ISSN: 0973-7510
E-ISSN: 2581-690X
The coronavirus disease of 2019 (COVID-19) emerged as a pandemic at the end of 2019. The clinical presentation of COVID-19 was comparable to bacterial infections, and due to the ambiguity of an effective treatment method, the healthcare professionals routinely used antibiotics to treat the patients. So, this study evaluated the antibiotic usage patterns at our hospitals among COVID-19 patients, considering the World Health Organization (WHO) AWaRe (Access, Watch, and Reserve) classification. The present study was a retrospective observational hospital record-based study on COVID-19 patients admitted from March-August 2020. A total of 256 COVID-19 patients were enrolled, considering inclusion and exclusion criteria. Data collection utilizing a standardized case record form to capture all information methodically based on age, sex, comorbidities, duration, and nature of the symptoms. Drug data, for example antibiotic usage patterns were collected with detailed prescription analysis, including route, dose, frequency and number of antibiotics used. A structured proforma was used to analyze data, and descriptive statistical analysis was performed and presented as Histograms. The WHO AWaRe antibiotic classification was used to determine the antibiotics’ suitability for empirical therapy. The mean age of the participants was 40.00 ± 15.46 years with male predominance, 188 (73.43%) in number, compared to females, with 68 (26.56%). Almost all the current study participants received one or more antibiotics during their hospital stay, and a maximum of the patients 126 (49.22%) received 2 antibiotics during treatment, 78 (30.47%) subjects who were managed with 3 antibiotics, and the rest, 52 (20.31%) participants received only 1 antibiotic during their hospital stay. To treat moderate to severely ill patients, the most used antibiotic was doxycycline, followed by meropenem and piperacillin/tazobactam. The findings of this study will provide baseline data that will help to generate strategies to reduce antibiotic prescriptions for the formulation of better antibiotic stewardship programs (ASP) and institutional policies to fight against any infection in a better way in the near future.
Antimicrobial Agent, Coronavirus Disease, COVID-19, SARS-CoV-2, Respiratory Infection, WHO AWaRe
Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) emerged as a pandemic at the end of 2019, and recently, the healthcare system of India has recovered from its associated challenges. In India, 44.9 million people had affected with COVID-19 infection as of May 2023, and 0.53 million people have died due to COVID-19 infection as of May 2022.1 The clinical picture of COVID-19 patients could be the same as that of bacterial infections like fever, acute onset and raised inflammatory markers, etc.2 Until now, there is no definite successful treatment protocol for SARS-CoV-2. Thus, clinical indecisiveness and the absence of any definite treatment protocol for SARS-CoV-2 have resulted in extensive early antibiotic use during the pandemic. World Health Organization (WHO), in May 2020, presented the first guidelines to fight COVID-19. However, the diverse nature of the virus, risk factors, health policies, environmental and socioeconomic conditions, and immunity of people affected worldwide led to national guidelines against this new infection. The GOI (Government of India), in the “Guidelines for the management of co-infection of COVID-19 with other seasonal epidemic-prone diseases,” recommended the prophylactic use of empiric antibiotic therapy based on the native antibiogram merely in the secondary bacterial infection cases.3,4 Though antibiotics are not the preferred choice for viral diseases, using antibiotics as prophylaxis is a widespread practice to avoid any secondary bacterial infection in high-risk patients.5 So, in the hospital settings during a pandemic, a huge rise in the usage of antibiotics was seen.6-8 Various studies have documented that during hospital stays of COVID-19 patients, around 70% were prescribed antibiotics3,9 due to bacterial co-infection concerns. However, on a thorough review of the data, it was seen that only 6% of them had confirmed bacterial co-infection.10,11 Since the onset, there exists a debate about the incidence of viral and bacterial co-infection in COVID-19 patients, as few studies have reported an incidence of <4%-8%.12-14 In other studies, globally it has been reported upto 28% in various countries worldwide, especially in Europe.15-17
