ISSN: 0973-7510
E-ISSN: 2581-690X
Gram-positive anaerobic cocci (GPAC) which are commonly known as Peptococci or Peptostreptococci belong to the genus Peptostreptococcus. Peptostreptococcus anaerobius is one of the most common GPAC known to be associated with infections of the abdominal cavity and the female genitourinary tract. The present study aims at determining the antimicrobial susceptibility profile of P. anaerobius isolates against various antimicrobials. This study was conducted over a period of three years from January 2015 to December 2017 in the Department of Microbiology of a tertiary care teaching hospital. Specimens like pus aspirates, soft tissue and body fluids were included in the study. P. anaerobius isolates were identified by standard methods and confirmed by automated mass spectrometry. Minimum inhibitory concentration was determined by the reference agar dilution method for different anti-anaerobic agents. A total of 30 P. anaerobius isolates were obtained from various infections with majority (n=21, 70%) of the isolates being recovered from infections of the anatomical sites below the waistline. All isolates showed excellent anti-anaerobic activity against metronidazole, penicillin G, cefoxitin and chloramphenicol. Clindamycin resistance was noted in 53.3% (n=16) of P. anaerobius isolates. None of the isolates were b-lactamase producers. Metronidazole which is considered as the empirical therapy of choice for anaerobic infections was found to have excellent activity. Significant resistance was noted towards clindamycin which is commonly used as an alternative to metronidazole in suspected anaerobic infections. Routine sensitivity testing of clinical isolates of anaerobes seems to be the need of the hour for effective patient management.
Agar dilution, Clindamycin, Metronidazole, Peptostreptococcus, Resistance
Gram-positive anaerobic cocci (GPAC) have influenced extensive taxonomic changes among anaerobic bacteria in the recent past due to advancement in molecular identification methods. Among the infections caused by Gram-positive anaerobic organisms, GPAC are the frequent isolates. They are present as commensal microbiota and account for about 20-30% of all isolated anaerobic bacteria from diverse clinical specimens1,2. They are mostly overlooked, as they are often isolated in polymicrobial infections involving pathogenic organisms. The most commonly isolated GPAC from clinical specimens include Finegoldia magna, Parvimonas micra, Peptoniphilus harei, and Peptostreptococcus anaerobius3.
P. anaerobius is one of the common GPAC, often associated with infections of the abdominal cavity and the female genitourinary tract1. They are found in polymicrobial infections although there have been reports of isolation in pure cultures1, 2. In spite of increase in the significance of GPAC, the genus or species level identification among this group is restricted to few Microbiology laboratories due to stringent methods associated with isolation and identification.
The frequency of antimicrobial resistance in anaerobic organisms is increasing globally in the past few decades4. Development of antibiotic resistance among anaerobic bacteria has tremendous impact on the selection of antimicrobial agents for empirical therapy. A prospective study was planned to analyse the susceptibility profile of P. anaerobius to anti-anaerobic antimicrobials in a tertiary care hospital setting.
The present study was conducted in the Department of Microbiology over a period of three years from January 2015 to December 2017 after obtaining ethical clearance from the Institutional Ethics Committee. The demographic and clinical details were collected for each case studied. Specimens including pus, soft tissue, abscess aspirates and body fluids received from consecutive patients with diverse anaerobic infections were analysed.
The specimens were directly inoculated into sterile wide mouth container and/or into Robertson’s cooked meat (RCM) medium. The specimens were initially subjected to Gram stain and then cultured anaerobically on 5% sheep blood agar, phenyl ethyl alcohol agar and neomycin blood agar with metronidazole disc (5 µg, HiMedia Labs, Mumbai). The inoculated culture plates were incubated anaerobically at 37ºC in anaerobic chamber (Whitley A35 Anaerobic workstation, Don Whitley Scientific, Shipley, UK) and inspected daily for five days.
Preliminary identification of anaerobes was done by zone of inhibition around metronidazole disc, colony morphology, Gram stain, susceptibility to special potency discs of vancomycin 5µg (BD Diagnostics, Sparks, MD, USA), kanamycin 1000µg (HiMedia Labs, Mumbai) and colistin 10µg (Oxoid Ltd, Basingstoke, Hamsphire, England) and susceptibility to 5% sodium polyanethol sulfonate (SPS)5.
