ISSN: 0973-7510
E-ISSN: 2581-690X
Escherichia coli is considered one of the most frequent causative agents of common bacterial infections worldwide. In addition, effective treatment and prevention measures are still in demand due to the rise of antibiotic resistance and the emergence of new virulent strains. In this work, we evaluated antibiotics and bacteriophages as interventions for controlling pathogenic E. coli. A total of 15 E. coli isolates were included in this study. The automated identification system, namely Vitek 2, has been utilized for the identification. Antibiotics susceptibility profiles of all isolates were confirmed by disc diffusion method. All strains exhibited resistance at least to one antibiotic (ampicillin) while 13 strains were resistant to Ampicillin/Sulbactam, Cefazolin, and Ceftriaxone. Except for two strains, no resistance to Amikacin was observed. On the other hand, bacteriophages designated øEU-3 and øEU-4 were isolated by single plaque isolation and investigated as antimicrobial agents against pathogenic E. coli. Phages morphology, determined by transmission electron microscopy, revealed a structure comprised of a head diameter (71.42 nm) and a tail length (214.28 nm). These features placed the øEU-3 phage in the family Siphoviridae while øEU-4 belonged to family Myoviridae with a head diameter (66.6 nm) and a contractile tail length (108.3nm). Phages susceptibilities were determined by spot test to fifteen E. coli isolates. Coliphage øEU-3 and øEU-4 had narrow host range. This work described the efficacy of antibiotics and bacteriophages as intervention strategies to control pathogenic E. coli and paved the way for depth studies to broaden the antimicrobial spectrum of øEU-3 and øEU-4 phages.
Escherichia coli, Antibiotic resistance, Coliphage, Siphoviridae, Myoviridae.
Escherichia coli is a gram-negative bacterium that is considered a part of the normal flora inhabiting the intestine and is thought to be harmless. However, pathogenicity mechanisms have been exhibited by many strains making them able to cause diseases in animals and humans. These diseases can be categorized into extra-intestinal (urinary tract infections (UTI), meningitis, septicemia, and pneumonia) and intestinal (diarrhea)1. Six pathotypes of E. coli causing intestinal diseases are known based on the pathogenicity profiles (clinical disease and virulence factors) and include enterohaemorrhagic E. coli , enteropathogenic E. coli , enteroinvasive E. coli, enterotoxigenic E. coli, enteroaggregative E. coli, diffusely adherent E. coli and the recently emerged; adherent invasive E. coli2–4. The spread of prolonged-spectrum beta-lactamase producing E. coli capable of resisting several antibiotics and even the emergence of resistance to drugs of last resort such as colistin has become a major health threat worldwide and motivated the challenge for antimicrobial alternatives such as bacteriophages5–7.
Bacteriophages are bacteria-specific viruses8. Félix d’Hérelle, a co-discoverer of bacteriophages, was the first to propose “phage therapy”9, 10 in the early twentieth century, however, the advent of antibiotics overtook the interest in phage therapy in that time. Nevertheless, phage therapy is now back in the headings11 because of the emergence of new infectious antibiotic-resistance strains. E. coli phages, termed T-even, T-odd and Lambda phages, are a group of ds-DNA bacteriophages that have a head capsid and tail. Phages of E. coli were firstly isolated from a raw sewerage by T.L. Rakietenin the late 1930s, and currently, they are the most studied among bacteriophages. They are commonly isolated from fecal samples, sewage, polluted rivers and hospital wastewater. Life cycles of E. coli phages could be lysogenic or lytic; however, for phage therapy, only lytic phages are used12. Currently, phage therapy is being used in Russia, Georgia, Poland. and U.S.A
The current manuscript was designed to isolate and identify Gram-negative bacilli from Sohag University Hospital, Sohag Governorate, Upper Egypt, and to evaluate their antibiotics sensitivity patterns, also we tried to isolate host-specific bacteriophages from sewage water to evaluate the efficacy of phage therapy against the isolated E. coli.
