Urinary Tract Infections (UTI) is one of the most common infections, especially among women. Presently accessible antibiotics are a clinician’s first line of defense to treat infections, but antimicrobial resistance menace to reduce their efficacy. The consequences of multi-drug resistance to antibiotics are enhanced morbidity and mortality rates. The yearly death toll is >700,000 population worldwide, rising to ~10 million by 2050. There is a lack of novel antibiotics for UTIs as the return on its investment is poor compared to medicines for lifestyle diseases. The three organisms of utmost worry are methicillin-resistant Staphylococcus aureus (MRSA), Carbapenems and third-generation Cephalosporins resistant Klebsiella pneumoniae, Fluoroquinolones and third-generation Cephalosporins resistant Escherichia coli (E. coli). Among these, Escherichia coli is the foremost cause of community-acquired UTI infections throughout the globe, mainly due to the absence of alertness and inappropriate wastewater treatment. The purpose of this review article is to explore literature on uropathogens, the pattern of their antimicrobial resistance, and the hospital practices concerning the spread, as inadequate studies have been carried out and published on this topic. Hospital personnel are usually familiar with the management of infections, but most do not understand the conditions in their hospital. Implications of hospital practices play a major role in controlling hospital-acquired UTIs and the burden of its antimicrobial resistance. A complete approach involving financial and human resources will improve the infection control practices in hospitals without a doubt. Strict infection control measures in hospitals can help to reduce the number of hospital-acquired infections in pregnant women.
Hospital infection control, Antibiotic resistance, Hospital wastewater, Antibiotic concentrations, urinary tract infection, nosocomial infection
Urinary tract infections (UTI) are among the most common infections, especially in women. They are typically treated with currently accessible antibiotics as a clinician’s first line of defense, but their efficacy is reduced by antimicrobial resistance. Multidrug resistance to antibiotics results in increased morbidity and mortality rates. Globally, the yearly death toll related to antimicrobial resistance is >700,000 population, which will increase to ~10 million by 2050. Furthermore, novel antibiotics for UTIs are insufficient because their return on investment is lower than that of medicines for lifestyle diseases.
The three most common organism resistant to antimicrobials are methicillin-resistant Staphylococcus aureus (MRSA), carbapenem- and third-generation cephalosporin-resistant Klebsiella pneumoniae, fluoroquinolone- and third-generation cephalosporin-resistant Escherichia coli. Among them, E. coli is the main cause of community-acquired UTI worldwide mainly because of the absence of alertness and inappropriate wastewater treatment.
Given that inadequate studies on this topic, this review article aimed to explore studies on uropathogens, their antimicrobial resistance pattern, and hospital practices concerning their spread. Hospital personnel are usually familiar with infection management; however, most of them do not understand the conditions in their hospitals. Implications of hospital practice play a major role in controlling hospital-acquired UTIs and the burden of antimicrobial resistance. Therefore, a complete approach involving financial and human resources will improve infection control practices in hospitals. Strict infection control measures in hospitals can also help reduce the number of hospital-acquired infections in pregnant women.
Antibiotic epoch
In the early 1900s, Paul Ehrlich, a German physician, discovered the first antimicrobial drug1 that led to the start of an innovative period when microbial infections could be treated for the first time and cured with a high success rate. In 1909, Ehrlich applied the concept of “magic bullet,” discovered the active compound 606 against Treponema pallidum, the cause of syphilis, which was sold later under the name Salvarsan.2 Motivated by Ehrlich’s victorious discovery, pharmaceutical industries and researchers focused on screening of drugs and compounds for antimicrobial activity; in 1932, dye manufactures at IG Farben in Germany introduced the first sulfa drug as an antimicrobial agent, which was patented under the name Prontosil in 1935.2,3 During the same period, Alexander Flemming found an antimicrobial compound produced by Penicillium in 1928 and named it penicillin.4 This breakthrough initiated the discovery of natural compounds and served as a basis for developing new antimicrobial agents.
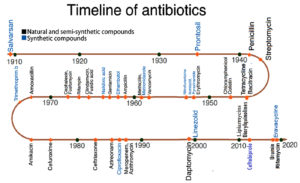
Fig. 1 shows the timeline of the evolution of various antimicrobial agents obtained either naturally or synthetically to fight various microbial infections. It clearly shows a decline in the development or discovery of new antimicrobial drugs in recent decades.
As the discovery of penicillin, scientists have actively been searching for microbes that produce antimicrobial compounds. In 1943, Albert Schatz, a student of Selman Waksman, discovered streptomycin in his laboratory. Similar to many other successful antibiotics that were later found, streptomycin is produced by Streptomyces, a bacterial genus, hence its name.5 It is the first antibiotic to show considerable efficacy against Mycobacterium tuberculosis, which causes tuberculosis. Furthermore, streptomycin was the first pharmaceutical medicine to undergo a randomized controlled trial.6 Over the next 40 years, new antimicrobial agents have emerged. Although the modification of natural chemicals has resulted in some effective semisynthetic derivatives, few synthetic substances have been created.7
Decline of the antibiotic era
In response to the amplified use of antimicrobial drugs throughout the 20th century, microorganisms have developed ways to defeat the effect of these drugs, consequently becoming resistant. As a result, formerly effective antimicrobial drugs are no longer effective in curing infections, leading to an increase in morbidity and death.8,9 Drug resistance remarkably varies among species and geographical locations. However, previously effective medications against some species have almost completely lost their clinical utility. For example, Staphylococcus aureus was once susceptible to ampicillin and penicillin; however, it is now entirely resistant to these antibiotics. With the increased prevalence of methicillin-resistant S. aureus (MRSA) in many countries, beta-lactamase-resistant compounds such as nafcillin and dicloxacillin have become ineffective.10,11 Similarly, among the most prevalent uropathogens, approximately half of E. coli isolates that were formerly responsive to sulfa medicines and ampicillin are now resistant.10 Fortunately, other available antibiotics are effective against these microorganisms, but resistance to these last-resort medications are frequently documented.12,13
Although antibiotics are still successful in treating most infections, further research on novel and safer antibiotics should be performed. Because drug development takes several years, it should ideally begin while resistance is still manageable. Over time, the scientific community has become increasingly focused on informing legislators and other decision-makers about the lack of progress in the development of new antibiotics.14,15 Professionals in hospitals worldwide encounter pan-resistant microorganisms such as multidrug-resistant Enterobacteriaceae and extensively drug-resistant tuberculosis. Consequently, pharmaceuticals that were no longer used because of their severe toxicity are considered as last-resort medications.3,17 Resistance does not usually occur as quickly as it did in the examples above. S. pneumonia, which causes pneumonia, has been successfully treated with penicillin for more than 60 years, but penicillin resistance in S. pneumonia rarely occurs.10,13 With these disparities in resistance development, variables that drive resistance evolution in different organisms should be determined in the development of strategies that prevent resistance emergence.
