ISSN: 0973-7510
E-ISSN: 2581-690X
A total of 79 endophytic fungal isolates were obtained from four wild medicinal plants – Tinospora cordifolia (Willd.) Hook.f and Thomson (Menispermaceae), Piper nigrum L., Piper longum L. (Piperaceae) and Zingiber officinale Roscoe (Zingiberaceae) from Western Ghats of Karnataka and screen them for antimicrobial and antioxidant potential. Among them, 16 isolates depicting good antimicrobial activity by agar plug method (2.33mm-20.66mm) and agar well diffusion method (2.66mm-21mm) against human pathogens were identified by molecular techniques and subjected to secondary metabolite extraction. The extracts were tested for their antioxidant potential by DPPH assay, ABTS assay, reducing power assay and total phenolic content. The isolate ABR4 (Fusarium solani) of Tinospora cordifolia showed remarkable antimicrobial activity against the human pathogens at tested concentrations of 20-100µg/ml. The isolate GKS (Aspergillus terreus) of Zingiber officinale demonstrated excellent antioxidant activity (IC50 – 3.34) as compared to standard Gallic acid (IC50 – 5.54) which has not been reported previously. The findings of the study indicate that endophytic isolates serve as a potential source of novel bioactive products.
Endophytic fungi, medicinal plants, antimicrobial, antioxidant, phenolic content.
Endophytic fungi reside within the host plants without causing any apparent symptoms of disease and are considered to be a rich source of bioactive metabolites.1 They produce a broad array of secondary metabolites possessing antioxidant, antimicrobial, antitumor activities that have applications in medicine, agriculture and industry.2 The bioprospecting of these endophytic fungi offers tremendous potential to discover natural products with therapeutic value.3 Approximately one million endophytic species have been described.4 Metabolites produced by endophytes could be influenced by the chemistry of their host plants.5 During the long period of coevolution, some endophytes have the ability to produce similar or identical bioactive compounds as their host plants.6 Endophytes help the host plant to tolerate biotic and abiotic stress, increase growth rate and extent of reproduction and hence improve the resistance of host medicinal plants by secretion of bioactive metabolites.7 Of late, medicinal plants have been exploited for their bioactive compounds having important biological activities.8 There has been an upsurge of interest among the research groups to obtain endophytic species from the Western Ghats, which is well known for its rich and unique assemblage of flora and harbors several ethno medicinal plants widely used in traditional practices. It is therefore recognized as one of the 34 hotspots of biodiversity.9 The global emergence of infectious diseases due to antibiotic resistant microorganisms results in mortality and morbidity in human health which necessitates for inventive measures to tackle the problem of drug resistance. The screening of antimicrobial compounds from endophytic fungi is a promising way to deal with drug resistant human and plant pathogens and results in the discovery and development of new drugs.10 The byproducts of biological reactions result in the generation of free radicals. These free radicals are highly unstable and the reactive nature of these free radicals can damage cellular macromolecules. The involvement of free radicals in the pathogenesis of a large number of diseases is well documented.11 An antioxidant is capable of inhibiting the oxidation of other molecules by the initiation of oxidizing chain reactions. During the normal cellular metabolism, reactive oxygen/nitrogen species (ROS/RNS) are produced which are essential for apoptosis, cell signaling, gene expression and ion transportation. However, ROS/RNS can result in oxidative stress if accumulated in excess amounts in the body resulting in a myriad of diseases in living systems.12 Recently, research is focused on endophytic fungi associated with medicinal plants as they are promising source of antioxidants and other bioactive metabolites.13 Antioxidants act as scavengers and prevent cell and tissue damage. Vitamin C, Vitamin E and flavonoids are some of the naturally occurring antioxidants used for the treatment and prevention of human diseases.14 In the present study, mycoendophytic isolates from four medicinal plants collected from the Bisle region, Western Ghats of Karnataka were evaluated for their antimicrobial and antioxidant potential.
Isolation and identification of endophytic fungi for antimicrobial activity
Endophytic fungi were isolated from fresh and healthy tissues of four wild medicinal plants collected from Bisle region, Western Ghats of Karnataka and identified as T. cordifolia (Willd.) Hook. f and Thomson, P. nigrum L., P. longum L. and Z. officinale Roscoe. Herbarium of plant samples were prepared and deposited to National Ayurveda Dietetics Research Institute (Central Council for Research in Ayurveda and Siddha), Department of AYUSH, Ministry of Health and Family Welfare, Govt. of India, (New Delhi) Jayanagar, Bangalore, India. Standard protocols have been followed for the isolation of endophytic fungi as reported in our previous work.15 The endophytic fungi were identified based on the cultural characteristics, morphology of the fruiting bodies and spores, using standard manuals.16
Preliminary screening for antimicrobial activity by agar plug method
The fungal isolates were screened for antimicrobial activity against the test human pathogenic bacteria- Staphylococcus aureus (NCIM No. 2079), Bacillus cereus (NCIM No. 2106), Escherichia coli (NCIM No. 2256), Salmonella typhimurium (NCIM No. 2501) and Pseudomonas aeruginosa (NCIM No. 2200) and test human pathogenic fungi Candida albicans (NCIM No. 3471). Cylindrical pieces were cut out from well grown culture of the endophytic fungi strain on potato dextrose agar medium (PDA). The blocks were placed on the Petri dishes deep inoculated with a fixed amount of test-microorganisms grown in nutrient broth medium for bacteria and Sabouraud Dextrose Agar medium (SDA) for yeast (106 cells/ml). The cultures were kept for 12 hours at 2-8ºC for the antibacterial substance diffusion and thereafter they were incubated for the growth of bacterial test-microorganisms at 37ºC for 24 hours and incubated for 48 hours at 28ºC for fungi. The antimicrobial activity was measured in mm.17
Production and extraction of secondary metabolites
The endophytic fungal isolates depicting good antimicrobial activity were subjected for the production of secondary metabolites. The fresh mycelia of endophytic fungi were grown on PDA plates at 28±2ºC for 3-6 days and were inoculated into 1000 ml flasks containing 200gms of unpolished rice, soaked with 200 ml distilled water (autoclaved twice at 121ºC for 20 min), followed by incubation for 30 days at 28±2ºC 18. The incubated flasks were filled with 300ml of ethyl acetate and allowed to stand for one day, shaken thoroughly and filtered. The above procedure was repeated until most of the metabolites were extracted. Finally ethyl acetate extract was treated with anhydrous Sodium sulphate to remove the moisture content and dried under rotary evaporator.19
Molecular identification of the potential endophytic fungi
The potential endophytic fungi were identified based on their ribosomal DNA (18srRNA gene) sequences. Total genomic DNA was extracted from fungal mycelia grown on PDA using the Cetyl trimethyl ammonium bromide (CTAB) method.20 Primers ITS5 (5’-TCCTCCGCTTATTGATATGC-3’) and ITS4 (5’-GAAGTAAAAGTCGTAAGG-3’) were used to amplify the 5.8S and ITS regions. The DNA fragment was amplified and sequenced.21
Antimicrobial activity of crude extract of the endophytic fungi by agar well diffusion method
The extracted secondary metabolites from the potential endophytic fungi were dissolved in DMSO and poured into the 5mm diameter well bored into petridishes containing Nutrient Agar (NA) for test bacteria and SDA for test fungi, inoculated with a fixed amount of test-microorganisms (106 cells/ml). The cultures were kept at 2- 8°C for 24 hours for the antimicrobial metabolite diffusion and thereafter they were incubated at an appropriate temperature for the growth of test-microorganisms. The zone of inhibition was measured in mm.22
Evaluation of the antioxidant potential of endophytic fungi
The endophytic fungal isolates were subjected to different antioxidant assays to determine their antioxidant potential.
DPPH assay
The free radical scavenging activity of the fungal extract was measured in terms of its hydrogen donating or radical scavenging ability using the stable DPPH (2,2-diphenyl-1-picrylhydrazyl) radical method.23 The DPPH solution (0.1mM) in methanol was prepared and 1.0mg/ml of this solution was added to 3.0 ml of fungal extract and standard in solvent at different concentrations (10µM-100 µM). Thirty minutes later, the absorbance was measured at 517 nm. Lower absorbance of the reaction mixture showed higher free radical scavenging activity. The capability to scavenge the DPPH radical was calculated using the following equation:
% inhibition = control – test/control × 100
Where ‘control’ was the absorbance of the control reaction and ‘test’ was the absorbance in presence of fungal extract. The mean values were calculated from three experiments. Standard trolox solution was used as the positive control.
