ISSN: 0973-7510
E-ISSN: 2581-690X
Urinary tract infections (UTIs) are predominantly caused by bacteria, with Escherichia coli and Klebsiella pneumoniae being predominant causative agents. This retrospective study evaluated the antibiotic susceptibility profiles of E. coli and K. pneumoniae isolates obtained from urine culture of patients with UTI symptoms presenting at the Pradyumna Bal Memorial Hospital, Kalinga Institute of Medical Sciences, Bhubaneswarr, Odisha. With increasing antibiotic resistance, particularly in bacterial strains that synthesize extended-spectrum beta-lactamases (ESBLs), identifying effective treatment options is crucial. From January to November 2023, 1,798 urine cultures were analyzed using the VITEK 2 Compact System, revealing E. coli 432 (60%) and K. pneumoniae 239 (33.2%) as the predominant pathogens. E. coli isolates exhibited high susceptibility to fosfomycin (98.6%), nitrofurantoin (80%), and amikacin (75.8%). K. pneumoniae showed high susceptibility to fosfomycin (73.9%) and amikacin (50.2%) but significantly lower susceptibility to nitrofurantoin (17.2%). Approximately 307 (58.03%) isolates were ESBL-synthesizing E. coli (57.9%) and K. pneumoniae (42.01%). ESBL synthesizing E. coli and K. pneumoniae were highly susceptible to fosfomycin (71.5%) and amikacin (62.4%). Our results emphasize the need to consider local resistance patterns when choosing empirical antimicrobial treatments for UTIs. Although fosfomycin demonstrated the highest sensitivity, being a reserve drug requires cautious use. Amikacin, with its favorable susceptibility profile is a valuable alternative especially for UTIs caused by ESBL-synthesizing, nitrofurantoin-resistant, and fosfomycin-resistant K. pneumoniae and E. coli, and offers a potential single-dose treatment option. This targeted approach can help preserve last-resort antibiotics in more critical cases.
Urinary Tract Infections, Extended-spectrum Beta-Lactamase, Amikacin, Antibiotic Susceptibility
Urinary tract infections (UTIs) are commonly associated with bacteria that can infect multiple regions of the urinary tract in both men and women, although women are more susceptible because of the short urethra and the anatomical proximity of the urethra to the anal opening.1,2 Gram-negative bacteria are generally responsible for the infection, with Escherichia coli being the primary causative agent, followed by Klebsiella pneumoniae, and uropathogenic E. coli responsible for 75-90% of UTIs.3,4 Bacteria colonize and then infect the urinary tract, resulting in symptoms such as dysuria, urine incontinence, and hematuria.5 The infections caused by these bacteria can be treated with beta-lactam antibiotics. Extended-spectrum beta-lactamases (ESBLs) are enzymes most commonly produced by K. pneumoniae and E. coli isolates, causing non-susceptibility to beta-lactam antibiotics, including third and fourth-generation cephalosporins, and monobactams. ESBL-producing bacteria that cause UTIs restrict the treatment options.6 The Indian Council of Medical Research (ICMR) antibiotic treatment guidelines 2019 report recommends nitrofurantoin and fosfomycin as first-line drugs, and amikacin, ertapenem, and cotrimoxazole as second-line antibiotics for the treatment of UTIs. However, the use of fosfomycin should be limited to avoid the risk of developing resistance.7,8 Some studies have indicated the use of amikacin as a monotherapy or as a single dose aminoglycoside for the treatment of UTIs, since it is eliminated in large concentrations in urine.9 This study aimed to investigate the prevalence and antibiograms of E. coli and K. pneumoniae urinary isolates, with a focus on amikacin as the preferred treatment option for UTIs caused by ESBL-synthesizing, nitrofurantoin, and fosfomycin-resistant isolates, with a preference over ertapenem and cotrimoxazole.
A retrospective study was conducted by retrieving data of all urine culture reports of 1244 patients from microbiology laboratory of KIMS, Bhubaneswar who presented to different departments (January to November 2023), with symptoms suggestive of UTI and were advised for urine culture.
Inclusion criteria
Data of all bacterial culture isolates obtained from urine samples with a bacterial count ≥105 CFU/ml.
Exclusion criteria
Data of repeat isolates from the same patient, samples that showed growth of more than two kinds of organisms, and contaminants.