Identification of P. anaerobius isolates
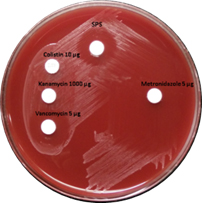
P. anaerobius isolates (Figure 1) were identified on 5% sheep blood agar as opaque, grey coloured colonies with a zone of inhibition around vancomycin (>10 mm) and SPS discs (>12 mm)5,6. The isolates were further confirmed by an automated microbial identification system, MALDI-TOF (Vitek MS, bioMérieux Inc.) mass spectrometry.
Fig. 1. Growth of P. anaerobius on 5% Sheep blood agar showing susceptible zone around Vancomycin 5µg and Sodium Polyanethol Sulfonate (SPS) disk
Antimicrobial Susceptibility testing
Chromogenic nitrocephin discs (Cefinase, BD Diagnostics, Sparks, MD, USA) were used for detection of b-lactamase activity among P. anaerobius isolates. Minimum inhibitory concentration (MIC) for P. anaerobius was determined by performing antimicrobial susceptibility testing by agar dilution (Wadsworth Method)7 on Wilkin-Chalgren agar with anaerobic supplement (HiMedia Labs, Mumbai). Antimicrobial susceptibility testing was performed against metronidazole, cefoxitin, penicillin G (Sigma Aldrich Ltd, USA), clindamycin and chloramphenicol (HiMedia Labs, Mumbai). Growth from 48 hours subculture on 5% sheep blood agar was inoculated into thioglycollate broth and incubated in anaerobic workstation to obtain adequate turbidity equivalent to 2 McFarland. Bacteroides fragilis ATCC 25285 was included as quality control strain in each batch. 2µL of this bacterial suspension was spot inoculated on to agar plates with varied concentrations of antibiotics. The plates were then incubated in the anaerobic workstation and results were read after 48 hours of incubation. The MICs were interpreted as susceptible or resistant as per Clinical & Laboratory Standard Institute (CLSI) guidelines7.
A total of 30 P. anaerobius isolates were recovered from various clinical specimens during the study period. These isolates were obtained from tissue specimens (n=18, 60%), pus aspirates (n=11, 36.6%) and pleural fluid (n=1, 3.3%). The most frequently affected age group was 41-60 years (n=15, 50%). Males (n=20, 66.6%) were predominantly affected than females (n=10, 33.3%). Diabetes mellitus (n=12, 40%) was the leading underlying illness observed in the present study. P. anaerobius isolates were majorly obtained from patients with deep-seated abscesses (n=10, 33.3%) (Table 1).
Table (1):
The clinical infection profile of patients with P. anaerobius isolates.
Clinical condition |
No (%) |
|---|---|
Deep-seated abscess |
10 (33.3) |
Diabetic foot |
4 (13.3) |
Necrotizing fasciitis |
4 (13.3) |
Osteomyelitis of lower limb joints |
4 (13.3) |
Gangrene of foot |
4 (13.3) |
Non-healing ulcer of leg |
3 (10) |
Pyometra |
1 (3.3) |
Among the 30 P. anaerobius isolates, monomicrobial growth was observed in 15 cases (50%). Polymicrobial anaerobic growth with Prevotella spp. was seen in 10% (n=3) of the samples. P. anaerobius along with aerobic pathogens was found in 40% cases (n= 12) with K. pneumoniae as the predominant aerobic pathogen (n=5, 41%).
Table (2):
Clinical Laboratory Standards Institute (CLSI) interpretive values for anaerobic bacterial pathogens.
