is diluted in MEM without fetal calf serum (FCS) and sterilized by a syringe filter of 0.2µm pore size (Millipore, MA) before each experiment (Sigma–Aldrich, St. Louis, MO). The cells were treated with different concentrations (0-25µg/ml) of the extract. MTT assay was used to determine the cytotoxicity.17 The absorbance values were used to calculate the MNCC50. The concentration of the extract which delivered least toxicity was recorded.
Antiviral activity by MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assay
MDCK cells were seeded in 96-well plate (1×104 cells/well) and incubated for 18 to 24 hrs for a monolayer formation. The non-cytotoxic concentration of the extract was prepared in MEM (MEM without FBS) and with 3 µg/mL L-tosylamido-2-phenyl ethyl chloromethyl ketone (TPCK- used to improve infection yield)-treated trypsin (Sigma-Aldrich). A virus dilution from 10-1 to 10-6 was prepared with serum free medium. The maximum non-cytotoxic concentration was determined with the ethyl acetate extract used in the MTT assay. After incubation, cell viability was determined using the MTT cell proliferation assay at 72 hrs. Images were documented every day with microscope (Nikon ECLIPSETs2). Necessary positive and cell controls were included for all the experiments. The experiment was performed in triplicates. The final average value was noted as probable concluding concentration.18
Plaque reduction assay
Antiviral activities of the tested fractions were evaluated by measuring the reduction in number of viral foci.19 Briefly, confluent monolayers of MDCK cells were prepared in 24 wells cell culture microplate. One hundred microlitres of Flu A/(H1N1)pdm09 (10-3 dilution) virus was prepared at the corresponding CCID50, allowed for absorbance by cells for 1h at 37°C. After incubation, the cells were treated with serial concentrations (ranging from 0 to 25 µg/mL) of S.jiangxiensis extract, and then incubated at 37°C further. After 3 days, the wells were overlaid with 1.5% of carboxy methyl cellulose (Sigma–Aldrich). Viral foci were visualized using crystal violet foci staining assay. The number of Flu A/(H1N1)pdm09 viral foci was counted and the titre of virus was expressed as Plaque-Forming-Unit (PFU). The percentage of reduction in viral foci (RVF%) compared with controls was calculated as follows:
RVF (%)=((C-T) x 100)/C
where, C is the mean of the number of foci for negative control well (without compound) and T is the mean of the number of foci in treated wells.
Quantitative RT-PCR
The concentration of the extracts which delivered inhibitory effect in the antiviral assay were further confirmed by qRT-PCR by TaqMan one-step RT-PCR master mix Reagents Kit (Applied Biosystems). Descriptions of primers and probes used are forward 5′ -GTGCTATAAACACCAGCCTYCCA-3′, reverse 5′-CGGGATATTCCTTAATCCTGTRGC-3′ and the probe is FAM specific 6-carboxyfluorescein-5′-CAGAATATACATCCRGTCACAATTGGARAA-3′ -BHQ-1.20
DAPI (4’,6-diamidino-2-phenylindole)staining assay
Cell nuclear morphology was evaluated by DAPI staining method and fluorescence microscopy. Gathering the supernatant from the antiviral assay plate includes Cell Control, Virus Control and various concentrations of drug and virus co-infected, were added to MDCK cells and incubated for 48 hrs and plate fixed with methanol. Finally, the cells were covered with DAPI and incubated for 15 min at 37°C in dark. After incubation, the DAPI stain was removed and the cells were washed with methanol and air dried.21 The 24-well plates were examined under a fluorescence microscope (Nikon Eclipse Ti, Nikon Instruments Inc., USA).
Gas Chromatography-Mass Spectrometry (GC-MS)
GC-MS analysis was performed at CLRI-CATERS (Central Leather Research Institute), Chennai. The fraction was dissolved in ethyl acetate and analyzed with GC-MS (CE GC 8000 top MSMD 8000 Tyson) with Db 35 mr column (10 mx0.5 mmx0.25 mm film thickness). The separation was carried with the aid of helium carrier gas from 100° to 250°C for three min.22
Secondary metabolites
Aqueous, methanol, ethanol and ethyl acetate extracts were included for Maximum Non Cytotoxic Concentration assay.