The most frequently prescribed antibiotics were macrolides, tetracyclines, cephalosporins, etc. Due to misuse of antibiotics, WHO 2022 global antimicrobial resistance & use surveillance system (GLASS) report highlighted many pathogenic bacteria have developed resistance against them.18-20 So, it is anticipated that overuse of antibiotics during COVID-19 must have noticeably added to the global antimicrobial resistance (AMR) burden. Globally, the monetary cost of AMR in GDP loss is predicted to be 3 trillion US dollars.19 So, the World Health Organization (WHO) has recognized AMR as one of the first 10 threats to worldwide public health.20 However, empiric treatment focused on wide coverage of various microbes could result in limited growth of drug-resistant bacteria. So, WHO 2017 proposed AWaRe (Access, Watch, Reserve), a new technique for antibiotic classification to enhance effective antibiotic usage and avoid overprescription21 and AMR burden.22 AWaRe has a list of 180 antimicrobial agents (AMAs), which are further classified based on their potential to develop resistance into “Access, Watch, and Reserve” groups in 2019 to decrease the risk of AMR, WHO declared that out of total AMAs used by a country, at least 60% must be from access group.23 However, it is unclear how strictly these restrictions are adhered to. Another main aspect guiding clinicians in selecting appropriate AMA is an Institute’s local antibiotic policy, which is responsible for the rational use of AMAs. Hence, studies should focus more on collecting data from different COVID-19-dedicated hospitals regarding antibiotic usage patterns to spot the challenges in rational antibiotic use. In countries like India, the task is more difficult as there is a paucity of information on antibiotic use among hospitalized COVID-19 patients in many tertiary care hospitals.24 Moreover, only a few point prevalence surveys (PPSs) have been conducted to evaluate antibiotic consumption patterns, with the maximum PPSs being before COVID-19.25,26 So, the current research was conducted to evaluate the antibiotic usage patterns at our hospital during COVID-19, considering the WHO’s AWaRe classification. This research can help the Institute to improve its native antibiotic stewardship program (ASP) along with the formulation of the institution’s antimicrobial policy’ shortly.
The present study was a retrospective observational record-based study conducted by collecting data on COVID-19 patients hospitalized between March-August 2020 at SDM College of Medical Sciences and Hospital, Karnataka, India. The study aimed to examine antibiotic consumption patterns in COVID-19 patients and evaluate the appropriateness of antibiotics used for empirical therapy using the WHO AWaRe (Access, Watch, and Reserve) antibiotic list. A total of 256 COVID-19 patients aged > 18 years of either sex were enrolled in the study after obtaining ethical clearance from the Institute Ethics Committee ref -SDMIEC2021/149.
Patients under 18 years and with insufficient data entry in the medical records were excluded from the study. We have used convenience sampling technique based on the duration that is the 6 months of data on the patients treated for COVID-19 infection meeting inclusion criteria were collected retrospectively from the research hospital’s medical records division, utilizing a standardized case record form to capture all information methodically. The case record proforma contains baseline demographic data such as age, sex, suspected environment of acquisition, comorbidities, duration, and nature of the symptoms. Severity markers were included like Glasgow Coma Scale (GCS) and vital signs like respiratory rate per minute (RR); heart rate per minute (HR); maximum temperature in Celsius scale (Tmax); systolic and diastolic blood pressure in mmHg (SBP/DBP) and oxygen saturation (SpO2). Drug data and antibiotic usage pattern, was collected with detailed prescription analysis, including route, dose, frequency, duration, and number of antibiotics used. Antibiotic usage was accessed using the WHO AWaRe antibiotic list, which determines the appropriateness of antibiotics used for empirical therapy. A structured proforma was used to analyze data, and descriptive statistical analysis was performed. Data was expressed as means and percentages. Strict confidentiality was maintained with the collected data.
