ISSN: 0973-7510
E-ISSN: 2581-690X
Antimalarial drug-resistance has become a critical global issue, thereby making the search for novel and effective antimalarials imperative. This study aimed at designing, synthesising, and evaluating the antiplasmodial properties of quercetin and some of its derivatives. Forty quercetin derivatives were designed with ChemDraw and docked on six antimalarial targets using AutoDock Vina. Two ligands with the best binding affinity were synthesised using standard methods and characterised using UV-Vis and FT-IR spectroscopy. The suppressive and curative models evaluated the antiplasmodial activity of dihydroartemisinin (DHA), quercetin, and its two synthesized derivatives. A similar administration design was adopted to determine the effects of quercetin and the two derivatives on the pharmacokinetics (PK) of DHA in albino rats. Blood was obtained from the hearts of the animals at 0.25, 0.5, 1, 2, 3, and 5 hours post-dose (n = 5 per time point). UV Spectrophotometer was used to analyse the concentration of DHA in the serum. The molecular docking studies of the synthesised compounds with 2ghu showed binding energy from -8.0 to -9.0 kcal/mol, with 3,5,7,3’,4′ -penta-acetoxyflav-3-ene (quercetin pentaacetate) and 3,3′,4′,5,7-pentahydroxyflavylium (cyanidin) having the best binding activity amongst other derivatives. Quercetin and the synthesized compounds exhibited a significant (p <0.05) suppressive and curative potential, with quercetin giving the highest parasitaemia clearance of 73.55 ± 3.06. There was a significant alteration of PK parameters of DHA by quercetin and the synthesized derivatives. The results confirm the antiplasmodial activity of quercetin and the synthesized quercetin derivatives.
Molecular Docking, Synthesis, Quercetin, Pharmacokinetics, Antiplasmodial Dihydroartemisinin
Plasmodium parasites cause malaria, one of the most common diseases, which leads to an annual mortality of over one million people in tropical and subtropical zones.1 The World Health Organization (WHO) recommends Artesunate for malaria treatment, which has been used worldwide for many years. Artesunate is a derivative of the “guinghaosu” or sweet wormwood plant (Artemisia annua).2,3 Dihydroartemisinin, a derivative of artesunate, has been shown to reduce fever in patients suffering from severe falciparum malaria within 16 to 28 hours following parenteral administration.2 However, resistance to all types of anti-malaria medications, including artemisinin, has developed and is contributing to the recent rise in malaria-related deaths, especially in Africa. The emergence of this resistance can be mitigated through the use of combinations of anti-malaria drugs, hence the need to design and develop new combination therapies with less resistance and better pharmacokinetics profile.4 Quercetin (Que) is widely recognised as one of the most prominent flavonoid antioxidants. Structurally, the compound consists of a polyhydroxylated chromen-4-one core, characteristic of flavonols, with a catechol group (ring B) attached at the 2-position. This compound is prevalent in diverse dietary vegetables, making it nearly omnipresent in raw foods. The typical American is believed to consume about 25 mg of it each day.5,6 Given that its oral bioavailability can reach up to 50% (including its phenolic metabolites), quercetin can significantly enhance the body’s antioxidant defences, particularly in vegetable-rich diets. Research has also linked quercetin to several health benefits, such as reducing inflammation, protecting blood vessels, stopping platelets from clumping together, fighting viruses, cataracts, and malaria, improving brain function, and fighting cancer.7-10
Structurally, quercetin consists of a polyhydroxylated chromen-4-one core, which facilitates its interaction with biological targets. Its widespread presence in dietary sources, such as fruits and vegetables, and its relatively high bioavailability make it a promising candidate for drug development. Recent studies have demonstrated quercetin’s potential to inhibit Plasmodium falciparum growth through multiple mechanisms, including interference with parasite enzymes and modulation of host immune responses.11 Molecular docking has emerged as a critical tool in drug discovery, enabling the prediction of ligand-protein interactions and the identification of potential drug candidates. This computational approach provides insights into the binding affinities and molecular interactions of compounds with target proteins, facilitating the rational design of derivatives with enhanced efficacy. In the context of quercetin, molecular docking studies have revealed its ability to bind to key Plasmodium proteins, such as falcipain-2 and histo-aspartic protease, with high affinity.12,13 These findings highlight the importance of molecular docking in optimizing quercetin derivatives for antimalarial activity.
The synthesis of quercetin derivatives has been explored using various methods, including reductive acetylation, hydroxylation, and glycosylation.14 For instance, Robertson and Robinson pioneered the synthesis of flavonoid derivatives through reductive acetylation, a method that has been widely adopted for modifying quercetin’s structure.15,16 More recently, advanced techniques such as microwave-assisted synthesis and enzymatic modifications have been employed to improve the yield and purity of quercetin derivatives. These synthetic approaches have enabled the development of derivatives with enhanced pharmacological properties, including improved solubility, stability, and target specificity.
This study aims to design and synthesize quercetin derivatives with improved antimalarial activity, evaluate their binding affinities with Plasmodium proteins using molecular docking, and assess their in vivo efficacy against Plasmodium berghei. Additionally, the effects of these derivatives on the pharmacokinetics of dihydroartemisinin (DHA) will be investigated to explore potential synergistic interactions for malaria treatment. By integrating computational and experimental approaches, this research seeks to contribute to the development of novel antimalarial agents that can address the growing challenge of drug-resistance.