Urinary tract infections
In hospital and community settings, urinary tract infections (UTIs) are among the most common bacterial infections.19-21 These are also the most common bacterial illnesses acquired by women.21 Because germs can easily enter the bladder through the shorter female urethra, women are more susceptible to UTIs than men,18,19 but UTIs are still more prevalent in men than in women.18 Although various bacterial and fungal pathogens can cause UTI, the most common pathogen isolated in patients is E. coli, a gram-negative bacterium.22,23 The health consequences of a UTI include 2.4 days of limited daily activity and loss of about 1.2 days of work. In patients with underlying health disorders such as diabetes, complicated infections (such as paraurethral or renal abscesses) may occur more frequently than in patients without other underlying health issues, resulting in higher morbidity and hospitalization.21,22 Furthermore, UTI can extend to the bloodstream, resulting in bacteremic UTI, which is linked to an increased death rate.24
Almost 50 % to 60 % of women experience a UTI at least once in their life.25 It is also typical for UTI episodes to recur if the influencing variables responsible for the occurrence of UTI are not properly diagnosed and treated.21,26 Untreated UTIs can lead to major problems such as kidney damage, renal scarring, and renal failure. Gram-negative bacteria such as E. coli, Enterobacter species, Citrobacter species, Acinetobacter species, Pseudomonas aeruginosa, Proteus species, and Klebsiella species are the most common causes of UTIs. Among gram-positive microorganisms, Enterococcus species, coagulase-negative Staphylococcus, and Staphylococcus saprophyticus are the common causes of UTIs.27,28
Table (1):
Microbiology of UTI.
| Organism Type | Name of the Organism that causes UTI |
|---|---|
| Gram-positive bacteria |
Staphylococcus aureus |
| Enterococcus faecalis | |
| Staphylococcus saprophyticus | |
| Coagulase-negative staphylococci (CoNS) | |
| Gram-negative bacteria | Escherichia coli |
| Acinobacter spp | |
| Proteus mirabilis | |
| Klebsiella pneumonia | |
| Pseudomonas aeruginosa | |
| Fungus | Candida albicans |
| Torulopsisglabrata | |
| Cryptococcus neoformans | |
| Candida parapsilosis | |
| Candida cystitis | |
| Candida tropicalis |
Table 1 presents a categorized list of various microorganisms that cause UTIs in humans. Among them, E. coli, K. pneumoniae, and Proteus mirabilis are the major gram-negative UTI-causing pathogens. Candida spp. are the most common fungi causing UTIs in pregnant women.26
Bacteria responsible for UTIs have more hostile virulence factors than nonpathogenic microorganisms, thereby improving their host cell attachment, colonization, and attack abilities. They evade their host’s immune system by using specific virulence factors, which include various cellular components, such capsules, pili, lipopolysaccharides, and other external cell structures.28 Certain human anatomical and physiological factors play a role in the incidence of UTIs. For example, the urethra is shorter in women than in men, thereby increasing the risk of UTI. Similarly, atrial bladder emptying, which occurs frequently in pregnant women, leads to the accumulation of residual urine in the bladder and causes vesicoureteral reflux; these conditions are important variables that can predispose a host to UTIs.29
UTIs are treated repeatedly by using broad-spectrum antimicrobial drugs; however, such treatments are administered empirically without prior culture sensitivity tests. This improper and nonjudicious use/practice of antimicrobial drugs significantly increases the global antimicrobial resistance of microorganisms, ultimately favoring the reproduction of multidrug-resistant bacterial strains.30 According to a review by the European Survey of Antibiotic Consumption, multidrug-resistant bacteria are responsible for the death rate of about 25,000 Europeans every year due to complications in UTIs.31 As a result, antibiotic overuse must be avoided to prevent resistance development, and the most appropriate antibiotics should be recommended as the first-choice empiric therapy for UTI. Antimicrobial susceptibility patterns among microorganisms differ across countries.32 According to the Infectious Diseases Society of America, provincial observation should be conducted to track changes in uropathogen vulnerability in certain regions.33
Etiology of UTI in pregnant women
Because pregnant women have a narrow urethra and their urinary tract can be easily contaminated with fecal microbes, they are more vulnerable to UTIs than men. Pregnancy and sexual activity are two other major variables that increase the risk of UTI in women. Women develop glycosuria during pregnancy as a result of the normal increase in plasma volume and decrease in urine concentration, eventually leading to bacterial growth in the urine. The three most common clinical manifestations of UTI in pregnancy are asymptomatic bacteriuria, acute cystitis, and acute pyelonephritis. Other symptoms include nausea, vomiting, frequent urination, dysuria, early birth, and low birth weight. Table 2 summarizes studies on the occurrence of UTIs in pregnant women and reveals that UTI is a common disease among pregnant women if no proper measurements are taken during pregnancy.