MTT assay
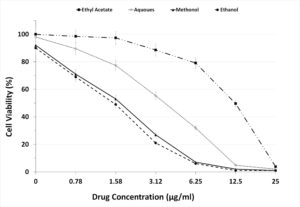
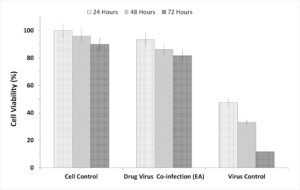
To evaluate the prepared fractions from S. jiangxiensis for their antiviral properties, a set of experiments in-vitro were carried out to conclude the nontoxic and effective antiviral dose of these fractions using MDCK cell line. Experimental results revealed that ethyl acetate fraction was found to be less toxic than others. To evaluate cytotoxicity, the viability of MDCK cells after incubation for 24, 48 and 72 hrs in MEM was determined by MTT assay. The viability of MDCK cells treated with fraction was unaffected up to 72hrs using the concentration of 25µg/ml suggesting the non toxic feature of the fraction in the optimised concentration. The viability extent was on par with cells exposed to DMSO control while using the fraction at non toxic doses. Hence, ethyl acetate fraction in the concentration range of 0-25µg/ml was chosen for further analysis including antiviral studies. As shown in the Figure 1, the inhibitory concentration value of 18.38µg/ml was found to be effective in inhibiting the growth of influenza viruses and the results were consistent with the microscopic observations (Figure 2 and 3).
Figure 1. Graph Depicting the Maximum Non Cytotoxic Concentration of the 4 extracts used in the present study
Figure 3. Graphic Presentation of the Cell Inhibitory based on the incubation hours of the extract and the virus
Plaque reduction assay
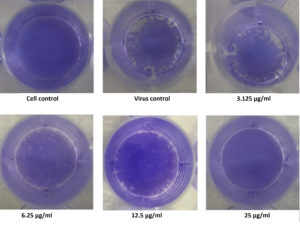
Among the four fractions, ethyl acetate exhibited significant levels of inhibitory effect on viral plaque formation as observed in the preliminary screening. Its inhibitory potential against Flu A/(H1N1)pdm09 virus was evaluated using the virus plaque assay, which is considered the gold standard for viral sensitivity test. Test fractions were shown to produce a visually noticeable reduction in CPE as well as a reduction in viral titer as plaques. The fraction was highly effective for inactivation of virus, producing a 50% viral inhibiting effect at concentration less than12.5µg/ml (Figure 4), which was considered for further characterization.
Figure 4. Illustration of the Inhibitory concentration of the extract against the virus by Plaque Reduction Assay
Quantitative RT-PCR
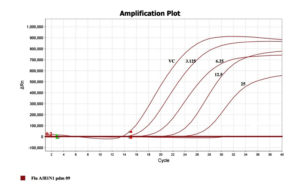
To confirm and quantitate the antiviral activity of the fraction, the RNA copy numbers was measured by qRT-PCR on Applied Biosystems Quant Studio. The RNA copies present in a given volume is relatively proportional to a measure of the degree of virus replication. Figure 5 depicts the decreasing mode in viral copy number estimated in cycle threshold (Ct) values as a function of concentration of the drug concentration. The RNA fractioned from virus stocks was used as a positive control, in the absence of drug which delivered an early Ct (14). The higher (Late) the Ct value was noticed in presence of drug i.e., in 12.5µg/ml concentration of drug, the ct value is 23 and in 25µg/ml concentration, the ct value noticed in 27th cycle which clearly indicates the inhibitory activity of the extraction (Figure 5). This show a leaner relationship between the drug concentration and the Ct values which demonstrates the virus growth inhibitory activity of the drugs chosen for the present study.