ABTS Radical Scavenging activity
The ABTS [2, 2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid)] radical scavenging activity of the fungal extract was measured.24 ABTS radical cation was produced by reacting ABTS solution (7 mM) with 2.45 mM ammonium per sulfate and the mixture was allowed to stand in dark at room temperature for 12-16hrs before use. Different concentrations (10-50µg/ml) of extract or standard Gallic acid (0.5ml) were added to 0.3 ml of ABTS solution and the final volume was made up with solvent to make up to 1ml. The absorbance was read at 745nm and the % inhibition of the ABTS radical was calculated using the following equation:
% inhibition = control – test/control × 100
Reducing power assay
The reductive potential of the fungal extracts were determined.25 Various concentrations of fungal methanolic extracts were mixed with 2.5mL of 0.2 M phosphate buffer (pH 6.6) and 2.5mL of 1% potassium ferricyanide. The mixture was incubated at 500C for 20 min. Post incubation, 2.5mL of 10% trichloroacetic acid (w/v) was added and the mixture was centrifuged at 1000 rpm for 10min. The supernatant (5mL) was mixed with 5 mL deionized water and 1mL of 0.1% of ferric chloride. The absorbance was measured at 700 nm using Gallic acid as a standard. Higher absorbance value of the reaction mixture indicates greater reductive potential. The assay was carried out in triplicate and the results are expressed as mean ± standard deviation.
Determination of total phenolic content
The amount of total phenolic was determined using the Folin–Ciocalteu reagent.26 One ml sample was dissolved in 1.5 ml distilled water and 0.5 ml Folin–Ciocalteu’s reagent. After 1 min, 1 ml of 20% sodium carbonate solution was added. The final mixture was shaken three times and incubated for 2 h in the dark at 250C. The absorbance of the mixture was measured at 760 nm. All tests were carried out in triplicate and the results were expressed as Gallic Acid Equivalents (mg GAE/mg of dry weight of the crude extract).
Isolation, identification and preliminary screening of endophytic fungi
A total of 79 endophytic fungal isolates were obtained from the four medicinal plants of Bisle region from the Western Ghats of Karnataka. The medicinal uses of the plants and their herbarium accession numbers are mentioned in (Table 1). The fungal isolates were identified by morphological characteristics. A majority of the fungal endophytes belong to the phylum Ascomycetes. Also, endophytic fungi belonging to the class Hyphomycetes, Zygomycetes and Coelomycetes were also obtained. The potential endophytic fungi were identified using molecular techniques by 18s rDNA analysis, submitted to Gen bank and their accession numbers were obtained (Table 2).
Table (1):
List of selected medicinal plants and their uses
Sl. No |
Medicinal Plant |
Family |
Herbarium Accession no. |
Medicinal uses of the plants |
References |
|---|---|---|---|---|---|
1 |
Tinospora cordifolia (Willd.) Hook.f & Thomson |
Menispermaceae |
RRCBI-8976 |
antipyretic, anti-asthmatic, hypoglycemic, hypotensive, analgesic, anti-inflammatory and anti-spasmodic properties |
8 |
2 |
Piper nigrum L. |
Piperaceae |
RRCBI-MUS 135 |
to treat vertigo, asthma, sinusitis, Fever, diarrhoea, arthritic disorders |
27 |
3 |
Piper longum L. |
Piperaceae |
RRCBI-AP-2591 |
Treatment of respiratory tract infections, chronic gut-related pain and arthritic condi-tions |
28 |
4 |
Zingiber officinale Roscoe |
Zingiberaceae |
RRCBI-AP.4046 |
Treatment of common colds, fever, to aid digestion, treat stomach upset, diarrhoea, nausea, rheumatic disorders and dizziness. |
29 |
Table (2):
List of endophytic fungi identified by molecular methods based on 18s rDNA and their accession numbers
Sl. no |
Endophytic culture |
Medicinal plant |
Identification of the fungal isolate |
Genbank accession number |
|---|---|---|---|---|
1 |
ABLS 2 |
T. cordifolia |
Aspergillus oryzae |
KR181978 |
2 |
ABLS3 |
T. cordifolia |
Penicillium rubens |
KR181979 |
3 |
PLLS 6 |
P. longum |
Aspergillus terreus |
KR181980 |
4 |
PLPS1 |
P. longum |
Aspergillus flavus |
KR181981 |
5 |
PKR1 |
P. nigrum |
Aspergillus flavus |
KR181982 |
6 |
PKR3 |
P. nigrum |
Sordaria tomento-alba |
KR181983 |
7 |
ABS1 |
T. cordifolia |
Aspergillus oryzae |
KJ729477 |
8 |
ABL1 |
T. cordifolia |
Fusarium solani |
KJ729475 |
9 |
ABS3 |
T. cordifolia |
Trichoderma asperellum |
KJ729478 |
10 |
ABR4 |
T. cordifolia |
Fusarium solani |
KJ729476 |
11 |
PKKR2 |
P. nigrum |
Aspergillus flavus |
KJ729481 |
12 |
PKKS1 |
P. nigrum |
Aspergillus terreus |
KJ729483 |
13 |
PKKS2 |
P. nigrum |
Lasiodiplodia theobromae |
KJ729484 |
14 |
PKKR3 |
P. nigrum |
Trichoderma virens |
KJ729482 |
15 |
PKS4 |
P. nigrum |
Aspergillus terreus |
KJ729485 |
16 |
GKS |
Z. officinale |
Aspergillus terreus |
KJ729479 |
The fungal endophytes were primarily screened for their antimicrobial activity against human pathogens by agar plug method wherein the zone of inhibition is detected around the endophytic fungal colony on test bacteria or test fungus swabbed plate. Agar plug diffusion method is often used to highlight the antagonism between microorganisms 30. Among the 79 endophytic fungal isolates, 41 isolates showed antimicrobial activity towards the tested microorganisms. The fungal isolates ABLS2, ABLS3 of T. cordifolia; PKR1, PKR3, PKKR2 of P. nigrum; PLLS6, PLPS1 of P. longum and GKS from Z. officinale exhibited good antimicrobial activity. None of the endophytic isolates of T. cordifolia depicted antibacterial activity against P. aeruginosa in the agar plug method. With regard to antifungal activity, only 2 isolates (ABL1 and ABR4) of T. cordifolia were found to be promising against C. albicans and one isolate each of P. longum (PLLS6), Z. officinale (GKS) and P. nigrum (PKR2) showed inhibitory activity against C. albicans (Table 3).
