ISSN: 0973-7510
E-ISSN: 2581-690X
Urinary tract infections (UTIs) are among the most frequently encountered infections for which individuals seek medical attention. They are usually ascending infections and if left untreated the causative agent can ascend the ureters causing pyelonephritis and, furthermore, enter the bloodstream causing systemic infections and urosepsis. Appropriate treatment prevents progression of disease. Escherichia coli is responsible for 80-95% cases of UTI. This study was undertaken to study the antimicrobial resistance among Escherichia coli isolated from patients suffering from UTI over 4 years at a tertiary level medical facility. A retrospective case series study was conducted. All UTI with significant bacteriuria due to E. coli were included in the study. Identification, speciation and antimicrobial susceptibility testing was done as per standard laboratory practices. A total of 555 strains of Escherichia coli were included in the study. Overall highest resistance rates for E. coli were seen among cephalosporins and fluoroquinolones while least overall resistance was seen for nitrofurantoin. There was gradual rise in resistance for carbapenem over the 4 years. The predominant microorganism responsible for all types of infections affecting the urinary tract is Escherichia coli. Empirical antimicrobial therapy for urinary tract infections may be unsuccessful, as resistance rates among E. coli are constantly increasing. The present study provides information regarding the antimicrobial susceptibility pattern over a period of 4 years. It shows that there is a gradual but consistent decrease in antimicrobial susceptibility among the isolates. Hence, it has become necessary that antimicrobial therapy be based on culture reports. Also, frequent and periodic updating of antimicrobial policy is essential.
Urinary Tract Infections, Uropathogenic E. coli, Escherichia coli, Antimicrobial Resistance
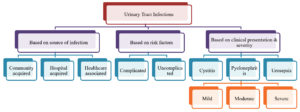
Urinary tract infections (UTI) is a hypernym used to denote the whole spectrum of infection, ranging from uncomplicated asymptomatic bacteriuria to complicated severe pyelonephritis and urosepsis. Underlying factors such as pregnancy, diabetes mellitus, hospital acquired infection (HAI), symptoms more than 7 days, renal failure, urinary tract obstruction, recent urinary tract instrumentation, presence of stent or urinary catheter or nephrostomy tube, anatomic or functional abnormality of the urinary tract, renal transplantation, history of childhood UTI, immunosuppression can be implicated in driving a disease course of uncomplicated UTI into a complicated one.1
UTI’s can be classified based on source of infection, risk factors or clinical presentation and severity as shown in the Figure 1.2 Nonetheless overall, the most common causative bacterial pathogen responsible for either uncomplicated or complicated UTI is Escherichia coli accounting for a staggering 80-95% of all UTI cases.3-5
UTI causing E. coli strains are distinct from the commensals E. coli and are referred to as Uropathogenic Escherichia coli (UPEC). An extragenetic material on pathogenicity-associated islands (PAIs) of UPEC strains allow to express gene products like secreted proteins, hemolysins, fimbrial adhesions, iron acquisition systems, specific lipopolysaccharides and capsule types that is lacking in commensals E. coli strains but certainly helps in weaponizing UPEC strains in bacterial pathogenesis as well as in disease progression.6
These pathogenic microorganisms initially establish colonization in the perineal area and then ascend the urethra to the bladder ultimately leading to cystitis. If unnoticed or untreated, cystitis develops into pyelonephritis caused by ascending bacteria through ureters to involve kidneys and in few cases can breach endothelial lining in the kidney to enter bloodstream causing serious and fatal complications like systemic infection and sepsis.7 The way to avoid the progression of disease is by early diagnosis and treatment with appropriate antibiotics. The treatment is complicated due to multi drug resistance among the causative agents. The objective of the current study is to examine the antimicrobial resistance trends among Escherichia coli isolated causing infections of the urinary tract over a span of 4 years.
Objectives
To study the antimicrobial susceptibility patterns in Escherichia coli causing UTI over 4 years.
A retrospective study was carried out within the central clinical laboratory under Department of Microbiology in a tertiary care hospital. Laboratory records for the last 4 years were scrutinized and all cases of urinary tract infection due to E. coli were included in the study. Urinary tract infection due to any other bacteria was not included in the study.