Figure 5. Representation of ct value in terms of Inhibition concentration of the extract and the positive virus controls
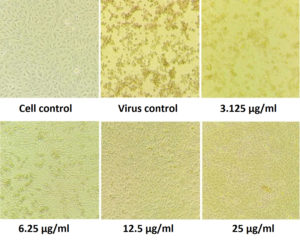
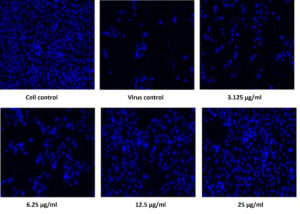
Figure 6. Pictorial image of the inhibitory concentration of the extract against the virus by DAPI tests
DAPI staining
To investigate whether the fractions inhibited the viral infection in cells, MDCK cells treated with a mixture of virus and fractions was examined by DAPI fluorescent staining method. The morphological changes such as nuclear fragmentation, cellular blebs and margination of nucleus were evident in untreated MDCK infected cells suggesting that the cells were undergoing apoptosis (Figure 6). Viability of ethyl acetate fraction treated viral infected cells was increased as shown by absence of CPE against untreated virus infected cells. This substantiated that the metabolite from S. jiangxiensis was able to inhibit the growth of virus and thereby possibly prevented the cell damage due to virus induced apoptosis.
Gas chromatography-mass spectrometry
The ethyl acetate fraction of S.jiangxiensis IMA1 was analyzed for the presence of virucidal compounds by GC-MS spectrum. Active compounds were predicted by their peak and molecular weight. Four different peaks were identified from chromatogram and the molecular weight 207, 210.27, 151.16 and 138.06 and the compounds are Streptazolin, Gancidin W, Streptokordin and Fosfomycin. Streptozolin and Gancidin exhibit anti-influenza activity by binding to viral Hemaglutinin protein domain having affinity with host cell receptor and thereby inhibits the binding of HA protein to host cell receptor subsequently preventing the entry of virus into the host cell.
Marine habitat is proving to be a rich source of novel microorganisms, particularly actinomycetes, whose metabolic products provide entirely new structural diversity with broad potential clinical applications.23-25 An array of novel compounds have been isolated from various marine organisms and evaluated for pharmacological properties. The screening of natural antiviral compounds from marine bacterial species has yielded positive outcome as there are over 40 compounds that are commercially made available in pharmacological markets. Several of the compounds have found applications in alternative antiviral medicines and some are being tested as potential antiviral drugs at the preclinical and clinical stages.26 Musacin C (Streptomyces griseovirdis), Fattiviracin A1(Streptomyces griseovirdis), FK 506 (Streptomyces tsukubaensis), Cyclomarin A (Streptomyces sp.), Resistomycin (Streptomyces corchorusii), Benzastatin C (Streptomyces nitrosporeus), MM461156 (Actinomadura pelletieri), Antimycin (S. kaviengensis) etc. produced by marine actinomycetes have exhibited potential antiviral effect against HSV-1, VZV, HIV, Orthopox, White Spot Syndrome virus, Hepatitis C, Western Equine Encephalitis virus etc. Their antiviral mechanisms broadly include the inactivation of the viral particles, inhibition of viral entry into host cells and inhibition of viral replication. These products continue to provide new drug leads for pharmaceutical development. However, reports on the compounds from natural sources especially marine actinomycetes exhibiting anti-Influenza viral activity are scarce.27
The immense capability of microorganisms presents an unrivalled opportunity to explore their unique properties towards the discovery of novel therapeutic agents. In this report, the potential anti-influenza feature of the solvent fractions derived from marine actinomycete S. jiangxiensis IMA1is explored in-vitro using MDCK cell line susceptible for influenza A/(H1N1)pdm09. This study optimizes the non-toxic dose of the ethyl fraction which was also found to possess anti-influenza activity as determined by antiviral studies. The ethyl acetate fraction was found to be more effective in terms of displaying non toxicity than the other solvent fractions such as methanol, ethanol, and aqueous derived from the marine actinomycete. Due to their toxic features as revealed by MTT assay and microscopic observations, further studies on evaluation of antiviral features were not planned for other fractions. Ethyl acetate is reported to be widely used for extracting the extra cellular antimicrobial secondary metabolites from actinomycetes.28-31 The compounds Streptozolin and Gancidin exhibit anti-influenza activity by binding to viral Hemaglutinin (HA) protein domain having affinity with host cell receptor and thereby inhibits the binding of HA protein to host cell receptor subsequently preventing the entry of virus into the host cell.