Sample Collection
Urine samples were collected using the midstream clean-catch technique in sterile containers and quickly transported to the central laboratory at KIMS to maintain quality and ensure accurate testing.
Bacterial culture and identification
For the semiquantitative determination of the colony counts, urine samples were plated on cystine lactose electrolyte deficient agar (CLED) (HiMedia Laboratories, Mumbai). The plates were then aerobically incubated at 37 °C for 18-24 hours. A bacterial colony count of ≥105 CFU/ml on the plates indicated significant bacteriuria. Single morphotypes or at least two forms were deemed significant and evaluated for identification and AST using the VITEK 2 compact system (BioMerieux, USA). AST results were categorized as sensitive, intermediate, or resistant, and interpretation was performed as per the Clinical and Laboratory Standard Institute (CLSI) 2024 guidelines.10 Urinary isolates that demonstrated intermediate sensitivity results were considered resistant during the statistical analysis.
Screening for ESBL production by E. coli and K. pneumoniae isolates
All E. coli and K. pneumoniae urinary isolates obtained were screened for possible ESBL production if they were resistant to aztreonam and the third-generation cephalosporins-ceftazidime, cefotaxime and ceftriaxone using the VITEK 2 compact system. As per the 2024 CLSI guidelines, resistance to at least one of these antibiotics was considered positive in screening tests for possible ESBL production.
Confirmation of ESBL production in E. coli and K. pneumoniae isolates
Screened ESBL-producing isolates were then phenotypically confirmed by the synergy disc-diffusion method using 30 µg ceftazidime and 30 µg cefotaxime discs alone and in combination with 10 µg clavulanic acid disc. The interpretation of ESBL production was done as per the CLSI 2024 guidelines.
Ethical Clearance
The Institute Ethics Committee of the Kalinga Institute of Medical Sciences approved this study (Ethical approval number: KIIT/KIMS/IEC/1857/2024) with a waiver of consent for retrospective data analysis provided with the de-identification of collected data.
Statistical analysis
All data are presented as numbers and percentages and recorded in the MS Excel spreadsheet. Data analysis was performed using the SPSS software version 26 (IBM). The chi-square test was performed to determine the differences in the data for statistical relevance. An unpaired t-test was conducted to compare the two groups of bacterial isolates. A p-value < 0.05 was considered statistically significant.
Among the 1798 urine samples processed; 1244 (69.2%) showed significant growth. Among the culture-positive isolates, 887 (71.3%) were Gram-negative bacteria (GNB). Enterobacterales were the most frequently identified uropathogens among GNB (81.1%, 720/887). The most frequently isolated bacteria were found to be Escherichia coli (432/720, 60%), followed by Klebsiella pneumoniae (239/720, 33.2%) (Table 1).
Table (1):
Enterobacterales isolates from the urine samples
Enterobacterales |
Number (%) |
|---|---|
Escherichia coli |
432 (59.9%) |
Klebsiella pneumoniae |
239 (33.1%) |
Citrobacter spp. |
13 (1.8%) |
Enterobacter spp. |
11 (1.5%) |
Serratia spp. |
8 (1.1%) |
Providencia spp. |
7 (0.9%) |
Proteus mirabilis |
3 (0.4%) |
Morganella morganii |
2 (0.2%) |
Shigella spp. |
2 (0.2%) |
Salmonella spp. |
2 (0.2%) |
Pantoea spp. |
1 (0.1%) |
Total |
720 |
The majority of the 671 E. coli and K. pneumoniae isolates were collected from female patients (54.6%) (Table 2).
Table (2):
Gender-specific distribution of E. coli and K. pneumoniae
E. coli (N = 432) |
K. pneumoniae (N = 239) |
Total (N = 671) |
Chi-square |
p–value |
|
|---|---|---|---|---|---|
Gender |
Number (%) |
Number (%) |
Total (%) |
2.298 |
0.076 |
Male |
187 (27.8%) |
118 (17.6%) |
305 (45.4%) |
||
Female |
245 (36.6%) |
121 (18%) |
366 (54.6%) |
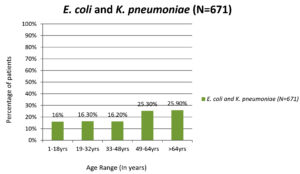
The age groups most commonly infected with UTIs caused by E. coli and K. pneumoniae was 49-64 (25.3%) and >64 (25.9%) years (Figure 1). The average age of the patients with UTIs caused by E. coli was 48 years (range: 1-96 years) and K. pneumoniae was 52 years (range: 1-92 years).