Following the laboratory protocol, all patients exhibiting signs and symptoms of urinary tract infection were given instructions to collect freshly voided clean catch mid-stream urine in sterile wide-mouth container with a screw cap. The specimens were labeled and transported promptly to the microbiology laboratory, where it was processed within half an hour. For culture, a modified semi-quantitative technique using a standard loop which is calibrated to 10 µL was used. Sample was inoculated on blood agar and MacConkey agar and incubated at 37°C for 24 hours. The plates were inspected for bacterial growth. The colonies were counted and the number of colonies was multiplied by 1000 to obtain the colony forming units per ml. Significant growth was considered if the number of colony-forming units was more than 105 per ml.8,9
The identification of the organism was determined by the presence of lactose fermenting colonies on MacConkey agar, presence of gram negative bacilli in gram stain that are catalase positive, oxidase negative and motile. Other confirmatory tests included a negative citrate utilization test and urease test, acid slant/acid butt with gas production in triple sugar iron test, fermented and motile in mannitol motility test and a positive indole test.8,9
Antimicrobial susceptibility test was done by modified Kirby Bauer’s disk diffusion technique on Muller Hinton agar. Selection of antibiotics to be tested and interpretation was done using Clinical Laboratory Standards Institute (CLSI) guidelines. The antimicrobials tested were nitrofurantoin, sulfamethoxazole-trimethoprim, amikacin, gentamicin, norfloxacin, ciprofloxacin, levofloxacin, cefotaxime, ceftazidime, cefepime, piperacillin-tazobactam and imipenem. Quality control was carried out using Escherichia coli ATCC 25922.10
Statistical analysis
Data analysis was performed using Microsoft Excel 2016. The resistance rates for E. coli, both annually and for the entire four-year period, were determined by dividing the count of urinary E. coli isolates exhibiting resistance to each antimicrobial agent by the total number of isolates tested against that specific antimicrobial.
In total, 555 strains of E. coli were isolated from urine samples in the Department of Microbiology from patients attending SNMC, Bagalkot from 1st January 2016 to 31st December 2019.
All 555 isolates were subjected to routine susceptibility testing against antibiotics as summarized in Table along with year-wise antibiotic sensitivity pattern.
Table:
Year-wise antimicrobial susceptibility pattern of urinary E. coli from 2016 to 2019
| 2016 | 2017 | 2018 | 2019 | |||||
|---|---|---|---|---|---|---|---|---|
| Antibiotics | Sensitive | % | Sensitive | % | Sensitive | % | Sensitive | % |
| Norfloxacin | 8 | 15.7 | 50 | 19.8 | 30 | 17.8 | NT | NT |
| Nitrofurantoin | 42 | 82.4 | 193 | 76.3 | 145 | 85.8 | 65 | 79.3 |
| Amikacin | 38 | 74.5 | 197 | 77.9 | 124 | 73.4 | 61 | 74.4 |
| Cefotaxime | 8 | 15.7 | 52 | 20.6 | 27 | 16.0 | 15 | 18.3 |
| Ceftazidime | 6 | 11.8 | 54 | 21.3 | 34 | 20.1 | NT | NT |
| Cefepime | 13 | 25.5 | 58 | 22.9 | 45 | 26.6 | 28 | 34.1 |
| Piperacillin-tazobactam | 41 | 80.4 | 201 | 79.4 | 126 | 74.6 | 43 | 52.4 |
| Imipenem | 50 | 98.0 | 242 | 95.7 | 166 | 98.2 | 61 | 74.4 |
| Levofloxacin | 15 | 29.4 | 141 | 55.7 | 57 | 33.7 | NT | NT |
| Ciprofloxacin | 6 | 11.8 | 47 | 18.6 | 36 | 21.3 | 14 | 17.1 |
| Gentamicin | 5 | 9.8 | 112 | 44.3 | 87 | 51.5 | 38 | 46.3 |
| Trimethoprim-sulfamethoxazole | 15 | 29.4 | 88 | 34.8 | 75 | 44.4 | 25 | 30.5 |
| TOTAL | 51 | 253 | 169 | 82 | ||||
NT-not tested
The highest resistance rates recorded in overall analysis of the urinary E. coli over the entire four-year period were seen for cephalosporins (cefotaxime-81.6%; ceftazidime-80.1%; cefepime-74%) as well as for fluoroquinolones (Ciprofloxacin-81.4%; Norfloxacin-81.3%; Levofloxacin-54.9%) while imipenem (6.4%) and nitrofurantoin (19.8%) showed least overall 4 year resistance rates (Figure 2). The resistance rate for imipenem exhibited a notable increased from 1.7% in 2017 to 25.6% in 2018 indicating a substantial increment of 1405.9%.