In the present study, it was shown that the ethyl acetate extract was found to apparently hinder at the viral entry stage of influenza A virus lifecycle. The MTT assay findings were further substantiated by the florescence assay (DAPI), qRT-PCR assay and plaque reduction assay. In addition, studies on mode of action would support the inference. Most important finding in the study is that this novel fraction has inhibitory effects on oseltamivir resistant strains of influenza viruses, suggesting that its antiviral activity may involve a different mode of action from current available antiviral agents besides its utility potential for treating oseltamivir resistant strains. Results from qRT-PCR also supported the findings from the viral foci reduction assays and demonstrated the significant reduction in Flu A/(H1N1)pdm09 virus specific RNA, suggesting that the fraction may probably target the virus replication machinery, mainly by inhibiting the RNA polymerase.32,33 Recent reports suggest the emergence of drug resistant pathogens and urge to search for the novel drugs against such pathogens. Takahashi and Nakashima stated the possibility of finding new and efficient bioactive metabolites from actinobacteria in under-explored resources.34
The present study identified that the fraction derived from marine actinomycetes, S. jiangxiensis IMA1 showed a promising anti-Influenza activity by means of reducing the viral load through blocking the amplification of oseltamivir resistant virus type A/(H1N1)pdm09. Ethyl acetate fractions were tested for their ability to block viral attachment/entry into the cells. The uniqueness of the present study remains that this represents the only study reporting anti-Influenza activity especially against oseltamivir resistant strain from marine actinomycete S. jiangxiensis IMA1.
ACKNOWLEDGMENTS
The authors would like to acknowledge the staff of the Department of Virology, King Institute of Preventive Medicine and Research, Guindy, Chennai, India, for their support. Dr. E. Kannapiran acknowledges the RUSA 2.0 grant from Alagappa University.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
KK conceptualized and designed the study. KR performed literature review and data acquisition. KR, PP and MK performed experimental studies. SS performed statistical analysis. PP performed data analysis. KR wrote the manuscript. KE reviewed the manuscript. SS and KR edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Bulla A, Hitze KL. Acute respiratory infections: a review. Bull World Health Org. 1978;56(3):481-498.
- World Health Organization. A manual for estimating disease burden associated with seasonal influenza.World Health Org. 2015.
- Smyk JM, Szydlowska N, Szulc W, Majewska A. Evolution of influenza viruses-drug resistance, treatment options, and prospects. Int J of Mol Sci. 2022;23(20):12244.
Crossref - Palese P. Influenza: old and new threats. Nat Med. 2004;10(12):S82-S87.
Crossref - Shao W, Li X, Goraya MU, Wang S, Chen JL. Evolution of influenza a virus by mutation and re-assortment. Int J Mol Sci. 2017;18(8):1650.
Crossref - Barik S. New treatments for influenza. BMC Med. 2012;10(1):1-15.
Crossref - Javanian M, Barary M, Ghebrehewet S, Koppolu V, Vasigala V, Ebrahimpour S. A brief review of influenza virus infection. J Med Virol. 2021;93(8):4638-4646.
Crossref - Xie XC, Mei WL, Zhao YX, et al. A new degraded sesquiterpene from marine actinomycete Streptomyces sp. 0616208. Chin Chem Lett.2006;17(11):1463-1465.
- Tziveleka LA, Vagias C, Roussis V. Natural products with anti-HIV activity from marine organisms. Curr Top Med Chem. 2003;3(13):1512-1535.
Crossref - Abad MJ, Bedoya LM, Bermejo AP. Marine compounds and their antimicrobial activities In: Méndez-Vilas A (ed.),. Science against microbial pathogens: communicating current research and technological advances, Formatex, Badajoz. 2011:51:1293-1306.
- Manikkam R, Parthasarathy K, Baskaran A, Dellibabu L. Inferences of actinobacterial metabolites to combat Corona virus. Advances in Traditional Medicine. 2022:1-8.
Crossref - Bhadury P, Mohammad BT, Wright PC. The current status of natural products from marine fungi and their potential as anti-infective agents. J Ind Microbiol Biotechnol. 2006;33(5):325-337.
Crossref - Arif JM, Al-Hazzani AA, Kunhi M, Al-Khodairy F. Novel Marine Compounds:Anticancer or Genotoxic? J Biomed Biotechnol. 2004;2004(2):93-98.
Crossref - Krishnamoorthy M, Dharmaraj D, Rajendran K, Karuppiah K, Balasubramanian M, Ethiraj K. Pharmacological activities of coral reef associated actinomycetes, Saccharopolyspora sp. IMA1. Biocatal Agric Biotechnol. 2020;28:101748.