Antibiotic |
Susceptible (µg/mL) |
Intermediate (µg/mL) |
Resistant (µg/mL) |
ATCC B. fragilis (252285) (µg/mL) |
|---|---|---|---|---|
Metronidazole |
<8 |
16 |
>32 |
0.25-1 |
Clindamycin |
<2 |
4 |
>8 |
0.5-2 |
Penicillin G |
<0.5 |
1 |
>2 |
8-32 |
Cefoxitin |
<16 |
32 |
>64 |
4-16 |
Chloramphenicol |
<8 |
16 |
>32 |
2-8 |
Antimicrobial Susceptibility Test
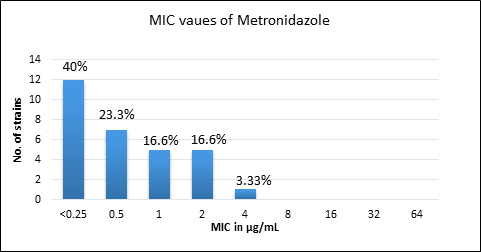
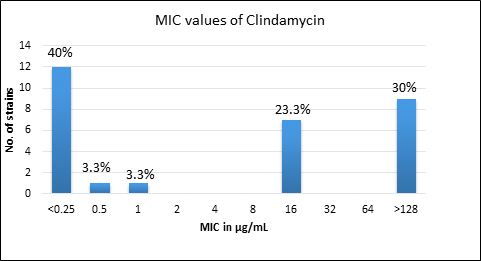
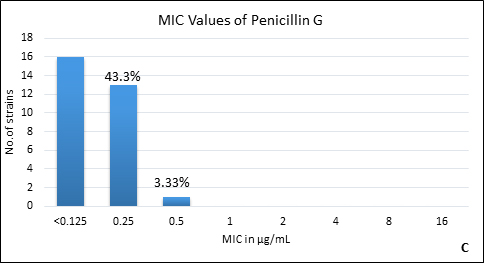
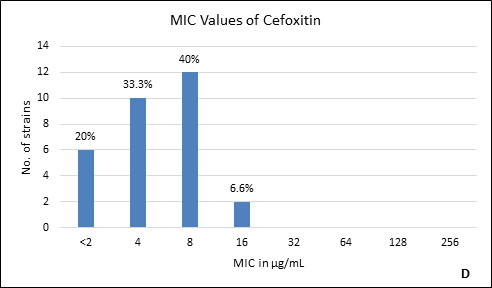
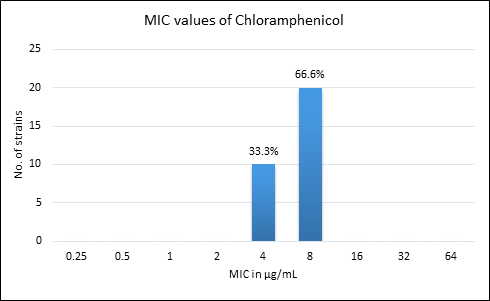
Quality control strain Bacteroides fragilis ATCC 25285 was tested along with the test strains for the five anti-anaerobic antimicrobials and the results were interpreted as per CLSI guidelines7 (Table 2). Clindamycin resistance was noted in 53.3% (n=16) of P. anaerobius isolates. None of the isolates tested were found resistant to metronidazole, cefoxitin, penicillin and chloramphenicol. The antimicrobial susceptibility results are summarized in Table 3 and Figure 2 (A to E).
Fig. 2. MIC distribution among P. anaerobius isolates to Metronidazole (A), Clindamycin (B), Penicillin (C), Cefoxitin (D) and Chloramphenicol (E)
Table (3):
Antimicrobial susceptibility profile of P. anaerobius isolates.
Antibiotic |
Range (µg/mL) |
MIC50 (µg/mL) |
MIC90 (µg/mL) |
% Resistance |
|---|---|---|---|---|
Metronidazole |
0.25-4 |
0.5 |
2 |
0% |
Clindamycin |
<0.25 – >128 |
16 |
>128 |
53% (n=16) |
Cefoxitin |
<2-16 |
4 |
8 |
0% |
Penicillin G |
<0.125-0.5 |
<0.125 |
0.25 |
0% |
Chloramphenicol |
4-8 |
8 |
8 |
0% |
Anaerobic bacteria form a vast majority of commensal flora inhabiting different body sites and are the source of diverse infections. Among various anaerobic pathogens, the importance of GPAC as a pathogen is increasing. P. anaerobius are a member of the genus Peptostreptococcus which form an integral part of the normal microbiota of gastrointestinal tract2.
P. anaerobius appear as Gram-positive cocci in pairs and short chains. On blood agar they form circular, entire edged, opaque shiny to off white colonies with wide zone of inhibition of >12mm around the SPS disc and show positive proline arylamidase test. However, it is to be noted that Peptostreptococcus stomatis, another species of genus Peptostreptococcus also produces a wide zone of inhibition around the SPS disc. The zone of inhibition is about 12-17 mm around SPS disc for P. anaerobius, whereas, P. stomatis give a wider zone of inhibition of 19-25mm8,9. In the present study all the 30 isolates of P .anaerobius had zone size ranging between 12-16 mm. However, the isolates were further confirmed by mass spectrometry.