Table (2):
Pervasiveness of UTI in pregnant women of various studies.
Reference |
% of UTI causing Uropathogens |
No. of Pregnant women tested |
Found UTI positive |
Percentage |
|---|---|---|---|---|
Nwachukwu E et.al (2018) 35 |
Escherichia coli-26%, S. aureus – 10%, Klebsiella aerogenes- 8%, Proteus mirabilis – 7%. Pseudomonas aerogenes- 5% |
200 |
112 |
56% |
Amiri M et.al. (2015)36 |
Escherichia coli – 57.25% Klebsiella species – 20.85% Coagulase-negative staphylococci– 8.39% Streptococus species– 6.63% Acinetobacter-2.47% Proteus Mirabilis – 2.38% Staphylococcus aureus – 1.68% Enterobacter aerogenes – 0.35% |
22,600 |
1,132 |
5% |
Lee AC et.al. (2020)37 |
Eschericia coli– 38% Staphylococcus species – 23% Klebsiella species – 12% Staphylococcus aureus – 12% Group B Streptococcus (GBS) – 5.3% |
4,034 |
360 |
8.9% |
Ranjan et.al. (2001)38 |
Eschericia coli – 43%, Enterococcus faecalis – 28% Klebsiella aerogenes – 8.5%, Pseudomonas aerogenes – 5.7% |
120 |
42 |
35% |
El-Kashif MM (2019)39 |
Eschericia coli– 37% Staphylococcus species – 3.7% Klebsiella species – 27% |
303 |
162 |
53.5% |
Turpin CA et.al (2007) 40 |
Eschericia coli– 37% Staphylococcus aureus – 31% |
220 |
16 |
7.3% |
Blas FH et.al (2007) 41 |
Escherichia coli – 76% Staphylococcus aureus – 5.5% Proteus sp – 2.7% Klebsiella sp – 4.1% |
874 |
73 |
8.4% |
Tadesse (2007)42 |
Escherichia coli – 47% Staphylococcus aureus – 18%) C. freundi– 12% |
173 |
17 |
9.8% |
UTIs in pregnancy are associated with early delivery, caesarean delivery, morphological abnormalities, low birth weight in newborns, and infant death. UTIs in pregnant women start during the sixth week of pregnancy and peak between weeks 22 and 24; about 90% of these women develop urethral dilatation. Because of an increase in urine volume and urethral dilation in pregnant women, this condition causes greater urine stasis in the bladder, urine reflux to the urethra, and a physiological increase in plasma volume, which reduces urinary concentration. Another prevalent explanation is glycosuria, which affects around 70% of pregnant women, causes an increase in progesterone and estrogen levels in the urine, and reduces a patient’s ability to fight invasive microorganisms. These factors may play a role in the development of UTIs during pregnancy.42,43 This condition in pregnancy has also been attributed to various factors. For example, resistant E. coli species have been the most common microbiological agent causing UTIs, and they require special attention.44,45
Control of hospital infections
Inadequate infection management consequently causes pathogen spread in healthcare settings, leading to healthcare-associated illnesses (HAIs).46 HAIs in turn result in increased antimicrobial treatments; consequently, bacterial antibiotic resistance occurs. The increasing number of germs and resistant infections in hospitals and other healthcare settings contributes significantly to the global burden of antibiotic resistance. The death toll from HAIs caused by multidrug-resistant microbes is predicted to exceed 25,000 per year in Europe, and this situation may worsen in other countries.47
In hospitals, the behavior of healthcare personnel plays a key role in executing infection control programs.48 “Compliance to infection management defense is globally suboptimal.”49 The primary problem is healthcare personnel’s insufficient use of infection control guidelines.49,50 Particularly, in middle- and low-income nations, the concern of inadequate healthcare staff needs important consideration.51 The lack of understanding and compliance with basic infection control procedures, such as hand hygiene, are major factors in the high prevalence of HAIs in these settings.46,52 As a result, evaluating the current information and behaviors among healthcare personnel is critical in designing an effective infection control strategy. Several studies have identified a know-do gap in infection control measures. For example, Paudyal et al.53 have found that even though all clinicians in two government and three private Nepalese hospitals understand the importance of handwashing, only around half of them follow the suggested practice. In China, a systemic study involving 1,444 nurses (2010) has indicated that their understanding of standard safety measures is average, but their acquiescence to standard safety measures is low.54 Tenna et al.55 have observed that healthcare staff from two higher education institute hospitals in Ethiopia have strongly comprehend the importance of hand cleanliness and tuberculosis infection control procedures. However, this awareness does not translate into efficient infection control procedures.55
Most studies have qualitatively evaluated healthcare workers’ understanding and behaviors of infection control. Qualitative advances are useful for characterizing background elements and hidden influences and gaining a better understanding of facilitators and barriers to healthcare person performance. Some qualitative studies have provided a comprehensive overview of hospital employees’ awareness of not only medical waste management or hand hygiene but also infection management issues.56,57 Woith et al.56 qualitatively investigated barriers and motivators affecting tuberculosis infection management practices of healthcare personnel in Russia and discovered that poor awareness and an unenthusiastic approach impede the implementation of good infection control practices. Ider et al.57 described the attitude of Mongolian healthcare professionals and their lack of understanding, along with other factors such as limited finance and management, as problems obstructing the efficient implementation of infection control programs.