Crossref - Hoffmann E, Stech J, Guan Y, Webster RG, Perez D. Universal primer set for the full-length amplification of all influenza A viruses. Arch Virol. 2001;146(12):2275-2289.
Crossref - World Health Organisation Staff. World Health Organization. Laboratory biosafety manual. 2004.
- Hosseini SM, Amini E, Kheiri MT, Mehrbod P, Shahidi M, Zabihi E. Anti-influenza activity of a novel polyoxometalate derivative (POM-4960). Int J Mol Cell Med. 2012;1(1):21-29.
- Shoji M, Arakaki Y, Esumi T, et al. Bakuchiol is a phenolic isoprenoid with novel enantiomer-selective anti-influenza A virus activity involving Nrf2 activation. J Biol Chem. 2015;290(46):28001-28017.
Crossref - Lai Y, Han T, Zhan S, Jiang Y, Liu X, Li G. Antiviral Activity of Isoimperatorin against Influenza A Virus in vitro and its Inhibition of Neuraminidase. Front Pharmacol. 2021;12:657826.
Crossref - Lan YC, Su MC, Chen CH, et al. Epidemiology of pandemic influenza A/H1N1 virus during 2009-2010 in Taiwan. Virus Res. 2013;177(1):46-54.
Crossref - Yasuhara-Bell J, Yang Y, Barlow R, Trapido-Rosenthal H, Lu Y. In vitro evaluation of marine-microorganism extracts for anti-viral activity. Virol J. 2010;7:1-11.
Crossref - Devakumar J, Keerthana V, Sudha SS. Identification of bioactive compounds by Gas Chromatography-Mass Spectrometry analysis of Syzygium Jambos (L) collected from Western Ghats Region Coimbatore, Tamil Nadu. Asian J Pharm Clinl Res. 2017;10(1):364-369.
Crossref - Bhatnagar I, Kim SK. Pharmacologically prospective antibiotic agents and their sources:a marine microbial perspective. Environ Toxicol Pharmacol. 2012;34:631-643.
Crossref - Gerwick WH, Fenner AM. Drug discovery from marine microbes. Microb Ecol. 2013;65:800-806.
Crossref - Mayer AM, Glaser KB, Cuevas C, et al. The odyssey of marine pharmaceuticals:a current pipeline perspective. Trends Pharmacol Sci. 2010;31(6):255-265.
Crossref - Raveh A, Delekta PC, Dobry CJ, et al. Discovery of potent broad spectrum antivirals derived from marine actinobacteria. PloS One. 2013;8(12):e82318.
Crossref - Yasuhara-Bell J, Lu Y. Marine compounds and their antiviral activities. Antiviral Res. 2010;86(3):231-240.
Crossref - Subramani R, Aalbersberg W. Marine actinomycetes:an ongoing source of novel bioactive metabolites. Microbiol Res. 2012;167(10):571-580.
Crossref - Berezin V, Abdukhakimova D, Trenozhnikova L, et al. Antiviral activities of extremophilicactinomycetes extracts from Kazakhstan’s unique ecosystems against influenza viruses and paramyxoviruses. Virol J. 2019;16(1):150.
Crossref - Liu M, Ren M, Zhang Y, et al. Antiviral Activity of Benzoheterocyclic Compounds from Soil-Derived Streptomyces jiujiangensis NBERC-24992. Molecules. 2023;28(2):878.
Crossref - Bernan VS, Greenstein M, Maiese WM. Marine microorganisms as a source of new natural products. Adv Appl Microbio. 1997;43:57-90.
Crossref - Ravikumar S, Gnanadesigan M, Saravanan A, Monisha N, Brindha V, Muthumari S. Antagonistic properties of seagrass associated Streptomyces sp. RAUACT-1:a source for anthraquinone rich compound. Asian Pac J Trop Med. 2012;5(11):887-890.
Crossref - Kwon HJ, Kim HH, Yoon SY, et al. In vitro inhibitory activity of Alpinia katsumadai extracts against influenza virus infection and hemagglutination. Virol J. 2010;7:1-9.
Crossref - Takahashi Y, Nakashima T. Actinomycetes, an inexhaustible source of naturally occurring antibiotics. Antibiotics. 2018;7(2):45.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.