Samples collection and bacterial identification
The present study was done in Sohag University Hospital; a teaching hospital in Upper Egypt, with more than 1000 bed capacity, and Faculty of Science, Benha University, Qalubiya governorate. Clinical specimens from the intensive care unit (ICU) were collected. Respiratory (R) secretions and tracheal aspirates (from intubated patients) were collected from patients with respiratory symptoms, urine (U) samples were collected aseptically in a sterile syringe from the demarcated site of catheterized patients, and swabs from surgical site infections (SSI) of patients were collected from deep incisions after skin antisepsis. The clinical specimens were collected under aseptic conditions and transferred immediately for culture and sensitivity during the period from March 2016 to February 2017. All Samples were collected aseptically after 48 hours admission in ICU; transferred immediately to be inoculated on routine culture media and incubated aerobically at 37°C for 24 hours. Identification and antimicrobial sensitivity of the isolated gram-negative bacilli were done by Vitek 2 system.
Antibiotic susceptibility testing
Gram-negative identification and antimicrobial susceptibility testing cards were used to determine the susceptibility of the isolates to different antimicrobial agents. The isolates were either susceptible, intermediate susceptible or resistant to the antimicrobials. Susceptibility testing was confirmed using the disc diffusion (modified Kirby Bauer) method for the following antibiotics (Oxoid, UK); Ampicillin (AMP 10 µg), Ampicillin/Sulbactam (SAM 20 µg), Amikacin (AK 30 µg), Aztreonam (ATM 30 µg), Cefazolin (CZ 30 µg , Cefepime (FEP 30 µg), Ceftriaxone (CRO 30 µg), Ciprofloxacin (CIP 5 µg), Ertapenem (ETP 10 µg), Meropenem (MEM 10 µg ), Gentamicin (CN 120 µg), Tobramycin (TM 10 µg), Moxifloxacin (MXF 5 µg), Nitrofurantoin (F 300 µg), Trimethoprim/ Sulphamethoxazole (SXT 25 µg), Tigecycline (TGC 15 µg), and Imipenem (IPM 10 µg). The results were inferred according to Clinical and Laboratory Standards Institute (CLSI) guidelines (CLSI, 2013).
Demonstration of Sewage E. coli phages
Samples of sewage water were collected in sterile dark containers from the sewage water treatment plant at Benha, Qalubiya Governorate, Egypt. The sample was first filtered through coarse filter paper to remove the debris, then the filtrate was serially diluted using 0.85% sterile saline, subsequently, the samples were filtered using 0.45 µm filters and stored at 4°C.
Isolation and propagation of E. coli phages
Fifty milliliters of filtered wastewater samples mixed with 50 ml of Luria Bertani (LB) broth inoculating with the log phase cells of E. coli. The culture media were incubated at 37°C for 24 h with speed of 120 rpm for bacteriophage enrichment. The media were centrifuged and filtered through a 0.45 µm membrane filter. The presence of lytic phage in the filtrate was examined by using the over layer method as described later.
One hundred µl of the filtrate was mixed with 300 µl of log phase culture of E. coli isolates and incubated at 37°C for 30 min. The mixture was added into a 3.5 ml of molten LB soft top agar (0.75% agar) which was already cooled down to 50°C, mixed gently and poured onto LB agar plate. The plates were left to stand at room temperature for 30 min to allow the top agar to solidify. The presence of lytic phages was distinguished according to the shape and size of plaques formed after incubation of the plates at 37°C for 24hr. Purification and propagation of E. coli phages were done by single plaque isolates according to (13).
Phage preparations
Stock lysates for the E. coli phages candidates were prepared by incubation of purified phage with the host in LB broth supplemented with CaCl2 (2 mM) for 24-48 h at 37°C with shaking, the cultures were centrifuged (6000 rpm, 10 min) then filter sterilized and the resulting crude lysate was stored at 4°C.
Phage tittering
Serial dilutions of the phages stock lysates were prepared and aliquots of these dilutions were incubated with the host at room temperature for 30 min. The incubated aliquots were added to molten LB soft agar and overlaid on an LB agar plate. Plates were incubated at 37°C for 24 h and the resulting plaques forming units (PFUs) were quantified to determine phage titer.