Residues of antimicrobial substances in hospital wastewater
Antimicrobial drugs are critical medicines used to treat infectious disorders such as HAIs in healthcare settings. Because of the antibacterial residual excretion and the improper disposal of unneeded compounds, large volumes of antibiotics can be released into hospital wastewater, which can then be discharged into the environment.58 The presence of antibiotics in aquatic environments can cause the spread of antibiotic-resistant microorganisms. In general, antibiotic pollution of the environment, as well as aquatic habitats, is a growing concern worldwide.59,60 Hospital wastewater treatment plants and effluents represent a significant source of antibiotics and antibiotic-resistant bacteria being released into the environment.61,62 Diwan et al.62 reported that “antibiotics are discharged in hospital wastewater continually.” The concentration of antibiotics in hospital effluents ranges from 1.4 µg/L to 236.6 µg/L.63
Ciprofloxacin is a fluoroquinolone antibiotic excreted as a parent component in urine in over 70% of cases. Antibiotics are not found in incoming safe water and groundwater sources (bore well and hand pump) in and around a hospital compound. Sabir et al.64 emphasized that the high incidence of UTIs caused by E. coli, E. cloacae, or K. pneumoniae displays a high intensity of biofilm formation that can then be discharged into wastewater. They compared the prevalence of AMR-resistant coliform bacteria in healthcare facilities and hospital effluents.59,63-68 Rodriguez-Mozaz et al.68 have found ofloxacin and ciprofloxacin at six different concentrations ranging from 13.78 µg/L to 14.38 µg/L in the sewage of a Spanish hospital. These antibiotics have also been detected at high concentrations in other hospital effluents.69-71 In Hanoi, Vietnam, Duong et al.72 discovered that norfloxacin and ciprofloxacin concentrations in hospital wastewater from 1.1 µg/L to 44 µg/L and 0.9 µg/L to 17 µg/L, respectively.
Hospital wastewater can act as an ideal growth medium for many pathogenic microorganisms, including bacteria, fungi, viruses, and parasites. It also consists of several antibiotic residues and resistant bacteria, which can prevent the growth of susceptible bacteria, thereby favoring the growth of resistant bacteria in water. Resistant microbes discharged into wastewater act as either reservoirs harboring antibiotic-resistance genes (ARGs) or vectors carrying a transmissible gene that can pose risks to public health. Furthermore, fungi that can grow at a quick rate and can spread their spores to the exterior atmosphere threaten human health and the environment.
Antibiotics contaminate water supplies in India and other countries because of improper wastewater facility maintenance, which is a cause for concern in terms of broad environmental and public health implications. Hospitals serve as biological habitats for AMR bacteria and play an important role in their emergence and spread. AMR bacteria can be found in infected patients in hospitals, but they can also be found in wastewater systems.61 This problem is exacerbated when hospital and healthcare facility effluents are dumped directly into wastewater systems without being treated first.73 As a result, high concentrations of antimicrobials are discharged in wastewater, thereby causing constant selective pressure to ARB. Heavy metals and disinfectants with antibacterial properties help ARB survive in the wastewater microbiota.74 The horizontal transfer of resistance genes within and between species is favored by antimicrobial selective pressure.75 Therefore, a precise and progressive machinery for hospital wastewater treatment must be developed to prevent the outspread of harmful microorganisms. Consequently, microbial presence in hospital wastewater must be detected to quantify contamination and establish specific treatment methods for environmental health protection.
Table 3 summarizes the concentrations of antibiotics accounted in various literature in hospital outlet wastewaters and effluents in respective hospitals. Many of these studies have found Ciprofloxacin to be amongst the most perceived in hospital effluents.
Table (3):
Concentrations of Antibiotics (ng/L) noticed in hospital wastewater/effluents from literature.
| Name of the antibiotic | Detected concentration in respective hospital wastewater bodies in (ng/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ujjain, India63 | Kalmar, Sweden76 | Hanoi, Vietnam77 | Spain78 | Italy70 | Norway69 | Portugal79 | France80 | China81 | |
| Ciprofloxacin | 31000-236600 | 3600-10100 | 42800 | 5329-7494 | 1400-26000 | 19235-41752 | 101-38689 | 590-5800 | 217 |
| Norfloxacin | 5700-22800 | — | — | — | — | — | — | — | — |
| Ofloxacin | 7500-66000 | 200-7600 | 4600 | — | 1300 | 12-2053 | — | — | — |
| Levofloxacin | 6800-70700 | — | — | — | — | — | — | — | — |
| Metronidazole | 2500-3800 | 100-90200 | 2600 | — | — | — | — | — | — |
| Tinidazole | 13600-88400 | — | — | — | — | — | — | — | — |
| Sulphamethoxazole | 5700-81100 | 400-12800 | 9800 | 65-200 | 940-3400 | 391-552 | 41-8714 | 330-550 | 1060 |
| Trimethoprim | — | 600-7600 | 7700 | 50-260 | 68-6800 | 3767-17993 | 12.5-3963 | 30-2500 | 174 |
| Erythromycin | 0 | — | — | — | 60-320 | — | 7545 | — | 13 |
| Azythromycin | — | — | — | 85-119 | 1040 | — | 7351 | — | — |
Antimicrobial-resistant strains in hospital wastewater
Antibiotic residues in the environment can have an indiscriminate effect on bacteria, contributing to the increase in antimicrobial resistance. In hospital wastewater, significant connections have been discovered between the concentration of drug residues and the incidence of antimicrobial-resistant microorganisms.82 Akter et al.83 observed that E. coli isolates in Bangladeshi hospital wastewater develop multidrug resistance to antibiotics used by practitioners to treat patients. In a similar investigation, Reinthaler et al.84 discovered a multidrug-resistant E. coli in hospital sewage in Southern Austria. Antimicrobial-resistant bacteria (S. aureus, E. coli, Shigella, and Salmonella) have also been detected in hospital effluents in South Ethiopia, and they are released into receiving water bodies.84
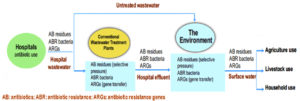
Fig. 2 depicts how antibiotics and their residues enter hospital water and are released into the environment due to a lack of effective hospital wastewater treatments, resulting in antimicrobial resistance from diverse pollution sources. National (and/or regional) policies have rarely outlined measures on how to treat hospital effluent for drug residues and antibiotic-resistant microbes before they are discharged into either community sewage for treatment at a municipal wastewater treatment facility or into a surface water body. In low- and middle-income nations, where wastewater treatment is less prevalent and direct discharge of raw hospital wastewater into surface rivers is common, the situation is likely worse. Such water can be dumped into water bodies, where it can be utilized for irrigation or eventually wind up in drinking water, resulting in resistant illnesses in humans and animals. Antibiotic residues, antibiotic-resistant bacteria, and ARGs that spread through hospitals can aggravate antibiotic resistance, thereby posing a public health risk.