This study utilized analytical-grade chemicals and reagents, all of which were acquired from Sigma-Aldrich in Germany. The refractive index and solubility of the synthesised compounds were assessed using standard techniques while the open capillary method was employed to determine the melting points and reported as uncorrected values. The purity of the synthesised compounds was evaluated with the use of pre-coated RP-18 TLC plates (F254, JDH) using a mobile phase of MeOH/dichloromethane in a 9:1 ratio. UV and FTIR spectra were also recorded with a UV-visible spectrophotometer (UH4100) and a Digilab FTIR spectrometer (Model FTS-14).
Design/Molecular Docking
Forty quercetin derivatives were created using ChemDraw Pro 12.0 (CambridgeSoft Corporation, USA) and saved in SDF format. The target proteins: histo-aspartic protease (HAP) from Plasmodium falciparum (3QVI), Plasmodium falciparum dihydroorotate dehydrogenase (6GJG), plasmepsin II from Plasmodium falciparum (4YA8), and falcipain-2 from Plasmodium falciparum (2GHU) were all obtained from the protein data bank (https://www.rcsb.org). Molecular docking of the ligands with each of the target proteins was done using AutoDock Vina to obtain their respective binding affinity. Discovery Studio was utilised to examine the interactions between ligands and proteins. The ligands with the best activity were selected and synthesised. The molecular and pharmacokinetics properties were obtained using molinspiration (https://molinspiration.com/). The synthesized compounds were evaluated for their drug-like potential using the pkCSM web server (https://biosig.lab.uq.edu.au/pkcsm/), utilising Lipinski’s Rule of Five as a guideline.
Synthesis of derivatives
The synthesis of quercetin derivatives was performed using a modified version of the method reported by Robertson and Robinson.15,16
Synthesis and isolation of 3,3′,4′,5,7,-Penta-acetoxyflav-3-ene (also known as pentaacetoxy quercetin)
A solution was prepared by dissolving 2.0 g of quercetin in 75 mL of acetic anhydride. 1.0 g of anhydrous sodium acetate and 2.0 g of technical-grade zinc dust (90% purity) were added to the solution and refluxed for one hour, after which an additional 2.0 g of zinc dust was incorporated, and refluxing continued for another hour. The solution was filtered and the filtrate was transferred into 500 mL water. The residue was washed with 50 mL of warm glacial acetic acid, and the resulting filtrates were combined with the aqueous solution. The mixture was cooled in a refrigerator at 5 °C for about three hours, and filtered again, yielding a solid (dark orange-red) which was washed multiple times with water. After about 48 hours of drying under vacuum with sodium hydroxide as a desiccant, the solid yielded 2.54 grams of crude product. Separation of the product was done using chromatographic techniques over a column created by pouring a slurry of silicic acid (Bio-Rad A, 200-325 mesh) mixed with a solvent of benzene and acetone in a ratio of 85:15 into a tubular Pyrex column measuring 19 cm by 122 cm. After allowing the stationary phase to settle for 12 hours, a solution of the reaction mixture (1.0 g in 5-10 mL of solvent) was applied to the top of the column using a pipette. To track the separation, small eluent samples (three to four drops) were periodically treated with 10% methanolic sodium hydroxide. Evaporation of the solvent under vacuum using a rotary evaporator then yielded 2.33 g of crude material. This crude product was recrystallised twice from methanol, forming colourless needle-like crystals of 3,5,7,3′,4′-penta-acetoxyflav-3-ene, which melted at 139 °C. The isolated crystalline product weighed 0.22 grams, corresponding to 38.3 percent.
Synthesis of 3,3′,4′,5,7-pentahydroxyflavylium (otherwise known as cyanidin)
A small sample (10 mg) of 3,3′,4′,5,7-penta-acetoxyflav-3-ene was dissolved in 10-15 mL of a 10% (w/w) hydrogen chloride solution in anhydrous methanol. After refluxing the mixture for 20-25 minutes, the red solutions that formed were evaporated to dryness with a rotary evaporator. The remaining residue was then dissolved in small volumes of 0.1% hydrochloric acid in 95% ethanol. This solution was then spotted on a thin layer chromatography (TLC) plate alongside standards of 3,3′,4′,5,7-penta-acetoxyflav-3-ene and quercetin for comparison. For UV analysis, the product was initially redissolved in 0.1% hydrochloric acid in 95% ethanol before being diluted with 50 mL of water. The resulting aqueous solution was extracted with ethyl acetate (2 x 25 mL), and these extracts were discarded. The n-butanol fractions from each extraction process were then combined and concentrated by rotary evaporation to remove all solvents, yielding a crude product weighing 70 mg (5 mL). This product was then dissolved again in 0.1% hydrochloric acid in 95% ethanol for further ultraviolet analysis.
Physico-chemical analyses of the synthesised derivatives
Melting point determinations of the synthesised compounds
A small amount of the synthesised derivatives was placed at the bottom of a melting point capillary tube. The tube was then inserted into the melting point apparatus. The melting point range was recorded using the thermometer, noting the temperature at which the test compound began to melt and form a liquid droplet and the temperature at which all of the compounds had completely melted in the tube.