Spot test
The spot assay was used to assess the prevalence and the bactericide ability of the sewage water E. coli phages against all the 15 isolated E. coli strains and was repeated three times for each bacterium. Five microliters of 45 µm filter-sterilized purified phages were spotted onto the surface of the plates with the different E. coli strains. The plates were left to dry and were inspected for lysis zones after an overnight incubation at 37°C. A positive spot test appeared as complete obliteration of the entire drop area, whereas a negative spot test resulted in the bacterial lawn growing normally in the region of the spot. positive samples in the spot tests were confirmed by plaque assay14.
Transmission electron microscopy (TEM)
Ten microliters of the purified phage lysate with a high titer were spotted onto carbon-coated grids and stained with 1% uranyl acetate. Phages particles were observed under a JOEL-JM-100-C Transmission Electron Microscope (TEM) (Japan Electron Optics Laboratory Co., Ltd.) in the Regional Center of Mycology and Biotechnology at Al-Azhar University, Egypt.
E. coli isolation and antibiotic susceptibility profiling
In this work, fifteen E. coli were isolated from clinical samples. They were designated as E. coli U-1 to E. coli U- 4, E. coli R-1, E. coli SI-1 to E. coli SI-10 as they were isolated from the urine samples, respiratory infections and surgical site infections, respectively. All strains were resistant to at least one antibiotic (ampicillin). Thirteen strains were also resistant to Ampicillin/Sulbactam, Cefazolin, and Ceftriaxone. Except for two strains, no resistance to Amikacin was observed. In addition, all strains were sensitive to Meropenem except for two strains. Nine strains were resistant to Aztreonam and six strains resist to gentamycin. One strain was resistant to Ertapenem and Imipenem while nine and twelve strains resisted to tobramycin and Ciprofloxacin, respectively. Furthermore, nine strains were resistant to Trimethoprim/ Sulphamethoxazole and out of six strains investigated for Tigecycline, no resistance was established. The antibiotic resistance patterns of all strains were summarized in Table 1.
Table (1):
Antibiotic resistance patterns of clinical E. coli isolates.
Sample source |
Isolate |
øEU-3 |
øEU-4 |
Two phages cocktail |
|---|---|---|---|---|
U |
E. coli U-1 |
– |
– |
– |
U |
E. coli U-2 |
– |
– |
– |
U |
E. coli U-3 |
+ |
+ |
+ |
U |
E. coli U-4 |
+ |
+ |
+ |
R |
E. coli R-1 |
– |
– |
– |
SSI |
E. coli SI-1 |
– |
– |
– |
SSI |
E. coli SI-2 |
– |
– |
– |
SSI |
E. coli SI-3 |
– |
– |
– |
SSI |
E. coli SI-4 |
– |
– |
– |
SSI |
E. coli SI-5 |
– |
– |
– |
SSI |
E. coli SI-6 |
– |
– |
– |
SSI |
E. coli SI-7 |
– |
– |
– |
SSI |
E. coli SI-8 |
– |
– |
– |
SSI |
E. coli SI-9 |
– |
– |
– |
SSI |
E. coli SI-10 |
– |
– |
– |
– denotes for resistant, +denotes for susceptible
Bacteriophage susceptibility
To investigate the prevalence of bacteriophages in municipal sewage water against the previously isolated bacteria, a spot technique was performed, and the results were summarized in Table 2. Out of the fifteen isolated E. coli strains, only two were shown to be sensitive to sewage bacteriophages, E. coli U-3 and E. coli U-4, as judged by spot test and later by plaque assay. The spot technique served as both an indicative experiment of bacteriophages presence and gave an idea of the host range of the resulted phages (data not shown), single isolated phages, as well as mixed cocktails of the two phages, were used in the preceding experiments. The accumulated data revealed a narrow-host-range of the isolated phages against the fifteen isolates. Single plaques were picked from the plates with visible clear plaques, from E. coli U-3 and E. coli U-4 lawns, the plaques were then propagated in liquid cultures. The previous phages, named øEU-3 and øEU-4 phages, were selected for imaging with transmission electron microscope.
Table (2):
In vitro susceptibility of different E. coli strains against sewage phages using spot assay..