Table (3):
List of endophytic fungi screened for antimicrobial activity by agar plug method
Sl No |
Medicinal plant |
Culture code |
Identification of the endophytic isolate |
S. aureus |
B. cereus |
E. coli |
S. typhi |
P. aeruginosa |
C. albicans |
|---|---|---|---|---|---|---|---|---|---|
1 |
T. cordifolia |
ABL |
Aspergillus niger |
0a |
0a |
0a |
0a |
0a |
0a |
2 |
T. cordifolia |
ABL1 |
Fusarium solani |
8.00d±0.51 |
0a |
0a |
8.33d±0.59 |
0a |
12.33a±0.78 |
3 |
T. cordifolia |
ABL2 |
Rhizopus sp. |
0a |
0a |
0a |
0a |
0a |
0a |
4 |
T. cordifolia |
ABL3 |
Aspergillus flavus |
0a |
0a |
0a |
0a |
0a |
0a |
5 |
T. cordifolia |
ABLS1 |
Mucor sp. |
0a |
0a |
0a |
0a |
0a |
0a |
6 |
T. cordifolia |
ABLS2 |
A. oryzae |
7.33c±0.29 |
6.33c±0.29 |
11.00c±0.89 |
10.66e±0.29 |
0a |
0a |
7 |
T. cordifolia |
ABLS3 |
Penicillium rubens |
10.00e±0.51 |
5.66b±0.29 |
6.33b±0.29 |
5.33b±0.59 |
0a |
0a |
8 |
T. cordifolia |
ABLS4 |
Mycelia sterilia |
0a |
0a |
0a |
0a |
0a |
0a |
9 |
T. cordifolia |
ABPS1 |
Cladosporium sp. |
4.33b±0-29 |
0a |
0a |
0a |
0a |
0a |
10 |
T. cordifolia |
ABPS2 |
Aspergillus sp. |
0a |
0a |
0a |
0a |
0a |
0a |
11 |
T. cordifolia |
ABPS3 |
Mycelia sterilia |
0a |
0a |
0a |
0a |
0a |
0a |
12 |
T. cordifolia |
ABS |
Curvularia sp. |
0a |
0a |
0a |
0a |
0a |
0a |
13 |
T. cordifolia |
ABS1 |
A. oryzae |
17.00f±0.51 |
0a |
0a |
11.00e±0.51 |
0a |
0a |
14 |
T. cordifolia |
ABS2 |
Mycelia sterilia |
0a |
0a |
0a |
0a |
0a |
0a |
15 |
T. cordifolia |
ABS3 |
Trichoderma asperellum |
0a |
0a |
0a |
12.66f±0.29 |
0a |
0a |
16 |
T. cordifolia |
ABSS1 |
Cladosporium sp. |
0a |
0a |
0a |
0a |
0a |
0a |
17 |
T. cordifolia |
ABSS2 |
Fusarium sp. |
0a |
0a |
0a |
7.00c±0.51 |
0a |
0a |
18 |
T. cordifolia |
ABSS3 |
Cladosporium sp. |
0a |
0a |
0a |
0a |
0a |
0a |
19 |
T. cordifolia |
ABSS4 |
Aspergillus sp. |
0a |
0a |
0a |
0a |
0a |
0a |
20 |
T. cordifolia |
ABSS5 |
A. terreus |
0a |
0a |
0a |
0a |
0a |
0a |
21 |
T. cordifolia |
ABR |
Phomopsis sp. |
0a |
0a |
0a |
0a |
0a |
0a |
22 |
T. cordifolia |
ABR1 |
Mycelia sterilia |
0a |
0a |
0a |
0a |
0a |
0a |
23 |
T. cordifolia |
ABR2 |
Colletotrichum sp. |
0a |
0a |
0a |
0a |
0a |
0a |
24 |
T. cordifolia |
ABR3 |
Mucor sp. |
0a |
0a |
0a |
0a |
0a |
0a |
25 |
T. cordifolia |
ABR4 |
F. solani |
0a |
0a |
6.66b±0.29 |
7.00c±0.51 |
0a |
12.33a±0.78 |
26 |
P. nigrum |
PKL1 |
Phoma sp. |
0a |
0a |
0a |
0a |
0a |
0a |
27 |
P. nigrum |
PKL2 |
Paecilomyces sp. |
0a |
20.33e±0.29 |
0a |
3.33b±0.29 |
4.33b±0.59 |
0a |
28 |
P. nigrum |
PKS1 |
Phoma sp. |
0a |
0a |
0a |
0a |
0a |
0a |
29 |
P. nigrum |
PKS2 |
Mycelia sterilia |
0a |
0a |
0a |
5.33c±0.29 |
19.00f±0.51 |
0a |
30 |
P. nigrum |
PKS3 |
Colletotrichum sp. |
0a |
0a |
0a |
0a |
0a |
0a |
31 |
P. nigrum |
PKKS1 |
A. terreus |
11.00e±0.51 |
12.33d±0.78 |
0a |
0a |
0a |
0a |
32 |
P. nigrum |
PKKS2 |
Lasiodiplodia theobromae |
3.33b±0.59 |
0a |
0a |
8.00d±0.51 |
0a |
0a |
33 |
P. nigrum |
PKS4 |
A. terreus |
3.33b±0.78 |
9.33c±0.29 |
6.00c±0.51 |
0a |
0a |
0a |
34 |
P. nigrum |
PKR1 |
A. flavus |
5.66c±0.59 |
12.33d±0.78 |
2.33b±1.30 |
12.66f±0.59 |
14.00e±0.51 |
0a |
35 |
P. nigrum |
PKR2 |
Penicillium sp. |
0a |
0a |
0a |
0a |
9.33d±0.59 |
2.33a±0.29 |
36 |
P. nigrum |
PKR3 |
Sordaria tomento-alba |
14.00f±0.51 |
0a |
2.33b±0.29 |
7.33d±0.29 |
6.33c±0.29 |
0a |
37 |
P. nigrum |
PKKR2 |
A. flavus |
16.66g±0.29 |
8.00b±0.51 |
26.00d±0.51 |
9.33e±0.59 |
0a |
0a |
38 |
P. nigrum |
PKKR3 |
T. virens |
9.66d±0.29 |
0a |
0a |
0a |
0a |
0a |
39 |
P. longum |
PLLS1 |
Mucor sp. |
7.00bcd±0.51 |
3.00bc±0.51 |
0a |
0a |
0a |
0a |
40 |
P. longum |
PLLS2 |
Penicillium sp. |
10.66e±0.29 |
5.66d±0.29 |
0a |
11.33i±0.51 |
0a |
0a |
41 |
P. longum |
PLLS3 |
A. flavus |
14.66g±0.78 |
11.66e±0.29 |
0a |
6.33ef±0.59 |
0a |
0a |
42 |
P. longum |
PLLS4 |
Colletotrichum sp. |
6.66bcd±0.29 |
0a |
0a |
5.33de±0.29 |
0a |
0a |
43 |
P. longum |
PLLS5 |
Penicillium sp. |
12.33ef±0.29 |
0a |
0a |
7.33fg±0.29 |
0a |
0a |
44 |
P. longum |
PLLS6 |
A. terreus |
7.66cd±1.19 |
3.33bc±0.29 |
7.00c±0 |
8.33gh±0.59 |
4.33b±0.29 |
3.00a±0.51 |
45 |
P. longum |
PLLS7 |
Dreshclera sp. |
0a |
0a |
0a |
0a |
0a |
0a |
46 |
P. longum |
PLLS8 |
Aspergillus sp. |
7.66cd±0.29 |
2.33b±0.29 |
0a |
6.00ef±0.51 |
0a |
0a |
47 |
P. longum |
PLLS9 |
Pithomyces sp. |
6.33bc±0.78 |
6.00d±0.51 |
0a |
7.33fg±0.29 |
0a |
0a |
48 |
P. longum |
PLLS10 |
Penicillium sp. |
5.33b±0.59 |
5.33d±0.59 |
0a |
0a |
0a |
0a |
49 |
P. longum |
PLLS11 |
Curvularia sp. |
0a |
0a |
0a |
7.33fg±0.78 |
0a |
0a |
50 |
P. longum |
PLSS1 |
Fusarium sp. |
0a |
0a |
0a |
2.66b±0.29 |
0a |
0a |
51 |
P. longum |
PLSS2 |
A. terreus |
13.33fg±1.40 |
0a |
0a |
3.00bc±0.51 |
0a |
0a |
52 |
P. longum |
PLSS3 |
Curvularia sp. |
0a |
0a |
0a |
0a |
0a |
0a |
53 |
P. longum |
PLSS4 |
A. terreus |
11.33e±0.78 |
5.66d±0.50 |
0a |
3.33bc±0.78 |
0a |
0a |
54 |
P. longum |
PLSS5 |
A. terreus |
6.66bcd±0.78 |
2.33b±0.78 |
0a |
6.33ef±0.29 |
0a |
0a |
55 |
P. longum |
PLSS6 |
Mycelia sterilia |
8.33cd±0.78 |
0a |
0a |
4.33cd±0.29 |
0a |
0a |
56 |
P. longum |
PLSS7 |
Mucor sp. |
0a |
0a |
0a |
0a |
0a |