Refractometric analysis of the synthesised compounds
A refractometer was employed to measure the refractive index of the synthesised compounds. The obtained results were carefully recorded.
Ultraviolet Spectrophotometric Analysis (UV)
Dichloromethane (2 mL) was used to dissolve roughly 0.1 mL of each test sample. The absorbance was measured using a UV-visible spectrophotometer (UH4100) in the range of 200-400 nm.
Fourier transform infrared spectrophotometric analysis (FTIR)
The FT-IR analysis of the synthesised compounds was conducted using a Digilab spectrometer (Model FTS-14).
Antimalarial studies
Ethical approval
Approval for scientific and ethical aspects of this research was granted by the ethics committee of the Faculty of Pharmacy at the University of Uyo, following established protocols for the appropriate care and use of laboratory animals.
Experimental animals
Sixty mice weighing 15 and 21 grams were utilised for the antiplasmodial study, while healthy Wistar rats weighing 120 and 165 grams were employed for pharmacokinetics studies. The antiplasmodial study utilized Swiss albino mice of both sexes, sourced from the Department of Pharmacology and Toxicology at the University of Uyo. These mice were housed under controlled laboratory conditions, with temperature maintained at 25 ± 2 °C, a 12-hour light/dark photoperiod, and humidity ranging from 55% to 69%. They received, ad libitum, pelleted grower mash and water.
Rodent malaria parasite
The Artesunate-resistant Plasmodium berghei NK-65 utilized in this research was obtained from the Nigeria Institute of Medical Research (NIMR) located in Lagos State, Nigeria. Throughout the experimental duration, the parasite was sustained via continuous intraperitoneal passage in mice. This method ensures the viability and pathogenicity of the parasite for subsequent research applications.17
Inoculum preparation and parasitaemia determination
A mouse previously infected intraperitoneally with chloroquine-sensitive Nk-65 strain Plasmodium berghei parasitised erythrocytes was used as the donor after confirming the desired level of parasitemia through microscopic examination. Infected blood was harvested from a chloroform-anesthetized donor mouse via cardiac puncture. A standard inoculum was prepared from this blood, adjusted to a concentration of 106 parasitized erythrocytes per millilitre in normal saline. The inoculum concentration was determined based on the parasitemia and erythrocyte count of the donor mouse’s blood, and each mouse received an intraperitoneal injection of this solution.17
Administration of synthesised products and drugs
Groups I, II, and III of animals were treated with 2.0 mg/kg body weight of quercetin and each of the two synthetic compounds via gavage. For positive controls, Groups IV and V received oral doses of chloroquine (35 mg/kg body weight) and dihydroartemisinin (DHA) (2.2 mg/kg body weight). Group VI served as the negative control and was given sterilized distilled water at a rate of 10 mL/kg body weight. All groups, including both control and test groups, were provided with standard animal feed that contained equal amounts of daily calories and nutrients to ensure consistency in dietary intake.17
Suppressive test (4 days test)
The Peters’ 4-day suppressive test was utilised to evaluate the efficacy of treatments against chloroquine-sensitive P. berghei NK-65 infection in mice. Swiss Albino mice were inoculated intraperitoneally with a standard inoculum containing 106 parasitised erythrocytes on day 1 (D0). The mice were grouped as previously described and treated for four consecutive days (D0-D3), with all treatments administered orally at a single daily dose. After the four-day treatment period, blood samples were collected from the tail vein of each mouse, and thin films were prepared on slides for microscopic examination to determine the level of parasitemia. This method allows assessing the antiplasmodial activity of the compounds tested by quantifying the parasitised erythrocytes in the blood.17-19
Curative treatment (Rane Test)
The mice were grouped as previously described and inoculated with the parasite on the first day (D0), allowing for maturation over three days (72 hours). Dihydroartemisinin (DHA) and chloroquine (CQ) were positive controls for the chloroquine-sensitive P. berghei. A blood film was taken on the fourth day (D3) to establish baseline parasitemia levels. Both drugs and the synthesised products were administered on the same day, with treatment continuing for five days (D3-D7) at a single dose per day. Daily blood smears were collected throughout the treatment period to monitor parasitemia levels. All treatments were administered orally to the mice at a single daily dose, consistent with earlier protocols. This experimental design follows Peters’ 4-day suppressive test methodology, which is widely used to assess the efficacy of antimalarial compounds against established infections in rodents.17,19
Microscopic examination and determination of parasitaemia
In all cases, thin blood smears were fixed with 70% methanol and stained with 3% Giemsa at pH 7.2 for 45 to 60 minutes. Examination of the slides was performed using an x100 oil immersion objective. The P. berghei parasites were counted per 200 leukocytes, which were then used to estimate the parasite density per microliter of blood. The parasite density was calculated using a specific formula that relates the number of parasites observed to the total leukocyte count. This method is consistent with established protocols for malaria diagnosis, as the Giemsa stain is widely recognised for its effectiveness in visualising malaria parasites in blood smears. The staining process enhances the parasites’ contrast against the blood cells’ background, allowing for accurate identification and quantification of parasitemia.20