Sources of specimens |
Isolate |
AMP |
SAM |
CZ |
CRO |
FEP |
ATM |
ETP |
IPM |
MEM |
AK |
CN |
TM |
CIP |
MXF |
TGC |
F |
SXT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
U |
E. coli U-1 |
R* |
R |
R |
R |
R |
R |
S** |
S |
S |
S |
S |
S |
S |
S |
S |
S |
S |
U |
E. coli U-2 |
R |
R |
R |
R |
I† |
I |
S |
S |
S |
S |
R |
R |
R |
R |
S |
S |
R |
U |
E. coli U-3 |
R |
NA |
NA |
I |
NA |
NA |
NA |
NA |
NA |
R |
NA |
NA |
R |
NA |
NA |
NA |
NA |
U |
E. coli U-4 |
R |
NA |
NA |
R |
NA |
NA |
NA |
NA |
NA |
R |
NA |
NA |
R |
NA |
NA |
NA |
NA |
R |
E. coli R-1 |
R |
R |
R |
R |
R |
R |
S |
S |
S |
S |
S |
S |
R |
R |
S |
I |
R |
SSI |
E. coli SI-1 |
R |
R |
R |
R |
R |
R |
R |
R |
R |
S |
S |
R |
R |
R |
S |
R |
S |
SSI |
E. coli SI-2 |
R |
R |
R |
R |
R |
NA†† |
NA |
NA |
S |
S |
R |
R |
R |
NA |
NA |
S |
R |
SSI |
E. coli SI-3 |
R |
R |
R |
R |
S |
NA |
NA |
NA |
S |
S |
R |
R |
R |
NA |
NA |
R |
R |
SSI |
E. coli SI-4 |
R |
R |
R |
R |
R |
R |
S |
S |
S |
S |
S |
R |
R |
R |
S |
S |
S |
SSI |
E. coli SI-5 |
R |
R |
R |
R |
R |
NA |
NA |
NA |
S |
S |
S |
S |
S |
NA |
NA |
S |
R |
SSI |
E. coli SI-6 |
R |
R |
R |
R |
R |
NA |
NA |
NA |
S |
S |
R |
R |
R |
NA |
NA |
NA |
R |
SSI |
E. coli SI-7 |
R |
R |
R |
R |
S |
I |
S |
S |
S |
S |
S |
S |
S |
S |
S |
S |
R |
SSI |
E. coli SI-8 |
R |
R |
R |
R |
R |
NA |
NA |
NA |
R |
S |
S |
R |
R |
NA |
NA |
S |
S |
SSI |
E. coli SI-9 |
R |
R |
R |
R |
R |
NA |
NA |
NA |
S |
S |
R |
R |
R |
NA |
NA |
NA |
R |
SSI |
E. coli SI-10 |
R |
R |
R |
R |
R |
NA |
NA |
NA |
S |
S |
R |
R |
R |
NA |
NA |
S |
R |
*denotes for Resistant, **denotes for Susceptible
† Intermediate †† Not tested
Morphology and diameters of øEU-3 and øEU-4 phages are shown in Fig.1 and Table 3. Electron micrographs of øEU-3phage showed an icosahedral head with a diameter of ~71.42 nm and a 214.28 nm long non-contractile tail (a character of Siphoviridae phages). Micrographs of øEU-4 phage showed that it has an icosahedral head with a diameter of ~66.6 nm and a 108.3 nm long contractile tail (a character of Myovirdae phages).
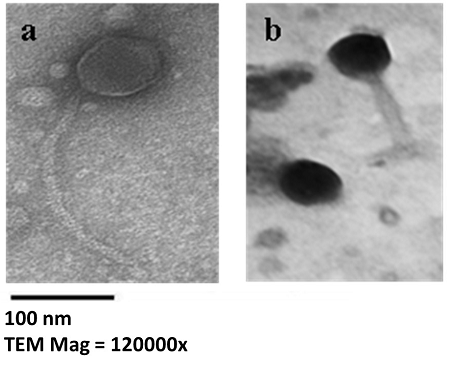
Fig. 1. Electron Micrographs of the isolate phages after negative staining of viral particles. (a) øEU-3 phage. (b) øEU-4 phage. The size bar corresponds to 100 nm.