The present study was conducted by collecting data from 256 COVID-19 patients hospitalized in the concerned hospital during the study period. The mean age of the participants was 40.00 ± 15.46 years. The current study had a predominance of males, with 188 (73.43%) in number, and females, with 68 (26.56%) in number. Table 1 highlighted the characteristics of the patients based on their hospital stay. The average length of hospital stay of the participants was observed to be around 7 days (± 2 days). Out of all the patients, 36 (14.06%) were critical and were on ventilators. The rest of the patients, i.e., 220 (85.93%), were stable and were under treatment in different wards. Most current study participants received one or more antibiotics during their hospital stay. During treatment, maximum 126 patients (49.22%) received 2 antibiotics, followed by 78 (30.47%) subjects who were managed with ≥3 antibiotics, and the rest, 52 (20.31%) participants received only 1 antibiotic during their hospital stay.
Table (1):
Fundamental questions and Characteristics feature of the patients based on data collection form where number of patients (n) having antibiotics is shown as %
| Parameter | Mean ± SD or n (%) | |
|---|---|---|
| Mean age | 40.00 ± 15.46 | |
| Sex | Males | 188 (73.43) |
| Females | 68 (26.56) | |
| Average length of hospital stays | 7 days ± 2 days | |
| Number of patients on ventilator | 36 (14.06) | |
| Number of patients treated with antibiotics | 256 (100) | |
| Patients treated with | 1 antibiotic | 52 (20.31) |
| 2 antibiotics | 126 (49.22) | |
| ≥3 antibiotics | 78 (30.47) | |
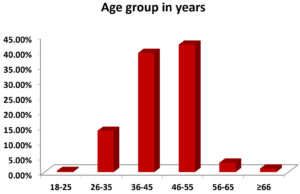
Further, patients were divided into different age groups. The maximum patients were from the age group 46-55 years, followed by 36-45 years, 26-35 years, 56-65 years, ≥66 years, and 18-25 years, with 108 (42.18%), 101 (39.43%), 35 (13.67%), 8 (3.12%), 3 (1.17%) and 1 (0.39%) patient respectively (Figure 1).
Figure 1. Age group distribution of the COVID-19 patient’s vs percentage histogram. 36-55 age group is at the highest risk of getting COVID-19 infection
Table 2 highlighted the access group of WHO’s AWaRe list and showed the maximum use of Doxycycline by 179 (69.92 %) patients. Table 3 highlights the appropriateness of taking antibiotics by COVID-19 patients.
Table (2):
Pattern of antibiotics used based on WHO AWaRe classification and distribution of patients (%) based on the antibiotic usage by ‘n’ number of patients
| WHO AWaRe classification | Antibiotic used | n (%) |
|---|---|---|
| Access Group | Doxycycline | 179 (69.92) |
| Watch Group | Piperacillin/Tazobactam, | 140 (54.68) |
| Ceftriaxone | 25 (9.76) | |
| Meropenem | 149 (58.20) | |
| Reserve group | Linezolid | 10 (3.90) |
| Colistin | 41 (16.01) | |
| Tigecycline | 52 (20.31) |
Table (3):
Analysis of appropriateness of the antibiotic used in all patients
Antibiotics |
Appropriate |
Non-appropriate |
|---|---|---|
Doxycycline |
150 |
29 |
Piperacillin tazobactam |
83 |
57 |
Ceftriaxone |
8 |
17 |
Meropenem |
96 |
53 |
Linezolid |
8 |
2 |
Colistin |
39 |
2 |
Tigecycline |
49 |
3 |
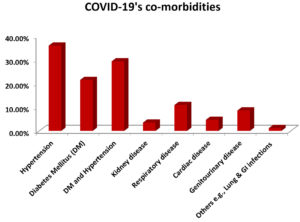
In the current study, the hospitalized COVID patients presented a few associated co-morbidities, The most common co-morbidity with the patients was hypertension, DM with hypertension, diabetes mellitus (DM), respiratory disease, genitourinary disease, cardiac disease, kidney disease and other diseases with 92 (35.93%), 75 (29.29%), 55 (21.48%), 28 (10.93%), 22 (8.59%), 12 (4.68%), 9 (3.51%) and 3 (1.17%) subjects respectively (Figure 2).