Parasites per microliter = Number of parasites counted x 8000 leucocytes per
microliter/Number of leucocytes counted
The percentage of parasitemia was calculated by comparing the levels of parasitemia in the control group with those in the treated groups, and the results were expressed as a percentage.17
% parasitaemia = Number of parasitized RBC x 100/Total number of RBC
Pharmacokinetics studies
Animal grouping and administration of synthesized products and drugs
Rats, separated by sex, were housed in standard laboratory conditions (25 ± 2 °C, 12-hour light/dark cycle, 55%-69% humidity) with free access to water and pelleted feed. The animals were divided into six groups of five. These groups received dihydroartemisinin (DHA) or one of the synthesized products. Blood samples were then collected from each rat at 0, 15, and 30 minutes, and 1, 2, 3, and 5 hours after treatment.14
Sample collection and serum extraction
In this study, a terminal blood collection method was utilized. The animals were anaesthetized in a closed chamber using 2% chloroform, after which blood was drawn from the heart via cardiac puncture with a 5 mL syringe. The collected blood was promptly placed into a labelled serum separator tube, which was then sealed securely. To promote clot formation, the tubes were left standing vertically for 30 minutes at room temperature. Following this period, the tubes were centrifuged at 2,000 RPM for 15 minutes. The serum that formed on top was carefully drawn into a plain sample bottle for subsequent analysis.21
Extraction of drug from serum
Dihydroartemisinin (DHA) was isolated from serum samples using a liquid-liquid extraction technique. The process began by transferring 2 mL of serum into a plain container, followed by the addition of an equivalent volume of acetonitrile to induce protein precipitation. The tubes were securely sealed and subjected to centrifugation at 4,000 RPM for 20 minutes. After centrifugation, the clear supernatant was carefully collected and transferred into a newly labelled container.
For the DHA-containing samples, a hexane and ethyl acetate mixture (60:40 v/v)22 was introduced, while pure diethyl ether was used for samples containing chloroquine.23 Each mixture was thoroughly vortexed for 10 minutes to ensure proper extraction. The organic layers were then separated and collected into distinct labelled containers. To maximize recovery, the aqueous phases underwent a second extraction with 2 mL of the respective solvents. Finally, the combined organic extracts were dried using a stream of air.
UV-VIS spectrophotometric analysis of DHA
Dihydroartemisinin (DHA) was reconstituted using simulated intestinal fluid (SIF). To each container holding the dried drug samples, precisely 4 mL of simulated intestinal fluid (SIF) was introduced. The mixture was then vigorously shaken for 10 minutes to achieve complete homogenization. The inclusion of SIF played a crucial role in both reconstituting the samples and supporting the derivatization of dihydroartemisinin (DHA). This process allowed DHA to exhibit enhanced absorption at a wavelength of 290 nm during subsequent UV-Vis spectroscopic analysis.24
Estimation of drug concentration
The concentration of dihydroartemisinin (DHA) recovered from serum samples was determined by directly extrapolating from the calibration graphs established for DHA. This process involved measuring the absorbance of the serum samples at the specific wavelength where DHA exhibits maximum absorbance, typically around 290 nm following derivatisation.24
Statistical analysis
The experimental results were expressed as the mean ± standard error of the mean (SEM), calculated from triplicate measurements. Statistical evaluations were carried out using GraphPad Prism software (version 7.03 for Windows). Variations in antiplasmodial activity across the treatment groups were analyzed using one-way ANOVA. For further comparison of group means, Tukey’s multiple comparison test was applied, with statistical significance defined at a p-value of less than 0.05. Additionally, the drug concentration-time graphs and semilogarithmic graphs used to extrapolate the initial dose (C0) of the drugs in serum were plotted using the same software, facilitating a comprehensive pharmacokinetic data analysis. This approach ensures robust statistical validation of the findings and aids in drawing meaningful conclusions regarding the efficacy of the treatments tested.
Molecular docking
The results of docking (Table S1) of the previously designed quercetin derivatives revealed that all the ligands showed a good binding affinity (ranging from -6.5 to -9.1) across the four target proteins. Among all the proteins, Plasmodium falcipain-2 (2GHU) had the best binding affinity (-8.0 to -9.0) with all the docked ligands compared with the other proteins, thus making it the desired protein for further studies. It was observed that the presence of an acetyl (OAc) group reduced the binding energy (Figure S1), thereby enhancing the binding interaction between the ligands and proteins. This reduction in binding energy typically indicates a more favourable interaction, which can lead to increased affinity of the ligands for their target proteins.
The different binding interactions of the selected ligands and Plasmodium falcipain (2GHU) Figures S2-S6 revealed the presence of some amino acid residue (Table S2) such as pi-pi interactions, van der waals forces and conventional hydrogen bonds, which is common among quercetin (Que), 3, 5,7,3’,4′ – penta-acetoxyflav-3-ene (Flav-3) and 3,3’,4′,5,7-pentahydroxyflavylium (Flav). At the same time, the pi-alkyl and pi-cations are peculiar to quercetin alone.