How to overcome challenges in infection control
Healthcare personnel have a good understanding of hospital infection control. Although their self-reported practices are acceptable or competent, infection control measures in hospitals appear poor. The reported infection control issues are insufficient resources, lack of awareness, and patient overload. Nevertheless, they can be overcome by providing sufficient data on HAIs to healthcare personnel to raise their awareness, motivate them to apply their existing knowledge into practice, and urge them to use infection-control facilities.
To overcome AMR-related challenges, healthcare staff and cleaning workers must undergo constant tailor-made education and training. A standard monitoring system for hospital infection control should be established; every hospital should incorporate tools and monitoring software. Various measures, such as training, examination, access to equipment and facilities, and recurrent control and audit, should also be implemented to improve microbial infection management. An all-encompassing strategy involving financial and human resources is also required to optimize such practices. After wastewater treatment, several crucial tests should be conducted to reduce antibiotic concentrations in hospital wastewater. Other suggestions are presented as follows:
- A multidimensional approach is required to optimize hospital infection management practices. It should involve sequencing infection control in hospital organization, implement infection control in medical curricula, provide adequate infrastructure, and continue patient and health professional education.
- Training healthcare staff to increase their knowledge of infection control, particularly HAIs in hospitals can be a viable intervention to improve their practices in current hospital conditions.
- Cleaning workers should undergo individualized training.
- Hospital wastewater treatment systems that are effective in removing antibiotic residues, antibiotic-resistant microorganisms, and antibiotic resistance genes must be developed.
- Further research should focus on the relationship between antibiotic residues discharged from hospitals and antibiotic-resistant bacteria, their occurrence in the surrounding environment, human acquisition of antibiotic-resistant bacteria upon exposure to this environment, and public health implications.
UTIs are caused by a wide range of gram- and gram-negative bacteria. To treat patients with UTI appropriately, clinicians must accurately identify the causal organism. Failure to do so not only prolongs sickness and exposes patients to complications, but also contributes to the development of bacterial resistance as a result of injudicious antibiotic use. The presence of clinical signs and symptoms, as well as a positive urine culture, are required for the diagnosis of UTI; nonetheless, in many healthcare settings, diagnosis and treatment are given without prior culture and antimicrobial sensitivity tests.
Further studies should be performed on a priority basis to advance rapid diagnostic tests (point of care testing) for timely targeted therapy amid the increasing antibiotic resistance. A drug monitoring system must also be established to supplement drug administration and aid in the development of a more tailored methodology for prescribing treatments. Furthermore, community-wide education initiatives should be implemented to reduce disease prevalence and improve the quality of life of patients in low- and middle-income areas.
Effluent water from healthcare settings is a major source of antibiotic-resistant bacteria, particularly multidrug-resistant microbes, such as gram-negative bacteria. However, further research is needed to assess the impact of wastewater treatment techniques on the total antibiotic resistance in aquatic environments.
Numerous measures such as training, surveillance, access to equipment and facilities, and periodic control and audit should be implemented to strengthen infection control methods. A holistic approach involving financial and human resources is also required to optimize such practices. After wastewater treatment, several crucial tests should be performed to reduce antibiotic concentrations in hospital wastewater. Further research should focus on the association of antibiotic residues discharged from hospitals, antibiotic-resistant bacteria, their presence in the surrounding environment, human acquisition of these bacteria upon exposure to this environment, and their impact on public health.
ACKNOWLEDGMENTS
The authors would like to express their gratitude to JSS College of Pharmacy, Ooty and JSS Academy of Higher Education and Research, Mysuru, for providing all the essential facilities and support to complete this article. We would also like to thank the physicians and consultants from the Government Medical College and Hospital, Ooty for their contributions and modifications to this manuscript.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
FUNDING
This work was supported by the Department of Science and Technology (DST), New Delhi, with the WOS-B fellowship (DST/WOS-B/HN-5/2021)
ETHICS STATEMENT
This is a review article that does not contain any studies with human participants or animals performed by any of the authors.
AVAILABILITY OF DATA
Not applicable.
- Dougherty TJ, Pucci MJ. Antibiotic Discovery and Development. Springer US. 2014.
Crossref - Wainwright M. An alternative view of the early history of microbiology. Adv Appl Microbiol. 2003 52:333-355.