The current research evaluated the antibiotic usage patterns at our hospital during COVID-19, considering the WHO’s AWaRe classification. Data from a total of 256 COVID-19 patients were taken in the study. The mean age of the participants in our study was much lower than the study by Kashyap et al.27 The reason could be the study population as they researched COVID patients admitted to ICU (Intensive care unit). As described earlier, it was a mixed population of COVID-19 patients. Out of all the patients, 36 (14.06%) were critical and were on ventilators. The rest of the patients, i.e., 220 (85.93%), were stable and were under treatment in different wards. However, only 36 (14.06%) patients were critical (on ventilators), and the antibiotic prescriptions were 100% in our study, which means all COVID-19 patients get at least one antibiotic.
Our study had a predominance of males, which is strongly supported by the findings of previous studies.27-29 However, a study by Al-Nuaimi et al. showed female dominance.30 The participants’ average length of hospital stay (LOS) was around seven days, which aligns with the study’s findings.28 Mechanical ventilation they observed during this period was slightly higher than in our study. The COVID patients, while hospitalized, were presented with few associated co-morbidities, and the most common co-morbidities were hypertension and diabetes mellitus (DM), which is similar to the study done by Kashyap A et al. A Singapore study reported more likely antibiotic usage in cases presented with associated co-morbidities. DM patients received more antibiotics due to some antibiotic resistance genes in them.31 In the current study, most patients received two antibiotics during treatment, which agrees with the previous findings.29 All the patients were managed by at least one antibiotic,32 and an average number of 4 antibiotics were given.27 The possible reason behind this variation could be the severity of the disease and co-morbidities associated with the COVID-19 patients. The percentage found in our study is nearly like the findings earlier, which reported the use of combination antibiotics in around 45% of cases.33 Further, patients were divided into different age groups, and most of the patients in our study were adults in the age group 46-55 years. This outcome is in concurrence with the previous studies29,30 as they also found adults of the same age group in the maximum number in their studies.
During the first COVID- wave, a strict lockdown was imposed in India, and patients with mild symptoms were strictly forced to be in home isolation. The patients with moderate or severe symptoms were admitted to COVID-19-dedicated hospitals to start their immediate medical care. So, the antibiotic prescription rates of the COVID-dedicated hospitals were relatively high. The present study has taken the data of the patients hospitalized during the peak transmission period, due to which almost every patient has received one or the other antibiotic upon admission. So, without a suitable control group, marking antibiotic usage as high or low would be indecisive. During the first wave of COVID-19, India was a developing and overpopulated country, and its healthcare system was overburdened. So, prescribing antibiotics based on bacteriological identification was impossible, particularly during the pandemic. Secondly, our center, the tertiary care hospital, received many patients already on antibiotics prescribed in the referred centers. Earlier studies supported our hypothesis by stating a few factors restricting the appropriate implementation of ASP during the pandemic in India.34 Another study also agrees with our results as they documented lower antibiotic prescription rates, 63.1 to 64.8% in developed countries during the COVID-19 pandemic compared to Southeast Asian developing countries with 87.5 % antibiotic prescription rates.9 Other studies have documented a wide range of 70-95% antibiotic prescription rates.3,34-36 Whereas, other studies reported meager antibiotic prescription rates, like 38% in Scotland.37 and as low as 6.2% in Singapore.38 The wide variation in antibiotic usage in different countries could be due to differences in their healthcare system, study population, social health determinants, degree of infection, hospital prescription policies, and local pharmaceutical companies.24,39-40 Studies documented a biphasic antibiotic usage pattern during pandemic wave 1 from March to April 2020.8,37 As Wave 1 was over, due to globally accumulated experience, local learning, and knowledge, the use of antibiotics drastically dropped during Wave 2 and Wave 3 without any dramatic change in in-hospital mortality.28 Research also reported a decline in antibiotic usage during the second wave compared to the first wave.41 China, during wave 1, reported 95% antibiotic prescription rates35,36 compared to other geographic areas with 70%.3,42-43 Furthermore, in March 2020, at the beginning of the pandemic, the guidelines given by WHO recommended antibiotic usage in COVID-19 patients presented with sepsis,44 and these guidelines were modified later when the clinical picture of COVID became clear. The antibiotics used to treat COVID-19 patients in the present study were influenced by the local antibiotic stewardship program (ASP) and the Institution’s antimicrobial policy, along with consideration of WHO AWaRe classification. Also, our study’s ward or ICU patients were managed with one or more antibiotics during their hospital stay based on the severity of the disease. The severity of the disease was assessed by clinically correlating the symptoms and laboratory parameters.