P. anaerobius remain a significant anaerobic pathogen with reported rates of isolation ranging from 6-7%10,11. They are often encountered from infections of genitourinary tract, abdominal cavity, skin and soft tissue, and bone and joint infections. Generally, they are known to be involved in causation of infections below the waistline and seldom isolated from oro-dental infections2,12. In the present study, majority (n=21, 70%) of P. anaerobius were isolated from infections involving lower extremities (Table 1). Mostly GPAC are known to be isolated from polymicrobial infections involving different anatomical sites2, where as in our study, 50% of P. anaerobius isolates were obtained as monomicrobial flora.
As antimicrobial susceptibility testing is not performed routinely in many laboratories, there is paucity of susceptibility data related to GPAC. However, they are often considered to be susceptible to most of the available anti-anaerobic drugs2,13. Agar dilution is the gold standard and recommended method for determining MIC among anaerobic bacteria. In this, suspension containing a defined number of bacterial cells are spotted on appropriate growth medium with different concentrations of the antibiotic. The MIC is defined as the lowest concentration of the antibiotic that prevents visible growth of the microorganism in the growth medium.
Metronidazole remains the empirical drug of choice for most of the anaerobic infections. In the present study, all the strains of P. anaerobius have shown excellent activity against metronidazole. Similar findings were reported in other studies10,11,12,14. However, few studies have shown varying rates of metronidazole resistance. Baquero F et al.15, Lee K et al.16 and Meyer L et al.17 have reported resistance rates of 5.5%, 7.4% and 41% respectively against metronidazole among Peptostreptococcus spp.
Clindamycin, a lincosamide antibiotic has good spectrum of activity against most of the Gram-positive aerobic and anaerobic organisms and acts by inhibiting bacterial protein synthesis4,18. There are reports of emerging resistance against clindamycin among anaerobes, which could be due to modification of the target site10. Studies conducted by Brazier J et al.10, Wybo I et al.14 and Koeth LM et al.11 have reported clindamycin resistance rates varying from 1.1% to 5%. Other studies have shown higher rates of resistance of 19-25% to clindamycin15,16,17,19. Remarkably, in this study 53.3% (n=16) of P. anaerobius were resistant to clindamycin which is much higher than the earlier reports and is a point of concern.
Anaerobes develop resistance to b-lactam antibiotics by b-lactamase enzymes including penicillinases and cephalosporinases, reduced permeability through alteration in porin channels and by low affinity of penicillin binding proteins. Peptostreptococcus spp. are known to be susceptible to b- lactam drugs like penicillin G15. In our study, we found all the isolates susceptible to penicillin with low MIC90. However, there are reports of penicillin resistance among GPAC in literature varying from 4% to 29%10, 14, 15-17. It was also observed in the present study that, another b-lactam drug, cefoxitin had excellent activity against all P. anaerobius strains which is in agreement with the previous reports16, 20. Although, Könönen E et al.12 and Baquero F et al.15 have reported cefoxitin resistant Peptostreptococcus spp. in their studies. None of our isolates were b-lactamase producers which is concordant with other studies10, 12, 14.
Chloramphenicol, a bacteriostatic agent, known to be active against most of the anaerobic bacteria has also shown good activity against all the P. anaerobius strains in our setup, in line with the findings of Lee K et al16. The development of resistance to chloramphenicol is rare, however Wybo I et al.14 have reported 3% of GPAC being resistant to chloramphenicol.
It is necessary that the anaerobic laboratory facilities be optimally utilized and susceptibility testing be performed for the frequently isolated anaerobic pathogens. Awareness of the emerging resistance among the anaerobic pathogens has to be created so as to provide appropriate antimicrobial therapy for better patient care.
P. anaerobius is one of the significant GPAC isolated from diverse clinical infections in our setting. A high rate of clindamycin resistance was seen which is noteworthy, considering the fact that, this is a frequently utilized antimicrobial for treating anaerobic infections. Periodic surveillance of antimicrobial susceptibility profile of anaerobic bacteria is the need of the hour to guide empiric antibiotic therapy and to formulate local antibiotic policy.
ACKNOWLEDGMENTS
We are grateful to Manipal Academy of Higher Education, Manipal, Karnataka for providing this opportunity and the constant support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
- Murphy EC, Frick IM. Gram-positive anaerobic cocci-commensals and opportunistic pathogens. FEMS Microbiol Rev. 2013; 37:520-53.