A maximum of E. coli urinary isolates were isolated from OPD (42.1%) and the majority of K. pneumoniae isolates were isolated from wards (45.6%) (Table 3).
Table (3):
ICU, OPD, and Ward-specific distributions of E. coli and K. pneumoniae
Organism |
ICU |
OPD |
WARD |
Chi-square |
p-value |
|---|---|---|---|---|---|
Escherichia coli (N = 432) |
82 (19%) |
182 (42.1%) |
168 (38.9%) |
15.744 |
|
Klebsiella pneumoniae (N = 239) |
65 (27.2%) |
65 (27.2%) |
109 (45.6%) |
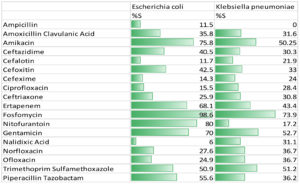
The sensitivity rate of the E. coli isolates to fosfomycin was 98.6%, followed by nitrofurantoin (80%), amikacin (75.8%), ertapenem (68.1%), and cotrimoxazole (50.9%).
For K. pneumoniae, the sensitivity rates were high to fosfomycin (73.9%), cotrimoxazole (51.2%), amikacin (50.2%), and ertapenem (43.4%), while nitrofurantoin showed less sensitivity (17.2%). The antibiotic susceptibility patterns of the urinary isolates of E. coli and K. pneumoniae is represented in Figure 2.
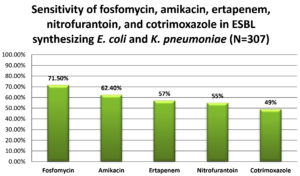
A total of 529 (78.8%) E. coli and K. pneumoniae isolates were found to be possible ESBL producers in the screening test. Of these, 307 (58.03%) isolates of E. coli and K. pneumoniae were confirmed to be ESBL producers by the combined disc-diffusion method. From the confirmed 307 ESBL producing isolates, 57.9% (178/307) and 42.01% (129/307) were E. coli and K. pneumonia, respectively. Among the 307 isolates, sensitivity rates to fosfomycin and amikacin were highest at 71.5% and 62.4%, respectively, followed by ertapenem (57%), nitrofurantoin (55%), and cotrimoxazole (49%) (Figure 3).
Globally, uropathogens have become increasingly resistant to various antimicrobials, posing a substantial challenge to clinicians because of limited treatment options available. Managing UTIs involves following a systematic process to identify the infection, and provide appropriate empiric treatment by choosing the appropriate antimicrobial.
In this study, the prevalence of Enterobacterales isolates from urine samples was 81.1%, which was slightly higher than the ICMR Antimicrobial Resistance Surveillance Network (AMRSN) report in 2022, where Enterobacterales comprised 76.4% of urinary isolates.3 Among them, Escherichia coli (60%) was found to be the most frequently isolated bacteria, which was consistent with the studies done by Asrat et al., Sarita et al., and Sneka et al., where 60.29%, 68.3%, and 61.2% of cultures showed growth of E. coli, respectively, and it was the predominant bacteria responsible for UTI in all of these studies.11-13 The second most prevalent pathogen in our study was K. pneumoniae (33.2%), which is consistent with the findings from other studies in which Klebsiella spp. were found to be the second most common pathogen in UTIs.14-18
Several key factors are responsible for the increase in UTIs caused by Gram-negative bacteria. These organisms have a special way of attaching to the lining of the urinary tract through adhesins, pili, and fimbriae. Overall, colonization of the urogenital mucosa and escape from the immune system responses by bacteria has substantially contributed to the increasing incidence of these infections.19
In the current study, we found that females had a higher infection rate (54.5%), indicating that they are more susceptible to UTIs. This was in accordance with the findings of an Egyptian study, in which 57.6% of UTIs in females were caused by E. coli and K. pneumoniae, whereas that in males was 42.3%. Additionally, an Indian study reported that UTIs were more common among the female patients (72.5%) compared with that in male patients (27.5%).12,20