The amino acid residue and bonding involved in 2GHU and the selected ligands (Table S2) revealed the following interactions: Asn, Ala: C, 110, Gln: A208, Gly: A:40, Gln: A23, Ala: A:157, among others. The molecular properties of the selected ligands (Table S3) show that dihydroartemisinin (DHA) has the highest number of non-acceptors of 8 acceptors, followed by quercetin and flav-3-ene of 7, while Chloroquine (CQ) and flav had the lowest non-acceptors. All the ligands evaluated in this study adhered to Lipinski’s Rule of Five, as none of the molecules violated more than one of these rules. This compliance suggests that the ligands are likely to be “druggable”, meaning they possess favourable oral bioavailability and pharmacokinetics properties.
The pharmacokinetics study profile (Table S4) confirmed that the intestinal absorption, Fraction unbound (human) (Fu), AMES toxicity, L50 (mol/kg), Hepatotoxicity, Total Clearance (log ml/min/kg), and most especially water solubility (log mol/L) are satisfactory.
Spectral characteristics
Quercetin (Que)
The UV and FTIR spectra are shown in Figures S7 and S8, respectively. Refractive index (R.I) 1.4163, melting point 306.5 °C, lmax (chloroform): 350.00 nm; IR absorption: 1664.57 (C=C stretch (Aromatic)), 1319.31 (C-H stretch (vinyl bend)), 3402.43 (broadband), 1319.31 (C-H (vinyl bend)), 2713.84 (C-H methyl asym/sym. stretch), 723.31 (cis C-H out of plane)
3,3′,4′,5,7,-penta-acetoxyflav-3-ene (Flav-3)
The UV and FTIR spectra are shown in Figures S9 and S10, respectively. Percentage yield 45%, refractive index (R.I) 1.4163, melting point 318.5 °C, lmax (chloroform): 450.00 nm; IR absorption: 1664.57 (C=C stretch (Aromatic)), 1319.31 (C-H stretch (vinyl bend)), 3402.43 (broadband), 1319.31 (C-H (vinyl plan bend)).
3,3′,4′,5,7-pentahydroxyflavylium (Flav)
The UV and FTIR spectra are shown in Figures S11 and S12, respectively.
Percentage yield 27%, refractive index (R.I) 1.4600, melting point 316.5 °C, lmax (chloroform): 430.00 nm; IR absorption: 1010.70 (C-H methylene bend), 16.10.56 (C=C alkyl ring stretch (Aromatic)), 1319.31 (C-H stretch (vinyl bend)), 3313.71 (broadband), 1166.93 (C-H (vinyl bend)).
In vivo evaluation of antimalarial activities
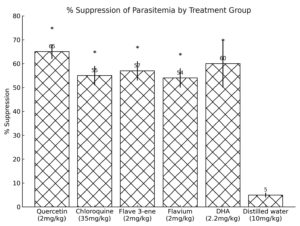
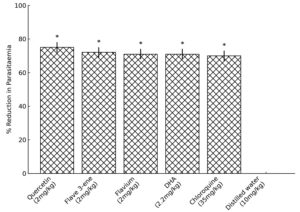
Figures 1 and 2 show the results of the suppressive and curative activities of quercetin, 3,5,7,3’,4′-penta-acetoxyflav-3-ene, 3,3’,4′,5,7-pentahydroxyflavylium, chloroquine (CQ) and dihydroartemisinin (DHA), respectively. The results of drug exposure and parasitaemia clearance are shown in Table S5.
Figure 1. Suppressive Activity of Quercetin, Dihydroartemisinin, Chloroquine and Synthesised Compounds In Mice.
*p <0.05 compared to distilled water group
Figure 2. Curative activity of Quercetin and Synthesised Derivatives In Mice.
*p <0.05 compared to distilled water
Pharmacokinetics studies
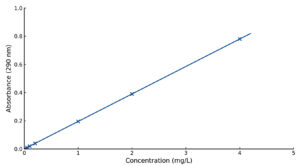
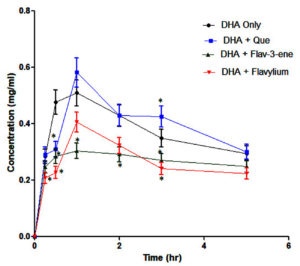
The calibration curve for derivatised dihydroartemisinin (DHA) in simulated intestinal fluid (SIF) is depicted in Figure 3. The pharmacokinetic effects of concomitant administration of quercetin and its synthesised derivatives on DHA were analysed and are detailed in Table. Figure 4 illustrates the concentration-time profiles of dihydroartemisinin (DHA) when administered alone and in combination with quercetin and its derivatives.