Among the bacterial infections reported to hospitals, UTIs are the most common infections. An estimated 50% of all women and 12% of men will suffer from at least one episode of symptomatic UTI in their lifetime.11,12 Among women who experience one episode of UTI, about 25% of those women experience a second episode within 6 months to 1 year, and 27-48% of women experience recurrent UTI’s.11,13
UTIs are caused by bacteria which belong to Enterobacteriaceae family, such as UPEC (most common), Klebsiella pneumoniae, Proteus mirabilis, Enterobacter spp, Citrobacter spp and non-fermenter gram negative bacteria such as Pseudomonas aeruginosa, Acinetobacter baumannii. Gram positive bacteria like Staphylococcus aureus, Staphylococcus saprophyticus, Enterococcus spp, Streptococcus bovis and fungi like Candida albicans are also known to cause UTIs.14,15 Furthermore, among UTIs, 50% of hospital-acquired, 80% of uncomplicated, 95% of community-acquired and most frequent cause of complicated UTIs are reportedly due to UPEC.16
The mainstay of successful UTI treatment depends on appropriate antimicrobial therapy, but most of the times antibiotics are prescribed without urine culture and antibiotic sensitivity testing at rural and urban health care centers, let that be private or government health care facilities/institutes across India. Around 65% of India population resides in rural area that is largely deprived of standard medical care.17
The recommended first-line antibiotics in uncomplicated cystitis is nitrofurantoin whereas Fluoroquinolones (ciprofloxacin and levofloxacin) and amoxicillin-clavulanic acid as alternative empirical therapy. In mild and moderate pyelonephritis, amoxicillin-clavulanic acid is first-line antibiotic and ciprofloxacin as second-line empirical therapy. For complicated UTI and severe pyelonephritis, recommended first-line antibiotics are amoxicillin along with gentamicin or an aminoglycoside with any second generation cephalosporin whereas intravenous 3rd generation cephalosporin as alternative empirical therapy.18
As per Indian Council of Medical Research (ICMR) annual report of Antimicrobial Resistance Research & Surveillance Network (AMRSN) for year 2021 showed susceptibility of urinary E. coli as follows- ciprofloxacin (28%), levofloxacin (25%), cephazolin (21%), cefotaxime (25%), piperacillin-tazobactam (63%), co-trimoxazole (43%), nitrofurantoin (83%), amikacin (83%), ertapenem (78%), imipenem (77%), meropenem (82%).19 In the present study, susceptibility of fluoroquinolones, 3rd generation cephalosporins, co-trimoxazole and gentamicin was found to be low and are not appropriate antibiotic agents to be considered as treatment options in seriously ill patients. This is certainly due to extensive and widespread use and abuse of these groups of antibiotics over couple of decades probably giving rise to multiple mutations in urinary E. coli producing multidrug resistant strains of UPEC. Hence, this rules out cephalosporins, fluoroquinolones, gentamicin or co-trimoxazole as empiric therapy in tertiary care set up for seriously ill patients. Fosfomycin can be an alternative antibiotic in empirical treatment of UTIs (3.0 gm single dose) because of its broad-spectrum activity against ESBL-producing E. coli.20,21
A successful antimicrobial therapy for UTIs is challenging task for clinicians due to increasing widespread drug resistance reported in urinary E. coli. This fact warrants judicious use of first and second-line antibiotics suggested for empirical treatment of UTIs. High resistance to cephalosporins, FQ’s, gentamicin and co-trimoxazole of UPEC in the present study is the critical evidence that should be considered while formulating rational antibiotic policy of regional health-care facilities. The knowledge of resistance pattern in UPEC has an important role in deciding the guidelines for use of first-line antibiotics as empirical treatment of UTIs; particularly policies with the objective of regulating prudent use of FQ’s, cephalosporins, gentamicin and co-trimoxazole.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors have made a substantial, direct and intellectual contribution to the work and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Urinary tract infections In: Torok E, Ed Moran, Cooke F. Oxford Handbook of Infectious Diseases and Microbiology, 2nd Ed. USA: Oxford University Press. 2017:678-91.