0a |
57 |
P. longum |
PLSS8 |
Penicillium sp. |
0a |
0a |
0a |
0a |
0a |
0a |
58 |
P. longum |
PLPS1 |
A. flavus |
20.66h±0.59 |
3.66c±0.29 |
6.66b±0.29 |
13.33j±0.78 |
15.33c±0.29 |
0a |
59 |
P. longum |
PLPS2 |
Pestalotiopsis sp. |
7.00bcd±0.51 |
0a |
0a |
0a |
0a |
0a |
60 |
P. longum |
PLPS3 |
Mycelia sterilia |
0a |
0a |
0a |
0a |
0a |
0a |
61 |
P. longum |
PLPS4 |
Bipolaris sp. |
8.66c±0.78 |
0a |
0a |
9.33h±0.50 |
0a |
0a |
62 |
P. longum |
PLPS5 |
Phomopsis sp. |
0a |
0a |
0a |
3.33bc±0.78 |
0a |
0a |
63 |
P. longum |
PLPS6 |
Penicillium sp. |
0a |
0a |
0a |
0a |
0a |
0a |
64 |
P. longum |
PLPS7 |
Aspergillus sp. |
0a |
0a |
0a |
0a |
0a |
0a |
65 |
P. longum |
PLPS8 |
Mycelia sterilia |
0a |
0a |
0a |
8.00gh±0.51 |
0a |
0a |
66 |
Z. officinale |
WGL |
Aspergillus sp. |
0a |
0a |
0a |
0a |
0a |
0a |
67 |
Z. officinale |
WGL1 |
Rhizopus sp. |
3.00c±0.51 |
0a |
0a |
0a |
0a |
0a |
68 |
Z. officinale |
WGMR1 |
Cladosporium sp. |
0a |
0a |
0a |
0a |
0a |
0a |
69 |
Z. officinale |
WGMR2 |
Cladosporium sp. |
0a |
0a |
0a |
0a |
0a |
0a |
70 |
Z. officinale |
WGMR3 |
Alternaria sp. |
0a |
0a |
0a |
0a |
0a |
0a |
71 |
Z. officinale |
WGMR4 |
Curvularia sp. |
0a |
0a |
0a |
0a |
0a |
0a |
72 |
Z. officinale |
GKS |
A. terreus |
0a |
1.66b±0.59 |
2.00b±0.89 |
0a |
5.66b±0.59 |
1.33a±0.29 |
73 |
Z. officinale |
GKS1 |
Mycelia sterilia |
0a |
0a |
0a |
0a |
0a |
0a |
74 |
Z. officinale |
GKS2 |
Cylindrocephalum sp. |
0a |
0a |
0a |
0a |
0a |
0a |
75 |
Z. officinale |
GKS3 |
Colletotrichum sp. |
0a |
0a |
0a |
0a |
0a |
0a |
76 |
Z. officinale |
GKKS |
Mycelia sterilia |
0a |
0a |
0a |
0a |
0a |
0a |
77 |
Z. officinale |
GKKS1 |
Mycelia sterilia |
1.33b±0.29 |
0a |
3.66c±0.29 |
0a |
0a |
0a |
78 |
Z. officinale |
GKKS2 |
Mucor sp. |
0a |
0a |
0a |
0a |
0a |
0a |
79 |
Z. officinale |
GKKP |
Rhizopus sp. |
0a |
0a |
0a |
0a |
0a |
0a |
Values represent mean ± SD of three parallel experiments. In each column, mean values followed by the same letter are not significantly different according to Duncan’s Multiple Range Test at p < 0.05
Production and extraction of secondary metabolites
The potential isolates were chosen after the preliminary screening and were selected for the production and extraction of secondary metabolites for further evaluation. The ethyl acetate extracts of the potential isolates yielded around 400mg/200g of red rice media.
Antimicrobial activity of crude fungal extract by agar well diffusion method
The potential endophytic fungal crude extracts were screened for their antimicrobial activity by agar well diffusion method. The crude extracts were added at concentrations of 20–100 µg/mL. The endophytic fungi exhibited a broad spectrum of antimicrobial activity against the test pathogens, when compared to that of standard positive control tetracycline (bacteria) and flucanazole (fungi). The zone of inhibition of test pathogens ranged from 2.66 mm to 23.33 mm at concentrations of 20–100 µg/mL of tested crude extracts (Table 4).
Table (4):
Antimicrobial activity of selected endophytic fungal extracts by agar well diffusion method
Sl. No |
Endophytic Fungi code |
Medicinal plant |
Conc. µg/mL |
Staphylococcus aureus |
Bacillus cereus |
Escherichia coli |
Salmonella typhi |
Pseudomonas aeruginosa |
Candida albicans |
|---|---|---|---|---|---|---|---|---|---|
1 |
ABL1 |
T. cordifolia |
20 |
7.00a±0.29 |
11.33a±0.59 |
0a |
0a |
0a |
0a |
40 |
7.00a±0.51 |
12.33a±0.29 |
0a |
0a |
0a |
2.56b±0.03 |
|||
60 |
9.66ab±0.51 |
12.33a±0.29 |
0a |
0a |
0a |
5.03c±0.29 |
|||
80 |
9.66ab±0.51 |
12.66a±0.59 |
0a |
0a |
0a |
7.30d±0.51 |
|||
100 |
11.00b±0.29 |
9.66a±0.29 |
0a |
0a |
0a |
10.05e±0.59 |
|||
2 |
ABS1 |
T. cordifolia |
20 |
8.66a±0.29 |
9.33a±0.59 |
0a |
0a |
0a |
0a |
40 |
9.00a±0.89 |
18.00d±0.51 |
0a |
0a |
0a |
0a |
|||
60 |
10.00a±0.29 |
13.66c±0.29 |
0a |
0a |
0a |
0a |
|||
80 |
10.00a±0.51 |
10.66ab±0.78 |
0a |
0a |
0a |
0a |
|||
100 |
10.33a±0.51 |
12.00bc±0.51 |
0a |
0a |
0a |
0a |
|||
3 |
ABS3 |
T. cordifolia |
20 |
0a |
0a |
5.66a±0.78 |
0a |
0a |
0a |
40 |
0a |
5.00b±0.51 |
12.00c±0.51 |
0a |
0a |
0a |
|||
60 |
0a |
7.66c±0.59 |
9.33b±0.29 |
0a |
0a |
0a |
|||
80 |
0a |
7.33c±0.29 |
9.33b±0.59 |
0a |
0a |
0a |
|||
100 |
0a |
5.33b±0.29 |
10.66bc±0.29 |
0a |
0a |
0a |
|||
4 |
ABR4 |
T. cordifolia |
20 |
11.33a±0.29 |
19.00b±0.51 |
0a |
7.66a±0.29 |
5.66ab±0.29 |
3.03a±0.03 |
40 |
10.66a±0.29 |
14.33a±0.59 |
0a |
6.33a±0.29 |
4.66b±0.29 |
5.56b±0.29 |
|||
60 |
15.33b±0.78 |
15.00a±0.51 |
0a |
10.33b±0.78 |
7.00b±0.51 |
7.50c±0.29 |
|||
80 |
11.00a±0.51 |
13.33a±0.29 |
7.33b±0.29 |
10.66b±0.29 |
9.00c±0.51 |
9.42d±0.51 |
|||
100 |
9.66a±0.29 |
17.33b±0.59 |
11.33b±0.59 |
22.33c±0.29 |
13.33d±0.59 |
10.55e±0.29 |
|||
5 |
PKS4 |
P. nigrum |
20 |
13.33b±0.78 |
12.33a±0.59 |
0a |
0a |
0a |
0a |
40 |
12.66b±0.59 |
16.00bc±0.51 |
0a |
0a |
0a |
0a |
|||
60 |
8.00a±0.51 |
14.33b±0.59 |
0a |
0a |
0a |
0a |
|||
80 |
13.66b±0.29 |
16.33c±0.29 |
0a |
7.33b±0.29 |
0a |
0a |
|||
100 |
17.33c±0.59 |
21.33d±0.59 |
0a |
11.33c±0.78 |
0a |
0a |
|||
6 |
PKKS1 |
P. nigrum |
20 |
11.66a±0.78 |
14.33a±0.59 |