Table:
Results of Co-Administration of Quercetin and Its Synthesized Derivatives on the Pharmacokinetic Profile of DHA
Parameter |
DHA Only |
DHA + Que |
DHA + Flv-3 |
DHA + Flv |
|---|---|---|---|---|
AUC (µg/h/mL) |
1.52 ± 0.136 |
1.71 ± 0.153 |
1.98 ± 0.177* |
2.03 ± 0.182* |
Cmax (µg/mL) |
0.44 ± 0.039 |
0.51 ± 0.046* |
0.23 ± 0.021* |
0.33 ± 0.030* |
Tmax (h) |
1.00 ± 0.089 |
1.00 ± 0.089 |
1.00 ± 0.089 |
1.00 ± 0.089 |
t½ (h) |
5.63 ± 0.503 |
7.06 ± 0.631* |
6.14 ± 0.549 |
6.57 ± 0.587 |
Ka (h) |
0.57 ± 0.051 |
0.64 ± 0.057 |
0.03 ± 0.003* |
0.04 ± 0.004* |
Kel (h) |
0.18 ± 0.016 |
0.10 ± 0.009* |
0.16 ± 0.014 |
0.12 ± 0.011* |
CL (mL/h/kg) |
0.22 ± 0.020 |
0.29 ± 0.026* |
0.34 ± 0.030* |
0.32 ± 0.029* |
Vd (L/h) |
1.22 ± 0.109 |
1.94 ± 0.173* |
2.06 ± 0.184* |
2.75 ± 0.246* |
F (%) |
55.44 ± 4.957 |
58.63 ± 5.243 |
49.54 ± 4.430 |
53.20 ± 4.757 |
*p <0.05 compared with DHA only
The molecular docking studies demonstrated significant interactions between quercetin derivatives and Plasmodium falciparum falcipain-2 (2GHU), with binding affinities ranging from -8.0 to -9.0 kcal/mol. These results were superior to the positive control, dihydroartemisinin (DHA), indicating strong in silico antiplasmodial potential. The amino acid residues involved in hydrophobic and hydrogen bonding interactions, as shown in Table S2, highlight the role of specific residues such as Asn, Ala, and Gln in stabilising the ligand-protein complexes. The introduction of acetyl groups enhanced the binding interactions. This agrees with the findings that acetylation improves ligand-target affinity, likely because of increased hydrophobic and hydrogen bonding with key amino acid residues.25,26
The FTIR spectra obtained for quercetin and in this study are identical to those of Quercetin and quercetin pentaacetate (i.e. 3,5,7,3’,4’-penta-acetoxyflav-3-ene) earlier reported.14 While that of 3,3′,4′,5,7-pentahydroxyflavylium (cyanidin) is confirmed by the FTIR earlier reported, with variations likely arising from synthesis conditions or measurement parameters.27
Drug-likeness analysis based on Lipinski’s rule (Table S3) revealed that the synthesised derivatives, which are 3,5,7,3’,4’-penta-acetoxyflav-3-ene (Flav-3) and 3,3′,4′,5,7-pentahydroxyflavylium (Flav), complied with oral drug criteria, confirming their potential as antimalarial candidates. According to Lipinski’s rule, orally active drugs typically adhere to specific criteria, with no more than one violation allowed. These guidelines include a molecular weight under 500 daltons, up to 5 hydrogen bond donors (nHBD), a maximum of 10 hydrogen bond acceptors (nHBA), a LogP value not exceeding 5, and a polar surface area (PSA) below 140 Å.
The two quercetin derivatives were successfully synthesised by reductive acetylation and acid hydrolysis using a similar method that Robertson and Robinson15,16 described (Figures S13 and S14). Low yields were recorded, probably due to the starting material used in the synthesis or an incomplete reaction during the synthesis. Therefore, using quercetin as the lead compound to chemically modify its structure and search for high bioavailability and stronger activity precursor drugs has become a research hotspot in the field of medicine. 3-OH is a unique hydroxyl group of quercetin, and introducing functional groups at this position often yields more active compounds.28
The suppressive study showed that quercetin had the highest suppressive activity (Figure 1) with a percentage reduction in parasitaemia of 66.67 ± 0.84, followed by DHA (61.59 ± 5.67) compared to other treatment groups. In contrast, chloroquine had the lowest with a percentage reduction in parasitaemia of 49.85 ± 3.44. This implies that quercetin may act through multiple mechanisms, including antioxidant, anti-inflammatory, and direct antiplasmodial effects.25 Statistically, there is no significant association between the potentiality of quercetin and its derivatives and DHA.
The treatment group that received both quercetin and dihydroartemisinin (DHA) exhibited the highest percentage of parasitemia clearance, recording values of 73.55 ± 3.06 and 71.55 ± 1.30, respectively, compared to the untreated infected groups in the curative study. In all treatment groups, the median survival time (MST) ranged from 18 to 30 days, while the group administered only distilled water had the lowest average MST of 11 days. No significant association (p >0.05) was observed between the efficacy of quercetin and its derivatives and DHA.
The AUC (Area Under the Curve) of DHA increased significantly when co-administered with Flav-3 (1.98 µg·h/mL) and Flav (2.03 µg·h/mL), indicating enhanced drug exposure, suggesting that these derivatives may improve the bioavailability of DHA. Quercetin and its derivatives may enhance DHA absorption by modulating intestinal transporters or improving solubility.29 Quercetin increased the Cmax (Maximum Concentration) of DHA (0.51 µg/mL), while Flav-3 and Flav reduced it (0.23 µg/mL and 0.33 µg/mL, respectively). This could be due to differences in absorption and metabolism pathways. These compounds may inhibit cytochrome P450 enzymes, thereby reducing the metabolism of DHA and increasing its bioavailability.30 The half-life (t½) of DHA increased when co-administered with quercetin (7.06 h) and its derivatives (6.14-6.57 h), probably as a result of prolonged drug retention in the body. Clearance (CL) and Volume of Distribution (Vd) increased with Flav-3 and Flav, indicating faster elimination and broader distribution of DHA in the presence of these derivatives. This suggests that these derivatives may alter renal or hepatic clearance pathways.