The antibiotics in our study were prescribed by considering the ASP, local antibiogram, co-morbidities, immune status of the patient, other clinical conditions, and severity of the disease. Table 2 shows the pattern of antibiotics used in the current study based on WHO AWaRe classification. The antibiotic used from the Access group of AWaRe was doxycycline, and the antibiotics from the Watch group were piperacillin/tazobactam, ceftriaxone, and meropenem. The remaining antibiotics, like linezolid, colistin, and tigecycline, were in the reserve group. The maximum patients, 179 (69.92%) in the present study, were given doxycycline in the access group, followed by meropenem and piperacillin/tazobactam in the watch group with 149 (58.20%) and 140 (54.68%) patients respectively. Tigecycline and colistin of the reserve group were given to 52 (20.31%) and 41 (16.01%) cases, respectively. Further, ceftriaxone of the watch group was administered to 25 (9.76%) subjects and linezolid to the reserve group to 10 (3.90%) patients. Table 3 depicts the maximum number of patients who received antibiotics from the access and watch group of WHO AWaRe classification of antibiotics.
At our tertiary care hospital, maximal doxycycline was given to the patients, followed by meropenem and piperacillin/tazobactam. Tigecycline and colistin were used moderately, whereas ceftriaxone and linezolid were sparsely given to the patients. The reason behind prescribing these injectables was that the hospitalized patients had moderate or severe COVID-19 disease with many cases associated with one or another co-morbidity, and injectable antibiotics are typically given to such patients to avoid any further deterioration in their condition.45 Doxycycline of the access group was given to 69.92% of the patients in our study, firmly in concurrence with prescribed doxycycline in 61% of the cases.27 Further, meropenem of Watch group was another antibiotic that was extensively prescribed in the present study, and the reason could be the version 7.00 guidelines given by the ‘Ministry of Health and Family Welfare (MOHFW)’ which have recommended the use of meropenem for the clinical management of severely ill COVID-19 cases. In the current study, the use of meropenem was followed by piperacillin/tazobactam, which is in harmony with 2020 research in Brazil and another study.27 Findings of the present study contrast with a study by Mustafa et al. as they sparingly used meropenem in their patients and instead used imipenem followed by ceftriaxone and fluoroquinolones.46 Saxena et al. also documented using beta-lactams and metronidazole in their patients.47 However, in our study, ceftriaxone and linezolid were rarely used. In the current study, colistin and tigecycline were used in moderation. However, in another study, colistin and polymyxin B were used.27 Comorbidities and sociodemographic factors influence COVID-19 patient mortality. The factors that were most obviously linked to a higher risk of death were being old age with a weak immune system, living in an urban region, and having diabetes and/or hypertension. The mortality risk is even enhanced with Infections or other diseased conditions like Kidney, Liver, and Lung, as highlighted in Figure 2. A recent study from Spain highlighted Sociodemographic determinants of intraurban variations in COVID-19 incidence.48