0a |
0a |
0a |
0a |
40 |
12.33a±0.59 |
15.33a±1.57 |
0a |
0a |
0a |
0a |
|||
60 |
12.66a±0.59 |
17.00a±0 |
0a |
0a |
0a |
0a |
|||
80 |
15.66b±0.29 |
14.33a±1.30 |
0a |
0a |
0a |
0a |
|||
100 |
16.66b±0.78 |
16.00a±1.79 |
0a |
0a |
0a |
0a |
|||
7 |
PKKS2 |
P. nigrum |
20 |
2.66a±0.59 |
0a |
0a |
6.66ab±0.59 |
0a |
0a |
40 |
3.33a±0.29 |
0a |
0a |
5.00a±0.51 |
0a |
0a |
|||
60 |
3.33a±0.29 |
0a |
0a |
7.66b±0.29 |
0a |
0a |
|||
80 |
5.66b±0.78 |
0a |
0a |
7.33b±0.78 |
0a |
0a |
|||
100 |
8.33c±0.29 |
0a |
0a |
14.33c±0.78 |
0a |
0a |
|||
8 |
PKKR3 |
P. nigrum |
20 |
5.66a±0.59 |
0a |
0a |
0a |
0a |
0a |
40 |
6.66a±0.59 |
0a |
0a |
0a |
0a |
0a |
|||
60 |
9.00b±0.51 |
0a |
0a |
0a |
0a |
0a |
|||
80 |
10.33b±0.29 |
0a |
0a |
0a |
0a |
0a |
|||
100 |
16.66c±0.78 |
0a |
0a |
0a |
0a |
0a |
|||
9 |
GKS |
Z. officinale |
20 |
13.33a±1.49 |
13.66ab±0.78 |
0a |
0a |
0a |
0a |
40 |
15.00ab±0.51 |
16.00bc±0.89 |
0a |
0a |
0a |
0a |
|||
60 |
16.00ab±0.89 |
18.66d±0.29 |
0a |
0a |
0a |
0a |
|||
80 |
18.33b±0.59 |
13.33a±0.78 |
0a |
0a |
0a |
0a |
|||
100 |
13.33a±0.78 |
18.00cd±0.51 |
0a |
0a |
0a |
0a |
|||
10 |
PKR1 |
P. nigrum |
20 |
0a |
4.00a±0.89 |
0a |
0a |
0a |
0a |
40 |
0a |
6.00ab±0.51 |
0a |
0a |
0a |
0a |
|||
60 |
0a |
5.66ab±0.29 |
0a |
0a |
0a |
0a |
|||
80 |
0a |
6.66b±0.78 |
0a |
0a |
0a |
0a |
|||
100 |
0a |
7.66b±0.59 |
0a |
0a |
0a |
0a |
|||
11 |
PKR3 |
P. nigrum |
20 |
0a |
4.66a±0.29 |
3.66ab±0.59 |
0a |
0a |
0a |
40 |
0a |
4.33a±0.78 |
1.66a±0.29 |
0a |
0a |
0a |
|||
60 |
0a |
7.33b±0.29 |
5.00b±0.51 |
0a |
0a |
0a |
|||
80 |
0a |
9.00b±0.89 |
11.66c±0.78 |
0a |
0a |
0a |
|||
100 |
0a |
13.00c±0.51 |
14.33d±0.59 |
0a |
0a |
0a |
|||
12 |
ABLS2 |
T. cordifolia |
20 |
5.00a±0.89 |
5.00a±0.51 |
5.66a±0.29 |
6.00a±0.89 |
0a |
0a |
40 |
6.33a±0.78 |
8.33b±0.29 |
7.66b±0.29 |
9.00b±0.51 |
0a |
0a |
|||
60 |
10.33b±0.29 |
11.00c±0.51 |
9.33b±0.78 |
13.00c±1.03 |
0a |
0a |
|||
80 |
12.00b±0.57 |
12.00c±0.57 |
12.33c±0.66 |
13.00cd±0.57 |
0a |
0a |
|||
100 |
19.00c±0.51 |
16.33d±0.78 |
15.33d±0.59 |
16.33d±0.59 |
0a |
0a |
|||
13 |
ABLS3 |
T. cordifolia |
20 |
3.66a±0.29 |
4.66a±0.59 |
3.33a±0.29 |
8.00a±0.51 |
0a |
0a |
40 |
6.33b±0.29 |
6.33a±0.59 |
8.33b±0.78 |
11.66b±0.78 |
0a |
0a |
|||
60 |
7.33b±0.29 |
6.33a±1.19 |
8.33b±0.59 |
12.33b±1.30 |
0a |
0a |
|||
80 |
10.66c±0.51 |
9.33b±0.29 |
13.33c±0.78 |
18.33c±0.29 |
0a |
0a |
|||
100 |
11.33e±0.59 |
12.66c±0.29 |
11.33e±0.59 |
21.00c±0.51 |
0a |
0a |
|||
14 |
PLLS6 |
P. longum |
20 |
6.33a±0.59 |
3.00a±0.51 |
3.33a±0.59 |
5.00a±0 |
4.33a±0.78 |
0a |
40 |
7.66a±1.19 |
5.66b±0.29 |
5.66ab±0.59 |
6.66a±1.19 |
6.00ab±0 |
0a |
|||
60 |
11.66b±0.59 |
6.66b±0.78 |
5.00ab±1.03 |
10.33b±0.59 |
5.66ab±0.78 |
0a |
|||
80 |
13.00b±0.51 |
13.00c±0.89 |
7.66b±1.19 |
12.00b±1.03 |
8.66b±1.57 |
0a |
|||
100 |
14.33b±0.78 |
17.33d±0.59 |
14.00c±1.03 |
20.00c±1.03 |
8.66c±1.19 |
0a |
|||
15 |
PLPS1 |
P. longum |
20 |
6.66a±0.59 |
2.66a±0.29 |
4.66a±0.29 |
6.33a±0.59 |
7.00a±0 |
0a |
40 |
11.33b±0.59 |
5.66b±0.78 |
5.00a±0.89 |
7.00a±0 |
11.33b±0.78 |
0a |
|||
60 |
13.33b±0.78 |
7.00bc±0.89 |
5.66a±0.78 |
9.00b±0.51 |
12.66bc±0.29 |
0a |
|||
80 |
17.66c±0.78 |
9.33c±0.29 |
10.66b±0.29 |
13.66c±0.78 |
14.00c±0.51 |
0a |
|||
100 |
21.00d±0.89 |
13.66d±0.78 |
15.00c±0.51 |
17.33d±0.29 |
17.33d±0.59 |
0a |
|||
16 |
PKKR2 |
P. nigrum |
20 |
12.33a±0.59 |
7.00a±0.51 |
4.33a±0.78 |
11.66a±0.59 |
0a |
0a |
40 |
14.00a±0.89 |
7.33a±0.59 |
5.66a±0.29 |
11.33a±0.78 |
0a |
0a |
|||
60 |
17.00b±0.51 |
6.66a±1.30 |
16.66b±0.59 |
14.33b±0.78 |
0a |
0a |
|||
80 |
16.33b±0.66 |
8.66a±0.33 |
18.00b±0.57 |
13.33ab±0.88 |
0a |
0a |
|||
100 |
18.33b±0.66 |
8.66a±0.66 |
18.33b±0.88 |
14.66b±0.66 |
0a |
0a |
|||
17 |
Positive control |
Tetracycline |
20 |
21.33e±0.88 |
19.33d±0.59 |
20.33d±0.89 |
23.33e±0.88 |
20.33d±0.89 |
0a |
18 |
Positive control |
Fluconazole |
20 |
– |
– |
– |
– |
– |
13.58e±0.58 |
Values represent mean±SD of three experiments. In each column, mean values followed by the same letter are not significantly different according to Duncan’s Multiple Range Test at p< 0.05.
Antioxidant potential of endophytic fungi
DPPH assay
DPPH is a stable free radical with absorption at 570nm, exhibits a deep purple color in methanol solution which gets reduced to a yellow colored product diphenyl picryl hydrazine.31 As antioxidants donate protons to DPPH radicals, the absorption decreases. The reduction in the number of DPPH molecules can be correlated with the number of available hydroxyl groups. The DPPH scavenging potential of endophytic extracts may be attributed to the hydroxyl groups present in the extracts.32 The sample was tested against this radical at different concentrations ranging from (10 to 100µg) and the readings were observed by decreasing the absorbance taken as a measure that indicates the extent of radical scavenging property. The antioxidant activity of the 16 potential endophytic fungi was determined and the results are tabulated in (Table 5). The ethyl acetate extracts of GKS, PKS4 and PKKR3 showed higher DPPH activity than the standard Trolox (Fig 1).