The synergistic antiplasmodial effect of quercetin + DHA is likely due to improved pharmacokinetics, including increased AUC and prolonged half-life. This ensures sustained drug levels in the bloodstream, enhancing parasite clearance. By modulating DHA pharmacokinetics, quercetin and its derivatives may reduce the risk of parasite resistance, a significant challenge in malaria treatment.31 The drug-likeness of quercetin and its derivatives ensures good oral bioavailability, which is critical for their antiplasmodial activity in vivo. This is consistent with studies showing that flavonoids enhance the efficacy of artemisinin derivatives by modulating drug metabolism and parasite resistance.32,33
The synthesised derivatives’ spectral characterisation and physicochemical analysis confirmed their purity and structural integrity. The UV and FTIR spectra revealed characteristic peaks of successful acetylation and hydroxylation, correlating with the expected molecular structures.
This study successfully designed and synthesised two quercetin derivatives-3,5,7,3′,4′-penta-acetoxyflav-3-ene (Flav-3) and 3,3′,4′,5,7-pentahydroxyflavylium (Flav)-and evaluated their antiplasmodial potential. Molecular docking studies revealed strong binding affinities with Plasmodium falciparum falcipain-2 (2GHU), indicating their potential as antimalarial agents. In vivo, studies demonstrated significant suppressive and curative activities against Plasmodium berghei, with quercetin achieving the highest parasitaemia clearance (73.55 ± 3.06%). Pharmacokinetic analysis showed that quercetin and its derivatives enhanced the bioavailability and elimination half-life of DHA, suggesting synergistic effects. These findings highlight the potential of quercetin derivatives as adjuncts to DHA in malaria treatment, offering a strategy to combat artemisinin resistance. Future research should focus on clinical trials to evaluate the long-term efficacy and safety of these compounds, as well as their interactions with other antimalarial drugs. This study provides a foundation for developing novel antiplasmodial agents to address the global burden of malaria.
Additional file: Additional Table S1-S5 and Figure S1-S14.
ACKNOWLEDGMENTS
The authors acknowledge the efforts and technical skills of Mr. Nsikak Malachy from the Department of Pharmacology and Toxicology at the Faculty of Pharmacy, University of Uyo, Nigeria. His contributions have been invaluable in advancing our research and understanding in this field.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
OE conceptualized the study. OE, ECJ and AE funding acquisition. OE, EA and SA performed supervision. PCA, OE, EA, AE, VA and ECJ applied methodology. PCA, AE, CG, FE, AO, EA and SA performed formal analysis. PCA wrote the original draft. OE and ECJ wrote, reviewed and edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
This work was supported by the Tertiary Education Trust Fund (TET Fund), National Research Fund (NRF) (Grant number TETF/DR&DCE/NRF/SETI/108).
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript and/or in the supplementary files.
ETHICS STATEMENT
This study was apporved by the Ethics Committee, Faculty of Pharmacy, University of Uyo, following established protocols for the appropriate care and use of laboratory animals.
- Rathore D, Jani D, Nagarkatti R, Kumar S. Heme detoxification and antimalarial drugs-known mechanisms and future prospects. Drug Discov Today. 2006;3(2):153-158.
Crossref - World Health Organization. WHO model prescribing information: Drugs used in parasitic diseases. 2nd ed. Geneva: World Health Organization; 1995. Available from: https://iris.who.int/handle/10665/41765
- Centre for Disease Control. Treatment of Severe Malaria. https://www.cdc.gov/malaria/hcp/clinical-guidance/treatment-of-severe-malaria.html. Accessed on February 12, 2025
- Wong NCW, Hansen HC, Chiacchia FS, Johansson JO. Pharmaceutical compositions for the prevention and treatment of complex diseases and their delivery by insertable medical devices. United States patent application publication US20060990162. 2006 Jul 28. http://v3.espacenet.com/textdoc?DB=EPODOC&IDX=US2009029987
- Memariani H, Memariani M, Ghasemian A. Quercetin as a Promising Antiprotozoan Phytochemical: Current Knowledge and Future Research Avenues. BioMed Res Int. 2024(1):7632408.
Crossref - Formica JV, Regelson W. Review of the biology of quercetin and related bioflavonoids. Food Chem Toxicol. 1995;33(12):1061-1080.
Crossref - Crozier A, Jaganath IB, Clifford MN. Dietary phenolics: chemistry, bioavailability and effects on health. Nat Prod Rep. 2009;26(1):1001-1043.
Crossref - Miles SL, McFarland M, Niles RM. Molecular and physiological actions of quercetin: need for clinical trials to assess its benefits in human disease. Nutr Rev. 2014;72(11):20-734.
Crossref - D’Andrea G. Quercetin: a flavonol with multifaceted therapeutic applications? Fitoterapia. 2015;106:256-271.