Crossref - Livermore DM, Warner M, Mushtaq S, Doumith M, Zhang J, Woodford N. What remains against carbapenem-resistant Enterobacteriaceae? Evaluation of chloramphenicol, ciprofloxacin, colistin, fosfomycin, minocycline, nitrofurantoin, temocillin and tigecycline. Int J Antimicrob Agents. 2011;37(5):415-419.
Crossref - Hare R. New light on the history of penicillin. Med Hist. 1982;26(1):1-24.
Crossref - Worthen DB. Streptomyces in nature and medicine: The antibiotic makers. J Hist Med Allied Sci. 2007;63(2):273-274.
Crossref - Marshall G, Blacklock JWS, Cameron C, et al. Streptomycin treatment of pulmonary tuberculosis: a medical research council investigation. Br Med J. 1948;2:769-82.
Crossref - Patnool RB, Wadhwani A, Balasubramaniam, Ponnusankar S. Need for the implementation of antibiotic policy in India: An overview. Int J Curr Res Rev. 2021;13(05):168-178.
Crossref - Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stays, and health care costs. Clin Infect Dis. 2006;42(Suppl 2):S82-S89.
Crossref - Maragakis LL, Perencevich EN, Cosgrove SE. Clinical and economic burden of antimicrobial resistance. Expert Rev Anti Infect Ther. 2008;6(5):751-763.
Crossref - DANMAP. DANMAP 2012 – Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Food Animals, Food and Humans in Denmark. (2013). Available from: https://www.danmap.org/-/media/sites/danmap/downloads/reports/2012/
danmap_2012.pdf?la=da&hash=C51FB755BE9AD990 B646729EE953CD842C7FA675 - Chambers HF. The changing epidemiology of Staphylococcus aureus? Emerg Infect Dis. 2001;7(2):178-182.
Crossref - Gupta N, Limbago BM, Patel JB, Kallen AJ. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis. 2011;53(1):60-67.
Crossref - Heuer O, Magiorakos AP, Gunell M, Economopoulou A, Blomquist PB, Brown D. Antimicrobial resistance surveillance in Europe: annual report of the European Antimicrobial Resistance Surveillance Network (EARS-Net.). 2010.
Crossref - Bush K, Courvalin P, Dantas G, et al. Tackling antibiotic resistance. Nat Rev Microbiol. 2011;9(12):894-896.
Crossref - Cooper MA, Shlaes D. Fix the antibiotics pipeline. Nature. 2011;472(7341):32.
Crossref - Organization WH. Multidrug and Extensively Drug-Resistant TB (M. World Health Organization). 2010.
- Kumarasamy KK, Toleman MA, Walsh TR, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10(9):597-602.
Crossref - Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002;113(Suppl 1):5-13.
Crossref - Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med. 2012;366(11):1028-1037.
Crossref - Mazzulli T. Diagnosis and management of simple and complicated urinary tract infections (UTIs). Can J Urol. 2012;19(Suppl 1):42-48. PMID: 23089347
- Stamm WE, Norrby SR. Urinary tract infections: disease panorama and challenges. J Infect Dis. 2001;183:S1-S4.
Crossref - Nicolle LE. Antimicrobial resistance in community-Acquired Escherichia coliIsolated from urinary infection: Good news or bad? Can J Infect Dis Med Microbiol. 2013;24:182615.
Crossref - Ronald A. The etiology of urinary tract infection: traditional and emerging pathogens. Am J Med. 2002;113(1A):14S-19S.
Crossref - American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 91: Treatment of urinary tract infections in nonpregnant women. Obstet Gynecol. 2008;111(3):785-794.
Crossref - Foxman B, Gillespie B, Koopman J, et al. Risk factors for second urinary tract infection among college women. Am J Epidemiol. 2000;151(12):1194-1205.
Crossref - Anturlikar SS, Trivedi NA. Antimicrobial Resistance Demonstrated by Uropathogenic Escherichia Coli at a Tertiary Care Hospital. Int J Cur Res Rev. 2016;8(15):33.
- Nguyen HT. Chapter 14. Bacterial Infections of the Genitourinary Tract. In: McAninch JW, Lue TF. eds. Smith and Tanagho’s General Urology, 18e. McGraw Hill; 2013. Accessed November 22, 2021.https://accessmedicine.mhmedical.com/content.aspx?bookid=508§ionid=4108809
- Momoh AR, Orhue PO, Idonije OB, Oaikhena AG, Nwoke EO, Momoh AA. The antibiogram types of Escherichia coli isolated from suspected urinary tract infection samples. J Microbiol Biotech Res. 2011;1(3).
- Schnarr J, Smaill F. Asymptomatic bacteriuria and symptomatic urinary tract infections in pregnancy. Eur J Clin Invest. 2008;38(Suppl 2):50-57.
Crossref - Spellberg B, Bartlett JG, Gilbert DN. The future of antibiotics and resistance. N Engl J Med. 2013;368(4):299-302.
Crossref - Haslund JM, Dinesen MR, Nielsen ABS, Llor C, Bjerrum L. Different recommendations for empiric first-choice antibiotic treatment of uncomplicated urinary tract infections in Europe. Scand J Prim Health Care. 2013;31(4):235-240.
Crossref - Goossens H, Ferech M, Vander Stichele R, Elseviers M; ESAC Project Group. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365(9459):579-587.
Crossref - Warren JW, Abrutyn E, Hebel JR, Johnson JR, Schaeffer AJ, Stamm WE. Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Infectious Diseases Society of America (IDSA). Clin Infect Dis. 1999;29(4):745-759.
Crossref - Nwachukwu E, Onyebuchi O, Michael O. Prevalence of urinary tract infections in pregnant women in Onitsha, Nigeria. J Bacteriol Mycol Open Access. 2018;6(5):284-285.