Table (3):
Dimensions and titration of the isolated phages.
Phage (PFU/ml) |
Prospected Family |
Head diameter (nm) |
Tail length (nm) |
Phage titer |
|---|---|---|---|---|
øEU-3 phage |
Siphoviridae |
71.42 |
214.28 |
2 x 107 |
øEU-4 phage |
Myoviridae |
66.6 |
108.3 |
2 x 105 |
Over the last decades, the prevalence of multidrug-resistant bacteria, particularly E. coli has gained a great interest due to their increasing rates on a daily basis15. The occurrence of extended-spectrum Beta-lactamase producing E. coli could be nearly 63% in many countries while the highest rates belong to E. coli from ICU patients16, 17. Our work indicated highly drug-resistant E. coli could be present in the ICU. Many E. coli isolates showed resistance to at least five antibiotics. In previous studies, high resistance to antibiotics has been described for E. coli isolated from UTI samples18. Moreover, high frequency of E. coli resistance to multiple antibiotics such as penicillin, co-trimoxazole, and nitrofurantoin has been documented19. In another study, 170 (43%) of E. coli isolates were multidrug resistant and showed high resistance to Ampicillin (82.53%), Amoxycillin-clavulanic acid (71.90%), Ceftriaxone (66.58%), Ciprofloxacin (65.82%)20. It was indicated that of total 328 strains of E. coli, 199 strains exhibited higher resistance rate to ampicillin, tetracycline, trimethoprim/sulfamethoxazole and cefazolin, and 34.6% of these E. coli strains exhibited resistance to at least four antibiotics. Three strains were resistant to seven antibiotics in the same study. The treatment of multidrug-resistant pathogens continues to be a challenging problem. Sustainable and effective alternative approaches have become a necessity. One of the most popular approaches is employing bacteriophages used as anti-infective agents to circumvent antibiotic resistance.
Phage therapy has various merits over antibiotics: (1) bacteriolysis mechanisms by phages differ from antibiotics mechanisms (21) so, it is very effective against antibiotic-resistant bacteria; (2) bacteriophages are very specific so, they will be harmless to other beneficial bacteria in human guts; (3) Phages undergo mutations so, they can respond quickly to phage-resistant mutants; (4) ease of isolation; (5) no side effects are known so far; (6) high therapeutic index, smaller effective dose, and a single shot is required; (7) production of phages are very quick and cheap as compared to development of a new antibiotic22, 23. The major obstacles against phage therapy are legislation which might take years to be approved. Currently, Russia, Poland, Georgia, China and U.S.A are using bacteriophages to treat systemic and enteric diseases that do not respond to conventional antibiotics. Bacteriophages are ubiquitous in different watersheds throughout the world and proposed to be most copious biological objects in the biosphere24, 25. Bacteriophages existed since the bacterial cells originated, wherever the bacteria are found, bacteriophages were found associated. In current study, we succeeded to isolate lytic bacteriophages, from sewage water, against two of the preceding E. coli isolates. Likewise our study, many researchers have isolated phages against E. coli from sewage26. The morphology of isolated E. coli phages, designated bacteriophages øEU-3 and øEU-4, showed that they belong to the family Siphoviridae and Myoviridae, respectively. Similar study reported isolation of E. coli siphophages from stool of pediatric patients with a complaint of diarrhea27 and others indicated in healthy subjects, as lambda-like Siphoviridae phages were mainly isolated from stool samples of healthy persons28, 29 while stools of diarrhea patients gave predominantly T4-like Myoviridae. But when using different indicator cells, different phages were also isolated from the same stool samples27. Our results showed that the two isolated phages (øEU-3 and øEU-4) were investigated for their potential use as antimicrobial agents against the isolated multidrug resistant clinical E. coli, whereas, E. coli U-3, E. coli U-4 isolates were susceptible to phages cocktail infection, but the rest of studied isolates revealed different patterns of resistance to antibiotics as well as phage cocktail which displayed a narrow host range.