Crossref - Khan H, Ullah H, Aschner M, Cheang WS, Akkol EK. Neuroprotective effects of quercetin in Alzheimer’s disease. Biomolecules. 2019;10(1):E59.
Crossref - Gogoi P, Shakya A, Ghosh SK, et al. In silico study, synthesis, and evaluation of the antimalarial activity of hybrid dimethoxy pyrazole 1,3,5-triazine derivatives. J Biochem Mol Toxicol. 2021;35(3):e22682.
Crossref - Ghosh S, Chetia D, Gogoi N, Rudrapal M. Design, molecular docking, drug-likeness, and molecular dynamics studies of 1,2,4-trioxane derivatives as novel Plasmodium falciparum falcipain-2 (FP-2) inhibitors. BioTechnologia (Pozn). 2021;102(3):257-275.
Crossref - Zothantluanga JH, Chetia D, Umbon Y, et al. Pharmacognostic analysis and antimalarial evaluation of quercetin in Ilex umbellulata bark using HPTLC, in vitro screening, molecular docking, and network pharmacology. Future J Pharm Sci. 2025;11:18.
Crossref - da Silva SVS, Barboza OM, Souza JT, et al. Structural Design, Synthesis and Antioxidant, Antileishmania, Anti-Inflammatory and Anticancer Activities of a Novel Quercetin Acetylated Derivative. Molecules. 2021; 26(22):6923.
Crossref - Robertson A, Robinson R. A Synthesis of pyrylium salts of the anthocyanidin type. J Chem Soc. 1927;1:2196-2106.
Crossref - Aft H, Grant RR, Molyneux RJ. The reductive acetylation of quercetin. Tetrahedron. 1967;23(4):1963-1970.
Crossref - Ebong AS, Eseyin OA, Iweh EE, et al. Telfairia occidentalis potentiates the antiplasmodial activity of artemisinins and amodiaquine combination therapy. Anti-Infective Agents. 2020;18(2):152-159.
Crossref - Okokon JE, Ekpo AJ, Eseyin OA. Antiplasmodial activity of ethanolic root extract of Telfairia occidentalis. Res J Parasitol. 2007;2:94-98.
Crossref - Peters W. Rational methods in the search for antimalarial drugs. Trans R Soc Trop Med Hyg. 1967;61(3):400-410.
Crossref - World Health Organization. Basic malaria microscopy: Part 1. Learner’s guide. 2nd ed. Geneva: World Health Organization; 2010.
- Ebong AS, Eseyin OA, Etim EI, et al. Effect of Telfairia occidentalis on the pharmacokinetic parameters and efficacy of dihydroartemisinin and amodiaquine combination therapy. World J Pharm Pharm Sci. 2019;8(11):1-20.
- Ortelli D, Rudaz S, Cognard E, Veuthey JL. Analysis of dihydroartemisinin in plasma by liquid chromatography-mass spectroscopy. Chromatographia. 2000;52(7):445-450.
Crossref - Adedeji ON, Bolaji OO, Falade CO, Osonuga OA, Ademowo OG. Validation and pharmacokinetic application of a high-performance liquid chromatographic technique for determining the concentrations of amodiaquine and its metabolite in plasma of patients treated with oral fixed-dose amodiaquine-artesunate combination in areas of malaria endemicity. Antimicrob Agents Chemother. 2015;59:5114-5122.
Crossref - Esimone CO, Omeje EO, Okoye FBC, Obonga WO, Onah BU. Evidence for the spectroscopic determination of artesunate in dosage form. J Vector Borne Dis. 2008;(45)4:281-286.
- Maleki SJ, Crespo JF, Cabanillas B. Anti-inflammatory effects of flavonoids. Food Chem. 2019;299:125124.
Crossref - Al-Khayri JM, Sahana GR, Nagella P, Joseph BV, Alessa FM, Al-Mssallem MQ. Flavonoids as potential anti-inflammatory molecules: a review. Molecules. 2022;27(9):2901.
Crossref - Suresh S, Vellapandian C. Assessment of oral toxicity and safety profile of cyanidin: acute and subacute studies on anthocyanin. Future Sci OA. 2024;10(1).
Crossref - Zhang W, Sun J, Zhang P, et al. Design, Synthesis and Antitumor Activity of Quercetin Derivatives Containing a Quinoline Moiety. Molecules. 2024;29(1):240.
Crossref - Muthaura CN, Keriko JM, Mutai C, et al. Antiplasmodial potential of traditional medicinal plants and their derivatives. J Ethnopharmacol. 2015;170:148-167.
Crossref - Kondža M, Brizić I, Jokić S. Flavonoids as CYP3A4 inhibitors in vitro. Biomedicines. 2024;12(3):644.
Crossref - Ashley EA, Dhorda M, Fairhurst RM, et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2014;371(5):411-423.
Crossref - Miron A, Aprotosoaie AC, Trifan A, Xiao J. Flavonoids as modulators of metabolic enzymes and drug transporters. Ann N Y Acad Sci. 2017;1398(1):152-67.
Crossref - Carrillo-Martinez EJ, Flores-Hernández FY, Salazar-Montes AM, Nario-Chaidez HF, Hernández-Ortega LD. Quercetin, a flavonoid with great pharmacological capacity. Molecules. 2024;29(5):1000.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.