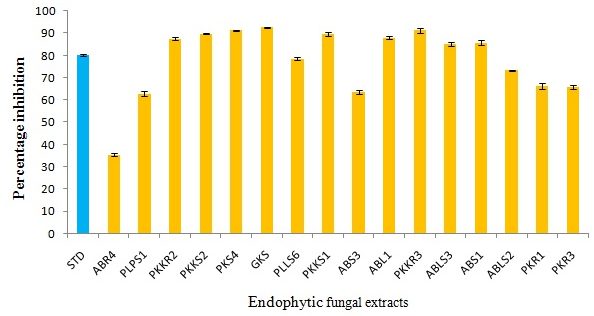
Fig. 1. DPPH activity of ethyl acetate extracts of selected endophytic fungi
Table (5):
Antioxidant activity and total phenolic content of selected fungal endophytes isolated from four medicinal plants
Sl. No |
Endophytic fungal extract |
DPPH scavenging assay (%) |
ABTS (IC50)(µg/ml) |
Total phenol content (mg/ml) |
Reducing power (µg/ml) |
|---|---|---|---|---|---|
1 |
ABR4 |
35.35±1.19 |
339 |
101±2.64 |
0.96±0.02 |
2 |
PLPS1 |
62.82±1.65 |
79 |
117.3±2.08 |
0.87±0.02 |
3 |
PKKR2 |
87.46±1.33 |
19.85 |
118.3±4.16 |
0.96±0.01 |
4 |
PKKS2 |
89.68±0.31 |
496 |
115±3.60 |
0.91±0.02 |
5 |
PKS4 |
91.22±0.36 |
116 |
129.6±1.52 |
0.91±0.009 |
6 |
GKS |
92.60±0.47 |
3.34 |
125.33±1.52 |
0.96±0.02 |
7 |
PLLS6 |
78.52±1.14 |
58.51 |
132.66±2.08 |
0.95±0.03 |
8 |
PKKS1 |
89.75±1.54 |
248 |
146.33±3.78 |
0.86±0.02 |
9 |
ABS3 |
63.63±1.37 |
97.48 |
130.33±2.51 |
0.95±0.02 |
10 |
ABL1 |
87.90±1.11 |
283 |
94.00±2.00 |
0.95±0.01 |
11 |
PKKR3 |
91.29±1.96 |
567.14 |
116.00±1.00 |
0.95±0.006 |
12 |
ABLS3 |
85.10±1.21 |
915 |
144.66±4.16 |
0.92±0.02 |
13 |
ABS1 |
85.74±1.64 |
412 |
101.66±2.08 |
0.90±0.01 |
14 |
ABLS2 |
73.23±0.69 |
342 |
96.66±3.21 |
0.92±0.01 |
15 |
PKR1 |
66.15±2.23 |
164 |
94.66±3.05 |
0.80±0.03 |
16 |
PKR3 |
65.65±1.70 |
128 |
76.66±2.08 |
0.78±0.02 |
17 |
Standard Trolox*/ Gallic acid # |
80.19±1.05* |
5.54# |
— |
0.97±0.01# |
Total phenolic content is expressed in mg Gallic acid equivalent (GAE) g/dw
Each result is expressed as mean ± S.D. (n = 3)
Radical scavenging activity against ABTS
ABTS is a stable free radical with the characteristic absorption at 745 nm and was used to study the radical scavenging effect of extracts. The results demonstrated that the extracts reacted with ABTS at different concentrations ranging from 200, 400, 600, 800 and 1600µg/ml respectively depending on the weight of the extracts. The readings were observed by measuring the reduction of radical cation generated by ABTS at 745 nm. The endophytic ethyl acetate extracts showed maximum decolorization at the maximum concentration of 1600µg/ml (Table 5). The extent of reduction in decolorization is directly proportional to the increased concentration of the extracts.
Total phenolic content
The Total phenolic content (TPC) was determined by the Folin Ciocalteu method. The fungal ethyl acetate extracts have been expressed as Gallic acid equivalent i.e., mg Gallic acid/g dry wt. A high phenolic content (146.33±3.78) was observed in the isolate PKKS1 (A. terreus) of P. nigrum (Table 5). Almost all the tested endophytic fungal isolates demonstrated a good phenolic content > 60 ± 1.00 mg gallic acid/g dw (Fig 2). Results showed that the levels of phenolic compounds in different endophytic fungi were significantly (p < 0.05) different from each other.
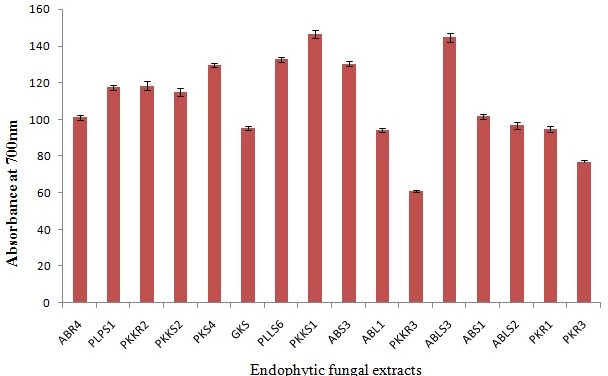
Fig. 2. Total phenolic content of selected endophytic fungal extracts
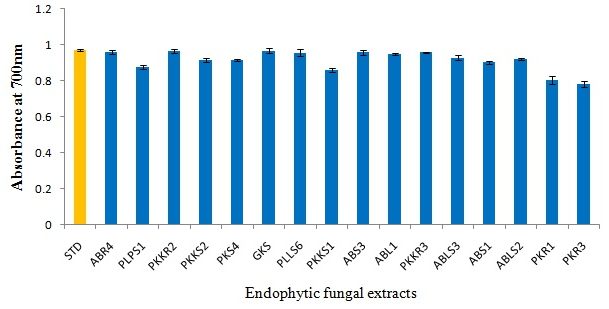
Fig. 3. Reducing power assay of selected endophytic fungal extracts
Reducing power assay
The reducing power of the ethyl acetate extracts of fungal strains increased with increasing concentration. The increase in absorbance of the reaction mixtures implies increased reducing power 33. In our study, most of the endophytic fungal extracts have demonstrated potent reducing activity comparable to the standard Gallic acid (Fig 3) (Table 5).
Endophytic fungi are ubiquitous in their distribution and are reported from almost every plant 34. The secondary metabolites from endophytic fungi from four medicinal plants from Western Ghats, Karnataka were investigated in a quest to search for novel antimicrobial and antioxidant agents. There is a dearth of information on the antioxidant activities from the medicinal plants Piper nigrum L, Piper longum L and Zingiber officinale Roscoe which prompted us to evaluate the antioxidant and antimicrobial potential of these medicinal plants. In this study, 79 endophytic fungal strains belonging to different taxa were obtained. Fungal endophytes are especially common among the Ascomycetes, representing at least five classes, dozens of families, and large numbers of previously unknown species 35. Most of the taxa were common endophytic fungi as observed earlier 36. Cultures which failed to sporulate or those lacking reproductive structures and which could not be identified to genus or species level without molecular analysis were grouped as Mycelia sterilia 37. There are a vast range of endophytic microorganisms which have been untapped for bioactive metabolite production and evaluation 38. Recent research has focused on finding an alternative to the problem of drug resistance acquired by the pathogens and endophytic metabolites have been investigated for their antimicrobial complement to tackle the drug resistance problem in pathogens 39. Antioxidants are effective in the management of reactive oxygen species- mediated impairments and antioxidant compounds are known to possess anti-inflammatory, anti-atherosclerotic, antitumor, anticarcinogenic, antibacterial and antiviral activities 40, 41. Natural products are the major sources of new drug molecules today. Plants and other natural sources can provide a huge range of complex and structurally diverse compounds. Recently, many researchers have focused on the investigation of plant and microbial extracts, bioactive secondary metabolites and new synthesized molecules as potential antimicrobial agents 42, 43.
In the present study, the fungal endophytes revealed good antimicrobial activities which can be utilized for the production of bioactive metabolites. Agar well diffusion method is widely used to evaluate the antimicrobial activity of plants or microbial extracts 44. The endophytic fungus F. solani (ABR4) of T. cordifolia inhibited both gram positive and gram negative organisms with effective inhibition. Also, ABR4 has demonstrated significant inhibitory activity against C. albicans at effective concentrations (20 µg/mL) indicating the ability of the endophyte to be utilized for pharmaceutical preparations. The extracts of P. longum -PLLS6 and PLPS1 demonstrated effective antibacterial activity by agar well diffusion to all the tested bacterial pathogens at concentrations of 20-100 µg/mL (Table 4). Several studies have reported novel compounds with antimicrobial activity from endophytic fungi 45. Endophytic fungi with anti- microbial, anti-cancer and anti-malarial activities were isolated from Thai medicinal plants 37. A vast majority of microorganisms remain unexploited for diverse and valuable bioactive metabolites 46. The extracts of ABLS2, ABLS3 of T. cordifolia and PKKR2 of P. nigrum exhibited antibacterial activity against the tested bacteria except P. aeruginosa when tested by agar well diffusion method. Further, the endophytic isolates ABL1, ABS1, PKS4, PKKS1 and GKS exhibited activity only against S. aureus and B. cereus at concentrations of 20-100 µg/mL; whereas PKR3 and ABS3 showed activity against B. cereus and E. coli demonstrating that it can be an effective antibacterial against both gram positive and gram negative organisms at the tested concentrations. Moreover, the extract ABL1 of T. cordifolia revealed antifungal activity by inhibiting C. albicans at concentrations of 40-100 µg/mL. The extract of PKR1 of P. nigrum exhibited antibacterial activity against B. cereus only and was ineffective against the other test pathogens. The endophytic fungi (SRVK 113 and SRVK 147) from T. cordifolia revealed good antibacterial activity against B. subtilis and S. aureus but did not show inhibitory activity against E. coli and P. aeruginosa. Also, none of the isolates showed antifungal activity against C. albicans and A. niger 8. The extract PKKS2 showed activity against S. aureus and S. typhimurium indicating a strong antibacterial potential of the extracts of P. nigrum. Also, PKKR3 from P. nigrum depicted inhibitory activity against S. aureus only at all tested concentrations but showed no activity against the other bacterial and fungal pathogens. However, little work has been done on the antimicrobial activity of endophytic fungal isolates from P. nigrum, P. longum and Z. officinale necessiating a need for the present study. The study demonstrates that crude extracts of endophytic fungi demonstrate effective antimicrobial activity which indicates the presence of an active principle in high concentrations which results in the biological activities of the fungal endophytes 47. Also, the isolates which demonstrated low anti-microbial activities may have the active principle in smaller amounts and may depict higher activity when purified. Another possibility can be that these extracts may show antimicrobial activity against other microbes which were not tested in the present study.