This study provided evidence that E. coli isolated from clinical specimens although being sensitive to certain antibiotics, they exhibited resistance to a wide range of other drugs. In addition, the use of lytic bacteriophage cocktail as antimicrobial agent against the isolated E. coli strains revealed the efficacy of the two isolated phages to control two out of fifteen strains used in this study and paved the way for future studies to focus on expanding their antimicrobial spectra by combining them with other antimicrobials and/or newly isolated phages.
ACKNOWLEDGMENTS
Funding for this study was partially supported by a grant from the Scientific Research Fund (SRF) from Benha University (A.G.A), other funds were from the Egyptian Ministry of Higher Education and Scientific Research.
- Cabal A, García-Castillo M, Cantón R, Gortázar C, Domínguez L, Álvarez J. Prevalence of Escherichia coli virulence genes in patients with diarrhea and a subpopulation of healthy volunteers in Madrid, Spain. Front Microbiol, 2016; 7.
- Mora A, Herrera A, López C, Dahbi G, Mamani R, Pita JM, Alonso MP, Llovo J, Bernárdez MI, Blanco JE, Blanco M, Blanco J. Characteristics of the Shiga-toxin-producing enteroaggregative Escherichia coli O104:H4 German outbreak strain and of STEC strains isolated in Spain. Int Microbiol, 2011; 14: 121–141.
- Agus A, Massier S, Darfeuille-Michaud A, Billard E, Barnich N. Understanding host-adherent-invasive Escherichia coli interaction in Crohn’s disease: Opening up new therapeutic strategies. Biomed Res Int 2014.
- Conte M, Longhi C, Marazzato M, Conte A, Aleandri M, Lepanto M, Zagaglia C, Nicoletti M, Aloi M, Totino V, Palamara A, Schippa S. Adherent-invasive Escherichia coli (AIEC) in pediatric Crohn’s disease patients: phenotypic and genetic pathogenic features. BMC Res Notes, 2014; 7: 748.
- Hawser SP, Bouchillon SK, Hoban DJ, Badal RE, Hsueh PR, Paterson DL. Emergence of high levels of extended-spectrum-â-lactamase-producing gram-negative bacilli in the Asia-Pacific region: Data from the Study for Monitoring Antimicrobial Resistance Trends (SMART) program, 2007. Antimicrob Agents Chemother, 2009; 53: 3280–3284.
- Schwaber MJ, Carmeli Y. Carbapenem-resistant Enterobacteriaceae: a potential threat. JAMA, 2008; 300: 2911–2913.
- Hasman H, Hammerum AM, Hansen F, Hendriksen RS, Olesen B, Agersø Y, Zankari E, Leekitcharoenphon P, Stegger M, Kaas RS, Cavaco LM, Hansen DS, Aarestrup FM, Skov RL. Detection of mcr-1 encoding plasmid-mediated colistin-resistant escherichia coli isolates from human bloodstream infection and imported chicken meat, denmark 2015. Eurosurveillance, 2015; 20: 1–5.
- Abedon ST, Kuhl SJ, Blasdel BG, Kutter EM. Phage treatment of human infections. Bacteriophage 2011.
- Shasha SM, Sharon N, Inbar M. Bacteriophages as antibacterial agents. Harefuah, 2004; 143: 121–5, 166.
- Peitzman SJ (Steven J. Felix d’Herelle and the Origins of Molecular Biology (review). Bull Hist Med, 2001; 75: 159–161.
- Merril CR, Scholl D, Adhya SL. The prospect for bacteriophage therapy in Western medicine. Nat Rev Drug Discov 2003.
- Khalifa L, Shlezinger M, Beyth S, Houri-Haddad Y, Coppenhagen-Glazer S, Beyth N, Hazan R. Phage therapy against Enterococcus faecalis in dental root canals. J Oral Microbiol 2016.
- Askora A, Merwad A, Gharieb RM, Maysa AIA. A lytic bacteriophage as a biocontrol for some enteropathogenic and enterohemorrhagic Escherichia coli strains of zoonotic risk in Egypt. Rev Med Vet (Toulouse), 2015; 166: 76–83.
- Baer A, Kehn-Hall K. Viral concentration determination through plaque assays: using traditional and novel overlay systems. J Vis Exp, 2014; e52065.