- Murdoch DA. Gram-positive anaerobic cocci. Clin Microbiol Rev. 1998; 11:81-120.
- Veloo AC, Welling GW, Degener JE. Antimicrobial susceptibility of clinically relevant Gram-positive anaerobic cocci collected over a three-year period in the Netherlands. Antimicrob Agents Chemother. 2011; 55:1199-203.
- Brook I, Wexler HM, Goldstein EJ. Antianaerobic antimicrobials: spectrum and susceptibility testing. Clin Microbiol Rev. 2013; 26:526-46.
- Jousimies-Somer H, Summanen P, Citron DM, Baron EJ, Wexler HM, Finegold SM. 2002. Wadsworth-KTL anaerobic bacteriology manual. 6th Ed. Star Publishing Company, California.
- Song Y, Liu C, Finegold SM. Development of a flow chart for identification of Gram-positive anaerobic cocci in the clinical laboratory. J Clin Microbiol. 2007; 45:512-6.
- Clinical and Laboratory Standards Institute. 2013. Methods for antimicrobial susceptibility testing of anaerobic bacteria; approved standard. 6th Ed. CLSI document M11-A6. Clinical and Laboratory Standards Institute, Wayne, Pennsylvania, USA.
- Downes J, Wade WG. Peptostreptococcus stomatis sp. nov., isolated from the human oral cavity. Int J Syst Evol Microbiol. 2006; 56:751-4.
- Song Y, Liu C, Finegold SM. Development of a flow chart for identification of Gram-positive anaerobic cocci in the clinical laboratory. J Clin Microbiol. 2007; 45:512-6.
- Brazier J, Chmelar D, Dubreuil L, Feierl G, Hedberg M, Kalenic S, et al. European surveillance study on antimicrobial susceptibility of Gram-positive anaerobic cocci. Int J Antimicrob Agents. 2008; 31:316-20.
- Koeth LM, Good CE, Appelbaum PC, Goldstein EJ, Rodloff AC, Claros M, et al. Surveillance of susceptibility patterns in 1297 European and US anaerobic and capnophilic isolates to co-amoxiclav and five other antimicrobial agents. J Antimicrob Chemother. 2004; 53:1039-44.
- Könönen E, Bryk A, Niemi P, Kanervo-Nordström A. Antimicrobial susceptibilities of Peptostreptococcus anaerobius and the newly described Peptostreptococcus stomatis isolated from various human sources. Antimicrob Agents Chemother. 2007; 51:2205-7.
- Boyanova L, Osmanliev D, Petrov D, Mitov I, Usunova I, Petrov S. Anaerobic cocci and their resistance patterns to penicillin, cefoxitin, clindamycin and metronidazole: a Bulgarian study. Clin Microbiol Infect. 2000; 6:623-4.
- Wybo I, Piérard D, Verschraegen I, Reynders M, Vandoorslaer K, Claeys G, et al. Third Belgian multicentre survey of antibiotic susceptibility of anaerobic bacteria. J Antimicrob Chemother. 2007; 59:132-9.
- Baquero F, Reig M. Resistance of anaerobic bacteria to antimicrobial agents in Spain. Eur J Clin Microbiol Infect Dis. 1992; 11:1016-20.
- Lee K, Chong Y, Jeong SH, Xu XS, Kwon OH. Emerging resistance of anaerobic bacteria to antimicrobial agents in South Korea. Clin Infect Dis. 1996; 23:S73-7.
- Meyer L, Brink D, Weldhagen D. Antibiotic susceptibility patterns of anaerobic bacteria isolated in Pretoria, South Africa, during 2003–2004. South Afr J Epidemiol Infect. 2006; 21:161-3.
- Smieja M. Current indications for the use of clindamycin: A critical review. Can J Infect Dis. 1998; 9:22-8.
- Jeverica S, Kolenc U, Mueller-Premru M, Papst L. Evaluation of the routine antimicrobial susceptibility testing results of clinically significant anaerobic bacteria in a Slovenian tertiary-care hospital in 2015. Anaerobe. 2017; 47:64-69.
- Roberts SA, Shore KP, Paviour SD, Holland D, Morris AJ. Antimicrobial susceptibility of anaerobic bacteria in New Zealand: 1999-2003. J Antimicrob Chemother. 2006; 57(5):992-8.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.