Crossref - Amiri M, Lavasani Z, Norouzirad R, et al. Prevalence of Urinary Tract Infection Among Pregnant Women and its Complications in Their Newborns During the Birth in the Hospitals of Dezful City, Iran, 2012 – 2013. Iran Red Crescent Med J. 2015;17(8):e26946.
Crossref - Lee AC, Mullany LC, Koffi AK, et al. Urinary tract infections in pregnancy in a rural population of Bangladesh: population-based prevalence, risk factors, etiology, and antibiotic resistance. BMC Pregnancy Childbirth. 2020;20(1):1.
Crossref - Ranjan A, Sridhar STK, Matta N, Chokkakula S, Ansari RK. Prevalence of UTI among pregnant women and its complications in newborns. Ind J Pharm Pr. 2017;10(1):45-49.
Crossref - Mohamed Labib El-Kashif M, Eaid Elgazzar S. Maternal markers for detecting urinary tract infection among pregnant women in port said city, Egypt. Am J Nurs Res. 2018;6(5):317-326.
Crossref - Turpin C, Minkah B, Danso K, Frimpong E. Asymptomatic bacteriuria in pregnant women attending antenatal clinic at komfo anokye teaching hospital, kumasi, ghana. Ghana Med J. 2007;41(1):26-29. PMID: 17622336
- Blas FH, Carmona JML, Moctezuma JRR, Pedrero MLP, Gutierrez RSR, Aguirre ARO. Frecuencia de bacteriuria asintomatica en embarazadas y sensibilidad antimicrobiana in vitro de los uropatogenos. Ginecologia y Obstetricia de Mexico. 2007;75(6).
- Tadesse A, Negash M, Ketema LS. Asymptomatic bacteriuria in pregnancy: assessment of prevalence, microbial agents and their antimicrobial sensitivity pattern in Gondar Teaching Hospital, northwest Ethiopia. Ethiop Med J. 2007;45(2):143-149. PMID: 17642170
- Emamghorashi F, Mahmoodi N, Tagarod Z, Heydari ST. Maternal urinary tract infection as a risk factor for neonatal urinary tract infection. Iran J Kidney Dis. 2012;6(3):178-180. PMID: 22555480
- Rizvi M, Khan F, Shukla I, Malik A, Shaheen. Rising prevalence of antimicrobial resistance in urinary tract infections during pregnancy: necessity for exploring newer treatment options. J Lab Physicians. 2011;3(2):98-103.
Crossref - Haider G, Zehra N, Munir AA, Haider A. Risk factors of urinary tract infection in pregnancy. J Pak Med Assoc. 2010;60(3):213-216. PMID: 20225781
- Rahman SR, Ahmed MF, Begum A. Occurrence of urinary tract infection in adolescent and adult women of shanty town in Dhaka City, Bangladesh. Ethiop J Health Sci. 2014;24(2):145-152.
Crossref - Allegranzi B, Bagheri Nejad S, Combescure C, et al. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet. 2011;377(9761):228-241.
Crossref - ECDC, EMEA. The bacterial challenge: time to react European Centre for Disease Prevention and Control European Medicines Agency. 2009.
- Duerink DO, Hadi U, Lestari ES, et al. A tool to assess knowledge, attitude and behavior of Indonesian health care workers regarding infection control. Acta Med Indones. 2013;45(3):206-215. PMID: 24045391
- Jenner EA, Fletcher BC, Watson P, Jones FA, Miller L, Scott GM. Discrepancy between self-reported and observed hand hygiene behaviour in healthcare professionals. J Hosp Infect. 2006;63(4):418-422.
Crossref - Gammon J, Morgan-Samuel H, Gould D. A review of the evidence for suboptimal compliance of healthcare practitioners to standard/universal infection control precautions. J Clin Nurs. 2008;17(2):157-167.
Crossref - Rowe AK, de Savigny D, Lanata CF, Victora CG. How can we achieve and maintain high-quality performance of health workers in low-resource settings? Lancet. 2005;366(9490):1026-1035.
Crossref - Borg MA. Prevention and control of healthcare associated Infections within developing countries. Int J Infect Control. 2010;6(1).
Crossref - Paudyal P, Simkhada P, Bruce J. Infection control knowledge, attitude, and practice among Nepalese health care workers. Am J Infect Control. 2008;36(8):595-597.
Crossref - Luo Y, He GP, Zhou JW, Luo Y. Factors impacting compliance with standard precautions in nursing, China. Int J Infect Dis. 2010;14(12):e1106-e1114.
Crossref - Tenna A, Stenehjem EA, Margoles L, Kacha E, Blumberg HM, Kempker RR. Infection control knowledge, attitudes, and practices among healthcare workers in Addis Ababa, Ethiopia. Infect Control Hosp Epidemiol. 2013;34(12):1289-1296.
Crossref - Woith W, Volchenkov G, Larson J. Barriers and motivators affecting tuberculosis infection control practices of Russian health care workers. Int J Tuberc Lung Dis. 2012;16(8):1092-1096.
Crossref - Ider BE, Adams J, Morton A, Whitby M, Clements A. Perceptions of healthcare professionals regarding the main challenges and barriers to effective hospital infection control in Mongolia: a qualitative study. BMC Infect Dis. 2012;12-170.
Crossref - Kummerer K. Antibiotics in the aquatic environment–a review–part II. Chemosphere. 2009;75(4):435-441.
Crossref - Kummerer K. Antibiotics in the aquatic environment–a review-part I. Chemosphere. 2009;75(4):417-434.
Crossref - Manzetti S, Ghisi R. The environmental release and fate of antibiotics. Mar Pollut Bull. 2014;79(1-2):7-15.
Crossref - Rizzo L, Manaia C, Merlin C, et al. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: a review. Sci Total Environ. 2013;447:345-360.