Different assays were employed to assess the comparative antioxidant potential of the endophytic fungal extracts. The total phenol content and the antioxidant activity are strongly linked as phenols possess strong scavenging activity for free radicals due to their hydroxyl groups and hence the phenolic content of the endophytic fungal extracts may contribute for their antioxidant nature 48. The total phenol content of the ethyl acetate extract in terms of GAE indicates high antioxidant potential of the extract. In our study, the isolates PKKS1 and ABLS3 presented with highest phenolic content followed by PLLS6, ABS3, PKS4, GKS, PKKR2, PLPS1 and PKKR3 which can be powerful antioxidants. In addition, all endophytic extracts were found to possess significant amount of phenolic content. DPPH assay serves as a quick and reliable factor to assess the antioxidant activity of natural products 49. In our study, the sample was tested at different concentrations of 10 – 100µg and the readings were observed by a decrease in the absorbance indicating the extent of radical scavenging property in comparison to the standard Trolox. The endophytes GKS and PKS4 (A. terreus) have demonstrated efficient DPPH activity as compared to the standard trolox and be considered as powerful antioxidant agents. All the endophytic fungi have shown antioxidant activity to some extent which is in agreement with the study made by Duan et al. 50. The endophytic extracts were tested for their radical scavenging activity against ABTS which is a reliable test of total antioxidant capacity 51. The results showed that most endophytic fungi have exhibited good antioxidant capacity especially the isolate GKS (A. terreus) from Z. officinale which has demonstrated strongest antioxidant activity when compared to the standard Gallic acid. This endophytic fungus will be further screened for bioactive compounds. To the best of our knowledge, this is a first report signifying the remarkable antioxidant potential of an endophytic fungal extract of A .terreus from Z. officinale which is higher than that of the standard. The reducing power evaluation of the test compounds is an important parameter related to assessing the antioxidant activity. The extracts act as reductones that inhibit lipid peroxidation by donating a hydrogen atom thereby terminating the free radical chain reaction 52. In our study, all the endophytic fungi assayed were found to possess reducing potential. The ethanolic extract of Aspergillus sp. isolated from Potentilla fulgens showed a potent reducing activity closer to ascorbic acid standard 53. Various endophytic fungal species isolated from different plants showed greater antioxidant potential. Phomopsis sp. and Xylaria sp. isolated from Emblica officinalis exhibited higher level of reducing potential with increase in concentration 54. The endophytic fungi have shown high phenolic content with good reducing power as well as DPPH and ABTS scavenging activity as compared to the standard Gallic acid which can be utilized to yield high amount of bioactive components with good antimicrobial and antioxidant activities.
Microbial infections pose a significant clinical threat with associated morbidity and mortality due to the development of microbial resistance to the existing antimicrobials. Therefore, the discovery of novel antimicrobial agents from endophytic fungi continues to be developed. The present study demonstrates that the fungal endophytes from medicinal plants produce bioactive compounds which can be both antimicrobial and antioxidant in nature. In our study, the endophyte ABR4 (F. solani) of T. cordifolia showed effective antimicrobial activity to the test microorganisms. Also, the endophytic A. terreus (GKS4) of Z. officinale showed highest antioxidant activity when compared to standard Gallic acid. Further investigations are needed to discover bioactive compounds from these endophytic fungi.
ACKNOWLEDGMENTS
The Financial assistance to Fazilath Uzma (F1-17.1/2012-13/MANF-2012-13-MUS-KAR-11899) granted by Maulana Azad National Fellowship (MANF), University Grants Commission (UGC), New Delhi is gratefully acknowledged.
- Aly, A.H., Debbab, A., Carol, C., RuAngelie., Edrada-Ebel., Barbora Orlikova., et al. NF kappa B inhibitors and anti trypanosomal metabolites from endophytic fungus Penicillium sp. isolated from Limonium tubiflorum. Bioorg Med Chem., 2011; 19(1):414-21.
- Strobel, G.A. Rainforest endophytes and bioactive products. Crit Rev in Biotechnol., 2002; 22(4): 315–333.
- Tan, R.X., Zou, W.X. Endophytes: a rich source of functional metabolites. Natural product reports., 2001; 18(4):448-59.
- Kusari, S., Zühlke, S., Spiteller, M. An endophytic fungus from Camptotheca acuminata that produces camptothecin and analogues. J Nat Prod., 2009; 72(1):2-7.
- Huang, W., Cai, Y., Hyde, K., Corke, H., Sun, M. Biodiversity of endophytic fungi associated with 29 traditional Chinese medicinal plants. Fungal Divers., 2008; 33:61-75
- Zhao, J., Shan, T., Mou, Y., Zhou, L. Plant-derived bioactive compounds produced by endophytic fungi. Mini Rev Med Chem., 2011; 11(2):159-68
- Uzma, F., Murthy, K.N., Srinivas, C. Diversity and extracellular enzyme activities of fungal endophytes isolated from medicinal plants of Western Ghats, Karnataka. Egyptian Journal of Basic and Applied Sciences., 2016a; 3(4): 335-342.
- Desai, S., Metrani, R., Vantamuri, S., Ginigeri, V., Padhke, K., Hungund, B. Phytochemical analysis, antimicrobial and antitumor screening of endophytes of Tinospora cordifolia. Intl J Pharma and Bio Sci., 2012; 3(4):533–540.
- Suryanarayanan, T.S., Murali, T.S., Thirunavukkarasu, N., Rajulu, M.B.G., Venkatesan, G., Sukumar, R. Endophytic fungal communities in woody perennials of three tropical forest types of the Western Ghats, Southern India. Biodiversity and Conservation., 2011; 20(5):913-928.
- Ayres, M.C., Brandão, M.S., Vieira-junior, G.M., Menor, J.C.A.S., Silva, H.B., Soares, M.J.S., et al. Antibacterial activity of useful plants and chemical constituents of the roots of Copernicia prunifera. Revista Brasileira de Farmacognosia., 2008; 18(1):90-97.
- Silva, F., Ferreres, J.O., Malva, Dias A.C.P. Phytochemical and antioxidant characterization of Hypericum perforatum alcoholic extracts. Food Chem., 2005; 90(1–2):157–167.
- Song, F.L., Gan, R.Y., Zhang, Y., Xiao, Q., Kuang, L., Li, H.B. Total phenolic contents and antioxidant capacities of selected chinese medicinal plants. Int. J. Mol. Sci., 2010; 11(6):2362-2372.
- Shukla, S.T., Kulkarni, V.H., Habbu, P.V., Jagadeesh, K.S., Patil, B.S., Smita, D.M. Hepatoprotective and antioxidant activities of crude fractions of endophytic fungi of Ocimum sanctum Linn. in rats. Oriental Pharmacy and Experimental Medicine., 2012; 12(2):81–91.
- Sukantha, T.A., Shubashini, K.S., Ravindran, N.T., Balashanmugam, P. Antioxidant and Antibacterial activities of Trianthema decandra Linn. Int J Pharm Pharm Sci., 2012; 4(1):410-413.
- Uzma, F., Murthy, K.N., Srinivas, C. Optimization of physiological conditions for L-asparaginase production by endophytic fungi (Fusarium solani) isolated from Tinospora cordifolia (Willd.) Hook. F & Thomson. Euro J Experimental Biol., 2016a; 6(3):37-45.