- Paterson DL, Bonomo RA. Extended-Spectrum beta-Lactamases/ : a Clinical Update. Clin Microbiol Rev, 2005; 18: 657–686.
- Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect, 2012; 18: 268–281.
- Nakai H, Hagihara M, Kato H, Hirai J, Nishiyama N, Koizumi Y, Sakanashi D, Suematsu H, Yamagishi Y, Mikamo H. Prevalence and risk factors of infections caused by extended-spectrum â-lactamase (ESBL)-producing Enterobacteriaceae. J Infect Chemother, 2016; 22: 319–326.
- Murugan T, Dereje Y, Tamiru A. Antibiotic resistant pattern of urinary tract infection causing Escherichia coli isolated from diabetic mellitus and non-diabetic mellitus patients with special reference to Rifampicin resistance. Int J Curr Microbiol Appl Sci, 2014; 3: 668–674.
- Mubita C, Syakalima M, Chisenga C, Munyeme M, Bwalya M, Chifumpa G, Hang’ombe BM, Sinkala P, Simuunza M, Fukushi H, Isogai H, Yasuda J, Isogai E. Antibiograms of faecal Escherichia coli and Enterococci species isolated from pastoralist cattle in the interface areas of the Kafue basin in Zambia – Short communication., 2008; Vet Arh 78:179–185.
- Kulkarni S, Peerapur B, Sailesh K. Isolation and antibiotic susceptibility pattern of Escherichia coli from urinary tract infections in a tertiary care hospital of North Eastern Karnataka. J Nat Sci Biol Med, 2017; 8: 176.
- Matsuzaki S, Rashel M, Uchiyama J, Sakurai S, Ujihara T, Kuroda M, Imai S, Ikeuchi M, Tani T, Fujieda M, Wakiguchi H. Bacteriophage therapy: a revitalized therapy against bacterial infectious diseases. J Infect Chemother, 2005; 11: 211–219.
- Pirnay JP, De Vos D, Verbeken G, Merabishvili M, Chanishvili N, Vaneechoutte M, Zizi M, Laire G, Lavigne R, Huys I, Van Den Mooter G, Buckling A, Debarbieux L, Pouillot F, Azeredo J, Kutter E, Dublanchet A, Górski A, Adamia R. The phage therapy paradigm: Prêt-à-porter or sur-mesure? Pharm Res 2011.
- Merabishvili M, de Vos D, Verbeken G, Kropinski AM, Vandenheuvel D, Lavigne R, Wattiau P, Mast J, Ragimbeau C, Mossong J, Scheres J, Chanishvili N, Vaneechoutte M, Pirnay JP. Selection and Characterization of a Candidate Therapeutic Bacteriophage That Lyses the Escherichia coli O104:H4 Strain from the 2011 Outbreak in Germany. PLoS One, 2012; 7: e52709.
- Bergh Ø, BØrsheim KY, Bratbak G, Heldal M. High abundance of viruses found in aquatic environments. Nature, 1989; 340: 467–468.
- Fuhrman JA. Marine viruses and their biogeochemical and ecological effects. Nature, 1999; 399: 541–548.
- Ruchi T, Hirpurkar SD, Shakya S. Isolation and characterization of lytic phage from natural waste material of livestock. indian Vet J, 2010; 87: 644–646.
- Chibani-Chennou S, Sidoti J, Bruttin A, Kutter E, Bru H. In Vitro and In Vivo Bacteriolytic Activities of. Antimicrob Agents Chemother, 2004; 48: 2558–2569.
- Dhillon TS, Dhillon EK, Chau HC, Li WK, Tsang AH. Studies on bacteriophage distribution: virulent and temperate bacteriophage content of mammalian feces. Appl Environ Microbiol, 1976; 32: 68–74.
- Furuse K, Osawa S, Kawashiro J, Tanaka R, Ozawa A, Sawamura S, Yanagawa Y, Nagao T, Watanabe I. Bacteriophage distribution in human faeces: Continuous survey of healthy subjects and patients with internal and leukaemic diseases. J Gen Virol, 1983; 64: 2039–2043.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