The average age of patients with UTIs caused by E. coli was 48 years (range: 1-96 years), whereas that of patients with UTIs caused by K. pneumoniae was 52 years (range: 1-92 years). Female patients with UTIs caused by either K. pneumoniae or E. coli had significantly higher infection rates in the age ranges of 19-32 years and 49-64 years. For males, infection rates were notably higher among those within 49-64 years and >65 years. This indicated that females are at a greater risk of infection during the younger and older years, while males experience higher infection rates primarily in older age groups. Similar results were also reported in an Indian study stating that infection rates in females were higher in the age group 31-45 years, whereas that in males infection rates were higher among patients aged >45 years.15 Veronica et al. noted that UTIs caused by E. coli and K. pneumoniae in males was higher (average age: 65.7 years), compared with that in female patients (average age: 57.2 years) (p-value < 0.01).21 Conversely, in an Indian study done by Rizwan et al., the majority of patients were in the age group 21-30 years.22
E. coli urinary isolates were mostly obtained from outpatients (27.1%), followed by wards (25%) and intensive care units (ICUs) (12.2%). Conversely, K. pneumoniae isolates were predominantly isolated from wards (16.2%) and equally isolated from outpatients and ICU patients (9.6% each). These results are consistent with other Indian studies, where E. coli causing UTIs were most frequently obtained from outpatients (66.1%) and K. pneumoniae was equally isolated from wards and OPDs (47.6%).23,24 According to the ICMR AMRSN 2022 report, E. coli was predominantly isolated from out-patients, whereas K. pneumoniae was mostly found in ICUs and wards.3 Additionally, Karuna et al. reported that E. coli was predominantly isolated from out-patient (32.1%).25
Antibiotic susceptibility test revealed that the E. coli urinary isolates exhibited high sensitivity to fosfomycin (98.6%), nitrofurantoin (80%), amikacin (75.8%), ertapenem (68.1%), and cotrimoxazole (50.9%). The ICMR AMRSN 2022 and CLSI 2024 guidelines, endorse fosfomycin as the preferred treatment for UTIs.3,10 The sensitivity of E. coli to nitrofurantoin and fosfomycin was similar to that observed in other studies, both in India and abroad, where it typically falls between 80% and 90%.15,17,26-28 The observed sensitivity to amikacin (75.8%) was consistent with studies from Bangladesh and Saudi Arabia, which reported that the E. coli sensitivity rates to amikacin were 88.6% and 88.5%, respectively.18,29 The high sensitivity levels of E. coli isolates to fosfomycin, nitrofurantoin, and amikacin are encouraging, as these antibiotics are recommended as empirical treatment options for suspected UTIs in India according to the ICMR antibiotic guidelines.7
The AST of K. pneumoniae isolates showed increased sensitivity to fosfomycin (73.9%), cotrimoxazole (51.2%), amikacin (50.2%), and ertapenem (43.4%), which are consistent with the findings of a study conducted in Bangladesh where amikacin exhibited a very high sensitivity rate of 85%.29 Similarly, an international study by Mukubwa et al. reported very high sensitivity rates to amikacin (91.9%), and slightly lower rates to fosfomycin and cotrimoxazole (59.3% and 54.5%, respectively), and a study done in Pakistan by Khan et al. found 78.7% of K. pneumoniae urinary isolates had high sensitivity to fosfomycin, which was also in congruence with our findings.30,31 The high resistance to nitrofurantoin among K. pneumoniae isolates can be attributed to improper use of the medication, which involves inadequate dosing and treatment duration.