- Bartoletti R, Cai T, Wagenlehner FM, Naber K, Bjerklund Johansen TE. Treatment of urinary tract infections and antibiotic stewardship. Eur Urol Suppl. 2016;15(4):81-87.
Crossref - Nicolle LE. Uncomplicated urinary tract infection in adults including uncomplicated pyelonephritis. Urol Clin North Am. 2008;35(1):1-12.
Crossref - Fasugba O, Mitchell BG, Mnatzaganian G, Das A, Collignon P, Gardner A. Five-year antimicrobial resistance patterns of urinary Escherichia coli at an Australian tertiary hospital: time series analyses of prevalence data. PLoS One. 2016;11(10):e0164306.
Crossref - Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13(5):269-84.
Crossref - Mobley HL, Donnenberg MS, Hagan EC. Uropathogenic Escherichia coli. EcoSal Plus. 2009;3(2).
Crossref - Mehnert-Kay SA. Diagnosis and management of uncomplicated urinary tract infections. Am Fam Physician. 2005;72(3):451-456.
- Forbes BA, Sahm DF, Weissfeld AS. Bailey and Scott’s Diagnostic Microbiology. 12th ed. USA: Elsevier;2007.
- Koneman EW, Allen SD, Janda WM, Schreckenberger PC, Winn WC. Koneman’s Colour Atlas and Textbook of Diagnostic Microbiology. 6th ed. Baltimore: Lippincott Williams and Wilkins; 2006.
- Clinical Laboratory Standards Institute: Performance standards for antimicrobial susceptibility testing; 23rd informational supplement, CLSI M100-S23, Vol. 33 No. 1. Wayne, PA: Clinical Laboratory Standards Institute. 2013.
- Foxman B, Brown P. Epidemiology of urinary tract infections: transmission and risk factors, incidence, and costs. Infect Dis Clin North Am. 2003;17(2):227-241.
Crossref - Bischoff S, Walter T, Gerigk M, Ebert M, Vogelmann R. Empiric antibiotic therapy in urinary tract infection in patients with risk factors for antibiotic resistance in a German emergency department. BMC Infect Dis. 2018;18(1):56.
Crossref - Micali S, Isgro G, Bianchi G, Miceli N, Calapai G, Navarra M. Cranberry and recurrent cystitis: more than marketing? Crit Rev Food Sci Nutr. 2014;54(8):1063-1075.
Crossref - Mann R, Mediati DG, Duggin IG, Harry EJ, Bottomley AL. Metabolic adaptations of uropathogenic E. coli in the urinary tract. Front Cell Infect Microbiol. 2017;7:241.
Crossref - Hof H. Candiduria! What now? Therapy of urinary tract infections with Candida. Urologe A. 2017;56(2):172-179.
Crossref - Tabasi M, Karam MR, Habibi M, Mostafavi E, Bouzari S. Genotypic characterization of virulence factors in Escherichia coli isolated from patients with acute cystitis, pyelonephritis and asymptomatic bacteriuria. J Clin Diagn Res. 2016;10(12):DC01-DC07.
Crossref - Rural population (% of total population) – India. World Bank Open Data. cited 2023. https://data.worldbank.org/indicator/SP.RUR.TOTL.ZS?locations=IN
- Kot B, Wicha J, Gruzewska A, Piechota M, Wolska K, Obrebska M. Virulence factors, biofilm-forming ability, and antimicrobial resistance of urinary Escherichia coli strains isolated from hospitalized patients. Turk J Med Sci. 2016;46(6):1908-1914.
Crossref - Division of Epidemiology and Communicable Diseases – ICMR. Antimicrobial Resistance Research and Surveillance Network – Annual Report. New Delhi: Indian Council of Medical Research; 2021
- Falagas ME, Roussos N, Gkegkes ID, Rafailidis PI, Karageorgopoulos DE. Fosfomycin for the treatment of infections caused by Gram-positive cocci with advanced antimicrobial drug resistance: a review of microbiological, animal and clinical studies. Expert Opin Investig Drugs. 2009;18:921-944.
Crossref - Gudiol C, Cuervo G, Shaw E, Pujol M, Carratala J. Pharmacotherapeutic options for treating Staphylococcus aureus bacteremia. Expert Opin Pharmacother. 2017;18(18):1947-1963.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.