- Barnett, H.L., Hunter, B.B. Illustrated Genera of Imperfect Fungi. APS Press, St. Paul, Minnesota, USA; 1998.
- Denitsa, N., Mariana, N. Screening the antimicrobial activity of Actinomycetes strains isolated from Antarctica. Journal of Culture Collections., 2004-2005; 4:29-35.
- Atalla, M.M., Hamed, E.R., El-Shami, A.R. Optimization of a culture medium for increased mevinolin production by Aspergillus terreus strain. Malaysian J Microbiol., 2008; 4(2): 6-10.
- Su, Y.C., Wang, J.J., Lin, T.T., Pan, T.M. Production of the secondary metabolites c-aminobutyric acid and monacolin K by Monascus. J Ind Microbiol Biotechnol., 2003; 30(1):41-46.
- Cui, J.L., Guo, S.X., Xiao, P.G. Antitumor and antimicrobial activities of endophytic fungi from medicinal parts of Aquilaria sinensis. Journal of Zhejiang University Science B(Biomed and Biotechnol)., 2011; 12(5): 385-392.
- Guo, L.D., Hyde, K.D., Liew, E.C. Identification of endophytic fungi from Livistona chinensis based on morphology and rDNA sequences. New phytologist., 2000; 147(3):617-30.
- Visalakchi. S, Muthumary, J. Antimicrobial activity of the new endophytic Monodictys castaneae SVJM139 pigment and its optimization. African Journal of Microbiology Research., 2009; 3(9):550-6.
- Shimada, K., Fujikawa, K., Yahara, K., Nakamura, T. Antioxidative properties of xanthone on the auto oxidation of soybean in cylcodextrin emulsion. J. Agr. Food Chem., 1992; 40:945–948.
- Arona, M.B., Cano, A., Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem., 2001; 73:239- 244.
- Yadav, M., Yadav, A., Yadav, J.P. In vitro antioxidant activity and total phenolic content of endophytic fungi isolated from Eugenia jambolana Lam. Asian Pacific journal of tropical medicine., 2014; 7:S256-61.
- Djeridane, A., Yousfi, M., Nadjemi, B., Boutassouna, D., Stocker, P., Vidal, N. Antioxidant activity of some algerian medicinal plants extracts containing phenolic compounds. Food Chem., 2006; 97(4):654–660.
- Karsha, P.V., Bhagya, L.O. Antibacterial activity of black pepper (Piper nigrum Linn.) with special reference to its mode of action. Ind J Nat Prod Res., 2010; 1(2):213-215.
- Kumar, V., Shriram, V., Mulla, J. Antibiotic resistance reversal of multiple drug resistant bacteria using Piper longum fruit extract. J App Pharma Sci., 2013; 3(3):112-116.
- Pereira, M.M., Haniadka, R., Chacko, P.P., Palatty, P.L., Baliga, M.S. Zingiber officinale Roscoe (ginger) as an adjuvant in cancer treatment: a review. J BUON., 2011; 16(3):414-24.
- Elleuch, L., Shaaban, M., Smaoui, S., Mellouli, L., Karray-Rebai, I., Fguira, L.F., et al. Bioactive secondary metabolites from a new terrestrial Streptomyces sp. TN262. Applied biochemistry and biotechnology., 2010; 162(2):579-93.
- Singh, N., Rajini, P.S. Free radical scavenging activity of an aqueous extract of potato peel. Food Chem., 2004; 85(4): 611-616.
- Gherraf, N., Segni, L., Brahim, L., Samir, H. Evaluation of antioxidant potential of various extract of Traganum nudatum. Del. Plant Science Feed., 2011; 1(9):155-159
- Kekuda, T.R.P., Shobha, K.S., Onkarappa, R. Studies on antioxidant and anthelmintic activity of two Streptomyces species isolated from Western Ghat soils of Agumbe, Karnataka. J. Pharm. Res., 2010; 3(1):26-29.
- Gond, S.K., Verma, V.C., Kumar, A., Kumar, V., Kharwar, R.N. Study of Endophytic Fungal Community from Different Parts of Aegle marmelos Correae (Rutaceae) from Varanasi (India). World J Microbiol Biotechnol., 2007; 23(10):1371- 1375.
- Petrini, O. Taxonomy of endophytic fungi of aerial plant tissues. In: Fokkema NJ, van den Heuvel, editors. Microbiology of the Phyllosphere. Cambridge: Cambridge University Press; 1986: 75–187.
- Photita, W., Taylor, P.W.J., Ford, R., Lumyong, P., McKenzie, E.H.C., Hyde, K.D., et al. Morphological and molecular characterization of Colletotrichum species from herbaceous plants in Thailand. Fungal Divers., 2005; 18:117-133.
- Wiyakrutta, S., Sriubolmas, N., Panphut, W., Thongon, N., Danwiserkanjana, K., Ruangrungsi, N., et al. Endophytic fungi with anti-microbial, anticancer, anti-malarial activities isolated from Thai medicinal plants. World J Microbiol Biotechnol., 2004; 20:265-272.
- Motta, A.S., Cladera-Olivera, F., Brandelli, A. Screening for antimicrobial activity among bacteria isolated from the Amazon basin. Braz. J. Microbiol., 2004; 35:307–10.
- Strobel, G., Daisy, B., Castillo, U., Harper, J. Natural products from endophytic microorganisms. J Nat Prod., 2004; 67(2):257-268.
- Sala, A., Recio, N.D., Giner, R.M., Manez, S., Tournier, H., Schinelia, G., et al. Anti-inflammatory and antioxidant properties of Helichrysum italicum. J Pharma Pharmacol 2002; 54:365-371.
- Cozma, L.S. The role of antioxidant therapy in cardiovascular disease. Current Opinion in Lipidology., 2004; 15:369-371.
- Berdy, J. Bioactive microbial metabolites. J. Antibiot., 2005; 58:1–26.
- Runyoro, D.K., Matee, M.I., Ngassapa, O.D., Joseph, C.C., Mbwambo, Z.H. Screening of Tanzanian medicinal plants for anti-Candida activity. BMC complementary and alternative medicine., 2006; 6(1):11.
- Valgas, C., Souza, S.M., Smânia, E.F., Smânia Jr, A. Screening methods to determine antibacterial activity of natural products. Brazilian Journal of Microbiology., 2007; 38(2):369-80.
- Shiono, Y. Anthracobic acids A and B, two polyketides, produced by an endophytic fungus Anthracobia sp. Chem Biodivers., 2006; 3:217-223.
- Rosalind, T., Dutta, B.K., Paul, S.B. Evaluation of in vitro antioxidant activity, estimation of total phenolic and flavonoid content of leaf extract of Eurya japonica Thunb. Asian J Pharm Clin. Res., 2004; 6:152-155.
- Radu, S., Kqueen, C.Y. Preliminary Screening of Endophytic Fungi from Medicinal Plants in Malaysia for Antimicrobial and Antitumor Activity. The Malaysian Journal of Medical Sciences/ : MJMS., 2002; 9(2):23-33.
- Bendini, A., Cerretani, L., Pizzolante, L., Gallina-Toschi, T., Guzzo, F., Ceoldo, S. et al. Phenol content related to antioxidant and antimicrobial activity of Passiflora spp. extracts. Euro Food. Res. Technol., 2006; 223(1):102–109.
- Koleva, II., van Beek, T.A., Linssen, J.P., de Groot, A., Evstatieva, L.N. Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochem. Anal ., 2002; 13(1):8-17.
- Duan, X.J., Zhang, W.W., Li, X.M., Wang, B.G. Evaluation of antioxidant property of extract and fractions obtained from a red alga, Polysiphonia urceolata. Food Chem., 2006; 95:37–43
- Shan, B., Cai, Y.Z., Sun, M., Corke, H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J Agricul Food Chem., 2005; 53:7749-7759.
- Chang, C.C., Yang, M.H., Wen, H.M., Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal., 2002; 10:178-182.
- Nath, A., Joshi, S.R. Bioactivity assessment of endophytic fungi associated with the ethnomedicinal plant Potentilla fulgens. World J Pharmaceu Res., 2013; 2(6): 2596-2607.
- Nath, A., Raghunatha, P., Joshi, S.R. Diversity and biological activities of endophytic fungi of Emblica officinalis, an ethnomedicinal plant of India. Mycobiol., 2012; 40(1): 8-13.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