Crossref - Diwan V, Lundborg CS, Tamhankar AJ. Seasonal and temporal variation in release of antibiotics in hospital wastewater: estimation using continuous and grab sampling. PLoS One. 2013;8(7):e68715.
Crossref - Diwan V, Tamhankar AJ, Aggarwal M, Sen S, Khandal RK, Lundborg CS. Detection of antibiotics in hospital effluents in India. Current Science. 2009;97(12):1752-1755.
- Sabir N, Ikram A, Zaman G, et al. Bacterial biofilm-based catheter-associated urinary tract infections: Causative pathogens and antibiotic resistance. Am J Infect Control. 2017;45(10):1101-1105.
Crossref - Martins AF, Vasconcelos TG, Henriques DM, Frank C da S, Konig A, Kummerer K. Concentration of ciprofloxacin in Brazilian hospital effluent and preliminary risk assessment: A case study. Clean (Weinh). 2008;36(3):264-269.
Crossref - Jarnheimer PA, Ottoson J, Lindberg R, et al. Fluoroquinolone antibiotics in a hospital sewage line; occurrence, distribution and impact on bacterial resistance. Scand J Infect Dis. 2004;36(10):752-755.
- Seifrtova M, Pena A, Lino CM, Solich P. Determination of fluoroquinolone antibiotics in hospital and municipal wastewaters in Coimbra by liquid chromatography with a monolithic column and fluorescence detection. Anal Bioanal Chem. 2008;391(3):799-805.
Crossref - Rodriguez-Mozaz S, Chamorro S, Marti E, et al. Occurrence of antibiotics and antibiotic resistance genes in hospital and urban wastewaters and their impact on the receiving river. Water Res. 2015; 69:234-242.
Crossref - Santos LHMLM, Gros M, Rodriguez-Mozaz S, et al. Contribution of hospital effluents to the load of pharmaceuticals in urban wastewaters: identification of ecologically relevant pharmaceuticals. Sci Total Environ. 2013;461-462:302-316.
Crossref - Thomas KV, Dye C, Schlabach M, Langford KH. Source to sink tracking of selected human pharmaceuticals from two Oslo city hospitals and a wastewater treatment works. J Environ Monit. 2007;9(12):1410-1418.
Crossref - Kovalyov L, Siegrist H, Singer H, Wittmer A, McArdell CS. Hospital wastewater treatment by membrane bioreactor: performance and efficiency for organic micropollutant elimination. Environ Sci Technol. 2012;46(3):1536-1545.
Crossref - Duong HA, Pham NH, Nguyen HT, et al. Occurrence, fate and antibiotic resistance of fluoroquinolone antibacterials in hospital wastewaters in Hanoi, Vietnam. Chemosphere. 2008;72(6):968-973.
Crossref - Berendonk TU, Manaia CM, Merlin C, et al. Tackling antibiotic resistance: the environmental framework. Nat Rev Microbiol. 2015;13(5):310-317.
Crossref - Oberle K, Capdeville MJ, Berthe T, Budzinski H, Petit F. Evidence for a complex relationship between antibiotics and antibiotic-resistant Escherichia coli: from medical center patients to a receiving environment. Environ Sci Technol. 2012;46(3):1859-1868.
Crossref - Cantas L, Shah SQa, Cavaco LM, et al. A brief multi-disciplinary review on antimicrobial resistance in medicine and its linkage to the global environmental microbiota. Front Microbiol. 2013; 4:96.
Crossref - Lindberg R, Jarnheimer PA, Olsen B, Johansson M, Tysklind M. Determination of antibiotic substances in hospital sewage water using solid phase extraction and liquid chromatography/mass spectrometry and group analogue internal standards. Chemosphere. 2004;57(10):1479-1488.
Crossref - Lien LT. Antibiotic resistance: Implications of hospital practices for public health: A study from Hanoi, Vietnam. Instforfolkhalsovetenskap/Dept of Public Health Sciences; 2018.
Crossref - Verlicchi P, Al Aukidy M, Galletti A, Petrovic M, Barcelo D. Hospital effluent: investigation of the concentrations and distribution of pharmaceuticals and environmental risk assessment. Sci Total Environ. 2012; 430:109-118.
Crossref - Pena A, Paulo M, Silva LJ, Seifrtova M, Lino CM, Solich P. Tetracycline antibiotics in hospital and municipal wastewaters: a pilot study in Portugal. Anal Bioanal Chem. 2010;396(8):2929-2936.
Crossref - Dinh Q, Moreau-Guigon E, Labadie P, et al. Fate of antibiotics from hospital and domestic sources in a sewage network. Sci Total Environ. 2017; 575:758-766.
Crossref - Chang X, Meyer MT, Liu X, et al. Determination of antibiotics in sewage from hospitals, nursery and slaughter house, wastewater treatment plant and source water in Chongqing region of Three Gorge Reservoir in China. Environ Pollut. 2010;158(5):1444-1450.
Crossref - Varela AR, Andre S, Nunes OC, Manaia CM. Insights into the relationship between antimicrobial residues and bacterial populations in a hospital-urban wastewater treatment plant system. Water Res. 2014; 54:327-336.
Crossref - Akter F, Amin MR, Osman KT, Anwar MN, Karim MM, Hossain MA. Ciprofloxacin-resistant Escherichia coli in hospital wastewater of Bangladesh and prediction of its mechanism of resistance. World J Microbiol Biotechnol. 2012;28(3):827-834.
Crossref - Reinthaler FF, Posch J, Feierl G, et al. Antibiotic resistance of E. coli in sewage and sludge. Water Res. 2003;37(8):1685-1690.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.