The current research was conducted retrospectively to evaluate the antibiotic usage patterns at our hospitals during the first wave of COVID-19, considering the WHO’s AWaRe classification. The study showed comparatively extensive use of antibiotics like doxycycline followed by meropenem and piperacillin/tazobactam in hospitalized patients. The reason could be the lack of prior information about the transmission, pathophysiology, mortality rate, and prognosis of the COVID-19 disease. So, to save the patients, the clinicians highly prescribed the recommended antibiotics by WHO and MOHFW during wave 1. The outcomes of our study imitate the impact of a pandemic on the quantity and quality of antibiotics prescribed in tertiary care hospitals. Hence, the findings of our current study will provide baseline data to our institute and other healthcare centers in India and help them generate strategies to reduce antibiotic prescription for the formulation of better ASP and Institutional policies to fight against any infection in a better way in the near future.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
GH conceptualized and designed the study. HH performed data collection. SH and RVN performed data analysis. RVN performed results interpretation. SH wrote the manuscript. HH reviewed the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Institutional Ethics Committee, SDM College of Medical Sciences & Hospital, Karnataka, India, with reference number SDMIEC2021/149.
- India COVID – Coronavirus Statistics – Worldometer. Available from: https://www.worldometers.info/coronavirus/country/India [Accessed June 16, 2023]
- Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of Coronavirus Disease 2019 in China. N Eng J Med. 2020;382(18):1708-1720.
Crossref - Rose AN, Baggs J, Wolford H, et al. Trends in Antibiotic Use in United States Hospitals During the Coronavirus Disease 2019 Pandemic. Open Forum Infect Dis. 2021;8(6):236.
Crossref - Guidelines for management of co-infection of COVID-19 with other seasonal epidemic prone diseases | Official Website of Department of Health & Family Welfare, Government of Puducherry, India. Available from: https://health.py.gov.in/guidelines-management-co-infection-covid-19-other-seasonal-epidemic-prone-diseases [Accessed June 23, 2023]
- Mirzaei R, Goodarzi P, Asadi M, et al. Bacterial co-infections with SARS-CoV-2. IUBMB Life. 2020;72(10):2097-2111.
Crossref - King LM, Lovegrove MC, Shehab N, et al. Trends in US outpatient antibiotic prescriptions during the COVID-19 pandemic. Clin Infect Dis. 2021;73(3):652-660.
Crossref - Buehrle DJ, Nguyen MH, Wagener MM, Clancy CJ. Impact of the Coronavirus Disease 2019 Pandemic on Outpatient Antibiotic Prescriptions in the United States. Open Forum Infect Dis. 2020;7(12):575.
Crossref - Abelenda-Alonso G, Padulles A, Rombauts A, et al. Antibiotic prescription during the COVID-19 pandemic: A biohasic pattern. Infect Control Hosp Epidemiol. 2020;41(11):1371-1373.
Crossref - Langford BJ,So M, Raybardhan S, et al. Antibiotic prescribing in patients with COVID-19: rapid review and meta-analysis. Clin Microbiol Infect. 2021;27(4):520-531.
Crossref - Lansbury L, Lim B, Baskaran V, Lim WS, et al. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81(2):266-275.
Crossref - Kubin CJ, H McConville T, Dietz D, et al. Characterization of Bacterial and Fungal Infections in Hospitalized Patients with Coronavirus Disease 2019 and Factors Associated with Health Care-Associated Infections. Open Forum Infect Dis. 2021;8(6):201.
Crossref - Westblade LF, Simon MS, Satlin MJ. Bacterial co-infections in coronavirus disease 2019. Trends Microbiol. 2021;29(10):930-941.
Crossref - Langford BJ, So M, Raybardhan S, et al. Bacterial co-infection, and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin Micro Biol Infect. 2020;26(12):1622-1629.