Currently, nitrofurantoin, fosfomycin, and cotrimoxazole have been safely used worldwide for the treatment of E. coli and K. pneumoniae causing UTIs.32 However, some urinary isolates of E. coli and K. pneumoniae that synthesize ESBL are not susceptible to most oral antibiotics, including nitrofurantoin, fosfomycin, and cotrimoxazole. According to the 2019 ICMR antibiotic treatment guidelines, nitrofurantoin and fosfomycin are considered first-line treatment options for UTIs, whereas amikacin, ertapenem, and cotrimoxazole are categorized as second-line options for treating UTIs.7 With an increase in resistance to these antibiotics, there are limited options for treating UTIs caused by E. coli and K. pneumoniae synthesizing ESBLs. In the current study, 529 (78.8%) isolates of E. coli and K. pneumoniae, were suspected to produce ESBL in the screening test. Of these, 307 were confirmed to be ESBL producers (58% were E. coli isolates while, 42.01% were K. pneumoniae). The prevalence of ESBL in other Indian studies ranges from 70% to 85%.33,34 ESBL-synthesizing E. coli and K. pneumoniae showed the highest sensitivity to fosfomycin (71.5%), followed by amikacin (62.4%), ertapenem (57%), nitrofurantoin (55%), and cotrimoxazole (49%). Although fosfomycin is the preferred drug, it should not be excessively used to avoid the risk of developing drug resistance.8 Ertapenem should be used cautiously to treat UTIs and to reduce the risk of resistance development. Carbapenems are regarded as the ultimate treatment option for infections caused by multidrug-resistant bacteria.35
Aminoglycosides are effective antimicrobial agents for the treatment of UTIs because of their increased excretion through urine.32 Recently, single-dose aminoglycosides has emerged as an ideal drug of choice for treating UTIs.36 Aminoglycosides are broad-spectrum antibiotics that kill bacteria by interfering with protein synthesis by attaching to the A-site of the smaller 30S ribosomal subunit. Amikacin is recommended for treating UTIs primarily caused by Gram-negative bacteria.37 In particular, amikacin can be administered as a single dose or in combination to treat UTIs caused by ESBL- synthesizing and nitrofurantoin-resistant Gram-negative bacteria.38 Goodlet et al. suggested a single-dose aminoglycoside therapy for the treatment of lower UTIs caused by Gram-negative bacteria with minimal toxicity, considering it to be an effective beta-lactam-sparing agent.9 The clinical and bacteriological effectiveness of amikacin as an efficient treatment option for adult patients with lower UTIs caused by E. coli or K. pneumoniae that produce ESBLs, has been demonstrated in few studies.32,39 This study explored the use of amikacin to treat UTIs caused by E. coli and K. pneumoniae, which are ESBL producers, offering a potential alternative to nitrofurantoin and fosfomycin.
Research limitations
The limitations of the study involves its retrospective design, which resulted in the exclusion of data due to the non-retrieval of records, including patient details and antibiotic susceptibility profiles for some antibiotics. Additionally, not all patient information, such as the risk factors influencing the growth of resistant Gram-negative bacteria, was available for analysis. This was a single-center study; therefore, collaborative research involving multiple centers would provide comprehensive data.
In conclusion, our study emphasizes the efficacy of amikacin as an efficient treatment option for infections of the urinary tract caused by ESBL-producing E. coli and K. pneumoniae, following fosfomycin treatment. Although bacteria showed the highest sensitivity to fosfomycin, its use requires caution. Amikacin, with its second-highest sensitivity, offers a practical alternative as a single-dose therapy, making it a suitable choice for managing resistant strains causing UTIs. This highlights the need for targeted antibiotic therapy, while preserving last-resort antibiotics in more critical cases.
ACKNOWLEDGMENTS
The authors would like to acknowledge Dr. Subhadra Priyadarshini, Research Associate (Biostatistics), Kalinga Institute of Medical Sciences, KIIT, Bhubaneswar, Odisha, India, for her constant support during statistical analysis.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Institutional Ethics Committee, Kalinga Institute of Medical Sciences, KIIT, Bhubaneswar, Odisha, India, with EC file number KIIT/KIMS/IEC/1857/2024.
- Vasudevan R. Urinary Tract Infection: An Overview of the Infection and the Associated Risk Factors. J Microbiol Exp. 2014;1(2):42-54.
Crossref - Bazzaz BSF, Fork SD, Ahmadi R, Khameneh B. Deep insights into urinary tract infections and effective natural remedies. Afr J Urol. 2021;27(1):6.
Crossref - https://www.icmr.gov.in/icmrobject/custom_data/pdf/resourceguidelines/AMRSN_Annual_Report_2022.pdf Accessed date, September, 2024
- Mohajeri P, Jalilian S, Farahani A. Antibiotic resistance in uropathogenic Escherichia coli isolated from urinary tract infections out-patients in Kermanshah. Int J Med Public Health. 2014;4(1):75-77.
Crossref - Sarwar MI, Sarwar I, Hussain MS, Sherwani SK, Hakim A, Kazmi SU. Frequency of urinary tract infection causing agents in pregnant women and their antimicrobial susceptibility profile. Pak J Biochem Mol Biol. 2013;46(3-4):107-110. http://pjbmb.org.pk/images/Current_Issue/06.pdf
- Vachvanichsanong P, McNeil EB, Dissaneewate P. Extended-spectrum beta-lactamase Escherichia coli and Klebsiella pneumoniae urinary tract infections. Epidemiol Infect. 2020;149:e12.