Crossref - Musuuza JS, Watson L, Parmasad V, Putman-Buehler N, Christensen L, Safdar N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: A systematic review and meta-analysis. 2021;16(5):2511-2570.
Crossref - Puzniak L, Finelli L, Yu KC , et al. A multicentre analysis of the clinical microbiology and antimicrobial usage in hospitalized patients in the US with or without COVID-19. BMC Infect Dis. 2021;21(1):227.
Crossref - Contou D, Claudinon A, Pajot O, et al. Bacterial and viral co-infections in patients with severe SARS-CoV-2 pneumonia admitted to a French ICU. Ann Intensive Care. 2020;10(1):119.
Crossref - Weiner-Lastinger LM, Pattabiraman V, Konnor RY , et al. The impact of coronavirus disease 2019 (COVID-19) on healthcare-associated infections in 2020: A summary of data reported to the National Healthcare Safety Network. Infect Control Hosp Epidemiol. 2022;43(1):12-25.
Crossref - Fadhil OQ, Jabbar SA, Tizkam HH, Allak W. Comparative study of antibiotic resistance pattern for gram-positive bacteria pre and post-COVID-19 pandemic. Journal of Communicable Diseases. 2022:49-55.
Crossref - Naylor NR, Atun R, Zhu N, et al. Estimating the burden of antimicrobial resistance: a systematic literature review. Antimicrob Resist Infect Control. 2018;7:58.
Crossref - Ten threats to global health in 2019. Available from: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 [Accessed March 06, 2024]
- WHO releases the 2019 AWaRe Classification Antibiotics. Available from: https://www.who.int/news/item/01-10-2019-who-releases-the-2019-aware-classification-antibiotics [Accessed June 16, 2023]
- Schouten J, Waele JD, Lanckohr C, et al. antimicrobial stewardship in the ICU in COVID-19 times: the known unknowns. Int J Antimicrob Agents. 2021;58(4):106409.
Crossref - Indicator Metadata Registry Details. Available from: https://www.who.int/data/gho/indicator-metadata-registry/imr-details/1 [Accessed March 10, 2024]
- Walia K, Ohri VC, Madhumathi J, Ramasubramanian V. Policy document on antimicrobial stewardship practices in India. Indian J Med Res. 2019;149(2):180-184.
Crossref - Panditrao AM, Shafiq N, Chatterjee S, et al. A multicentre point prevalence survey (PPS) of antimicrobial use amongst admitted patients in tertiary care centers in India. J Antimicrob Chemother. 2021;76(4):1094-1101.
Crossref - Singh SK, Sengupta S, Antony R, et al. Variations in antibiotic use across India: multi-centre study through Global Point Prevalence survey. J Hosp Infect. 2019;103(3):280-283.
Crossref - Kashyap A, Nath S. Antimicrobial Usage during COVID-19 Pandemic in Intensive Care Unit at a Tertiary Care Hospital in Eastern India: A Retrospective Study. J Clin Diagn Res. 2023;17(12):11-16
Crossref - Henig O, Kehat O, Meijer SE , et al. Antibiotic Use during the COVID-19 Pandemic in a Tertiary Hospital with an Ongoing Antibiotic Stewardship Program. Antibiotics. 2021;10(9):1056.
Crossref - Molla MMA, Yeasmin M, Islam MK, et al. Antibiotic Prescribing Patterns at COVID-19 Dedicated Wards in Bangladesh: Findings from a Single Center Study. Infect Prev Pract. 2021;3(2):100134.
Crossref - Al-Nuaimi S, Alkuwari S, Al-Jubouri AM , et al. Antibiotics Prescriptions Pattern among Patients Visiting Primary Health Care Centers (PHCC) before and during COVID-19 Pandemic: A Cross-Sectional Population-Based Study from Qatar. Antibiotics. 2023; 12(8):1228.
Crossref - Akash MSH, Rehman K, Fiayyaz F, Sabir S, Khurshid M. Diabetes-associated infections: development of antimicrobial resistance and possible treatment strategies. Arch Microbiol. 2020;202(5):953-965.
Crossref - Sheikh S, Vishwas G, Aggarwal M , et al. Antibiotic point prevalence survey at a tertiary healthcare hospital in India: Identifying strategies to improve the antibiotic stewardship program immediately after a COVID-19 wave. Infect Prev Pract. 2022;4(4):1002-1053.
Crossref - Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395(10223):507-513.
Crossref - Kakkar AK, Shafiq N, Singh G, et al. Antimicrobial Stewardship Programs in Resource Constrained Environments: Understanding and Addressing the Need of the Systems. Front Public Health. 2020;8:140.
Crossref - Wu J, Liu J, Zhao X, et al. Clinical Characteristics of Imported Cases of Coronavirus Disease 2019 (COVID-19) in Jiangsu Province: A Multicentre Descriptive Study. Clin Infect Dis. 2020;71(15):706-712.
Crossref - Zhou F, Yu T, Du R, et al. Clinical course, and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062.
Crossref - Seaton RA, Gibbons CL, Cooper L, et al. Survey of antibiotic and antifungal prescribing in patients with suspected and confirmed COVID-19 in Scottish hospitals. J Infect. 2020;81(6):952-960.
Crossref - Tan SH, Ng TM, Tay HL, et al. A point prevalence survey to assess antibiotic prescribing in patients hospitalized with confirmed and suspected coronavirus disease 2019 (COVID-19). J Glob Antimicrob Resist. 2021;24:45-47.
Crossref - Saleem Z, Hassali MA, Godman B, et al. Point prevalence surveys of antimicrobial use: a systematic review and the implications. Expert Rev Anti Infect Ther. 2020;18(9):897-910.
Crossref - Seethalakshmi PS, Charity OJ , Giakoumis T, et al. Delineating the impact of COVID-19 on antimicrobial resistance: An Indian perspective. Sci Total Environ. 2022;818:1517.
Crossref - Calderon-Parra J, Muino-Miguez A, Bendala-Estrada AD , et al. Inappropriate antibiotic use in the COVID-19 era, Factors associated with inappropriate prescribing and secondary complications. Analysis of the Registry SEMI-COVID. 2021;16(5):0251340.
Crossref - Martinez-Guerra BA, Gonzalez-Lara MF, de-Leon-Cividanes NA, et al. Antimicrobial Resistance Patterns and Antibiotic Use during Hospital Conversion in the COVID-19 Pandemic. Antibiotics. 2021;10(2):182.
Crossref - Townsend L, Hughes G, Kerr C, et al. Bacterial pneumonia coinfection and antimicrobial therapy duration in SARS-CoV-2 (COVID-19) infection. JAC Antimicrob Resist. 2020;2(3):71
Crossref - Clinical Management of Severe Acute Respiratory Infection (SARI) When COVID-19 Disease Is Suspected. https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf.
- Rawson TM, Moore LSP, Zhu N, et al. Bacterial and Fungal Coinfection in Individuals with Coronavirus: A Rapid Review to Support COVID-19 Antimicrobial Prescribing. Clin Infect Dis. 2020;71(9):2459-2468.
Crossref - Mustafa L, Tolaj I, Baftiu N, Fejza H. Use of antibiotics in COVID-19 ICU patients. J Infect Dev Ctries. 2021;15(04):501-505.
Crossref - Saxena S, Priyadarshi M, Saxena A, Singh R. Antimicrobial consumption and bacterial resistance pattern in patients admitted in ICU at a tertiary care center. J Infect Public Health. 2019;12(5):695-699.
Crossref - Lopez-Gay A, Spijker J, Cole HVS, et al. Sociodemographic determinants of intraurban variations in COVID-19 incidence: the case of Barcelona. J Epidemiol Community Health. (2022);76(1):1-7.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.