Crossref - https://www.icmr.gov.in/icmrobject/custom_data/pdf/resourceguidelines/Treatment_Guidelines_2019_Final.pdf (Accessed date September, 2024)
- Gardiner BJ, Stewardson AJ, Abbott IJ, Peleg AY. Nitrofurantoin and fosfomycin for resistant urinary tract infections: old drugs for emerging problems. Aust Prescr. 2019;42(1):14-19.
Crossref - Goodlet KJ, Benhalima FZ, Nailor MD. A Systematic Review of Single-Dose Aminoglycoside Therapy for Urinary Tract Infection: Is It Time To Resurrect an Old Strategy? Antimicrob Agents Chemother. 2018;63(1):e02165-18.
Crossref - CLSI Performance standards for antimicrobial susceptibility testing, 34th Edition. CLSI M100. Clinical and Laboratory Standards Institute. 2024.
- Abejew AA, Denboba AA, Mekonnen AG. Prevalence and antibiotic resistance pattern of urinary tract bacterial infections in Dessie area, North-East Ethiopia. BMC Res Notes. 2014;7:687.
Crossref - Mohapatra S, Panigrahy R, Tak V, et al. Prevalence and resistance pattern of uropathogens from community settings of different regions: an experience from India. Access Microbiol. 2022;4(2):000321.
Crossref - Sneka P, Mangayarkaras V. Bacterial pathogens causing UTI and their antibiotic sensitivity pattern: a study from a tertiary care hospital from South India. Trop J Pathol Microbiol. 2019;5(6):379-385.
Crossref - Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13(5):269-284.
Crossref - Pardeshi P, Prevalence of urinary tract infections and current scenario of antibiotic susceptibility pattern of bacteria causing UTI. Indian J Microbiol Res. 2018;5(3):334-338.
Crossref - Farfour E, Dortet L, Guillard T, et al. Antimicrobial Resistance in Enterobacterales Recovered from Urinary Tract Infections in France. Pathogens. 2022;11(3):356.
Crossref - Mitiku A, Aklilu A, Tsalla T, Woldemariam M, Manilal A, Biru M. Magnitude and antimicrobial susceptibility profiles of Gram-Negative bacterial isolates among patients suspected of urinary tract infections in Arba Minch General Hospital, southern Ethiopia. PLoS One. 2022;17(12):e0279887.
Crossref - Almutawif YA, Eid HMA. Prevalence and antimicrobial susceptibility pattern of bacterial uropathogens among adult patients in Madinah, Saudi Arabia. BMC Infect Dis. 2023;23(1):582.
Crossref - Terlizzi ME, Gribaudo G, Maffei ME. UroPathogenic Escherichia coli (UPEC) Infections: Virulence Factors, Bladder Responses, Antibiotic, and Non-antibiotic Antimicrobial Strategies. Front Microbiol. 2017;8:1566.
Crossref - Al Yousef SA, Younis S, Farrag E, Moussa S, Bayoumi FS, Ali AM. Clinical and Laboratory Profile of Urinary Tract Infections Associated with Extended Spectrum b-Lactamase Producing Escherichia coli and Klebsiella pneumoniae. Ann Clin Lab Sci. 2016;46(4):393-400.
- Zanichelli V, Huttner A, Harbarth S, Kronenberg A, Huttner B, Swiss Centre For Antibiotic Resistance Anresis. Antimicrobial resistance trends in Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis urinary isolates from Switzerland: retrospective analysis of data from a national surveillance network over an 8-year period (2009-2016). Swiss Med Wkly. 2019;149:w20110.
Crossref - Rizwan M, Akhtar M, Najmi AK, Singh K. Escherichia coli and Klebsiella pneumoniae Sensitivity/Resistance Pattern Towards Antimicrobial Agents in Primary and Simple Urinary Tract Infection Patients Visiting University Hospital of Jamia Hamdard New Delhi. Drug Res (Stuttg). 2018;68(7):415-420.
Crossref - Chooramani G, Jain B, Chauhan PS. Prevalence and antimicrobial sensitivity pattern of bacteria causing urinary tract infection; study of a tertiary care hospital in North India. Clin Epidemiol Global Health. 2020;8(3):890-893.
Crossref - Angami S, Jamir N, Sarma PC, Deka AC. Urinary tract infection, its causative microorganism and antibiotic susceptibility in Nagaland. Arch Med Health Sci. 2015;3(1):40.
Crossref - Karuna T, Kumar S, Garg R, et al. Changing Patterns of Antimicrobial Susceptibility of Uro-pathogens in Community-acquired Urinary Tract Infections in Central India: Two Year Prospective Surveillance Report. Infect Dis Diag Treat. 2023;7(3).
Crossref - Kumar KSH, Asina AP, Theckel PG, et al. Prevalence of multidrug resistant uropathogens isolated from different age groups in South-India: a cross-sectional study. Int J Res Med Sci. 2022;10(4):905.
Crossref - Kibret M, Abera B. Prevalence and antibiogram of bacterial isolates from urinary tract infections at Dessie Health Research Laboratory, Ethiopia. Asian Pac J Trop Biomed. 2014;4(2):164-168.
Crossref - Al Wutayd O, Al Nafeesah A, Adam I, Babikir I. The antibiotic susceptibility patterns of uropathogens isolated in Qassim, Saudi Arabia. J Infect Dev Ctries. 2018;12(11):946-952.
Crossref - Rahman F, Akhter H, Hasnat S, Begum N. Prevalence and antimicrobial sensitivity profile of uropathogens in a tertiary care hospital of Dhaka city. Bangladesh J Med Microbiol. 2020;14(2):3-11.
Crossref - Mukubwa GK, Lukusa FN, Kavulikirwa OK, Liesse Ji, Tshilolo Lm, Memvanga PB. Resistance profiles of urinary Escherichia coli and Klebsiella pneumoniae isolates to antibiotics commonly prescribed for treatment of urinary tract infections at Monkole Hospital Center, Kinshasa, Democratic Republic of the Congo. Afr J Clin Exper Microbiol. 2023;24(1):51-60
Crossref - Khan MA, Rahman AU, Khan B, et al. Antibiotic Resistance Profiling and Phylogenicity of Uropathogenic Bacteria Isolated from Patients with Urinary Tract Infections. Antibiotics. 2023;12(10):1508.
Crossref - Ipekci T, Seyman D, Berk H, Celik O. Clinical and bacteriological efficacy of amikacin in the treatment of lower urinary tract infection caused by extended-spectrum beta-lactamase-producing Escherichia coli or Klebsiella pneumoniae. J Infect Chemother. 2014;20(12):762-767.
Crossref - Govindaswamy A, Bajpai V, Khurana S, et al. Prevalence and characterization of beta-lactamase-producing Escherichia coli isolates from a tertiary care hospital in India. J Lab Physicians. 2019;11(2):123-127.
Crossref - Verma S, Kalyan RK, Gupta P, Khan MD, Venkatesh V. Molecular Characterization of Extended Spectrum b-Lactamase Producing Escherichia coli and Klebsiella pneumoniae Isolates and Their Antibiotic Resistance Profile in Health Care-Associated Urinary Tract Infections in North India. J Lab Physicians. 2022;15(2):194-201.
Crossref - Sheu CC, Chang YT, Lin SY, Chen YH, Hsueh PR. Infections Caused by Carbapenem-Resistant Enterobacteriaceae: An Update on Therapeutic Options. Front Microbiol. 2019;10:80.
Crossref - Liu S, She P, Li Z, et al. Drug synergy discovery of tavaborole and aminoglycosides against Escherichia coli using high throughput screening. AMB Express. 2022;12(1):151.
Crossref - Hartinger JM, Dvorackova E, Kratky V, et al. Elimination and penetration of amikacin into urine in patients with decreased glomerular filtration rate. Clin Kidney J. 2024;17(1):sfae002.
Crossref - Hamza NS, Khalil A. Resistant Gram-Negative Urinary Tract Bacterial Infections. Urinary Tract Infection – The Result of the Strength of the Pathogen, or the Weakness of the Host. InTech. 2017.
Crossref - Cho SY, Choi SM, Park SH, Lee DG, Choi JH, Yoo JH. Amikacin therapy for urinary tract infections caused by extended-spectrum b-lactamase-producing Escherichia coli. Korean J Intern Med. 2016;31(1):156-161.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.