ISSN: 0973-7510
E-ISSN: 2581-690X
Investigating new antimicrobial agents from various biological sources is necessary due to the rise of bacteria that are resistant to drugs. A neglected source of bioactive substances with possible antibacterial qualities is fish, especially Oreochromis niloticus (Nile tilapia). This study identifies the bioactive components of extracts made from O. niloticus and examines their antibacterial activity. PBS and sterile water were used to remove fish mucus using a solvent. Using the agar well diffusion method, extracts were tested for antibacterial activity against a panel of Gram-positive bacteria (Staphylococcus aureus, B. cereus), Gram-negative bacteria (Klebsiella pneumoniae, P. aeruginosa), and fungus (Candida albicans). The presence of hydroxyl and amide functional groups, which are suggestive of proteins and polyphenolic substances, was further validated by Fourier-transform infrared spectroscopy (FTIR). The study demonstrates O. niloticus’s antibacterial potential, with its mucus being an exceptionally abundant source of bioactive substances. These results highlight the potential of fish-derived antimicrobial compounds as substitutes for traditional antibiotics. The isolation, structural clarification, and possible therapeutic uses of these chemicals should be the main areas of future study. Furthermore, to guarantee the efficacy and safety of these natural compounds, knowledge of their toxicity profiles and mechanisms of action is crucial. In the fight against antibiotic resistance, this study helps to establish sustainable bioresources and expands our knowledge of O. niloticus’s antibacterial potential.
Nile Tilapia, Well Diffusion Assay, Aqueous Extract, Lyophilised Mucus, Physical Barrier
The effective reemergence and discovery of newer pathogens at a rate much higher than the discovery of new antimicrobials has caused a major downhill to human and animal health in fighting against infectious diseases causing microbes. This is primarily due to the growing resistance against antibiotics, antifungals, and antivirals. Furthermore, by adversely upsetting the balance of the pathogen-host-environment interaction, variables like population growth, intensive farming, globalization, pollution, and climate change significantly contribute to this problem.1 There is a rapid search for more natural antimicrobials to combat pathogenic agents and improve their capabilities by strengthening the now-existing and newer technologies.2 The main issue in aquaculture is infectious diseases brought on by bacteria, viruses, and other parasite organisms. One of the most important factors for aquaculture success is the efficient treatment of viral illnesses. Maintaining much fish in a limited space creates an environment favorable to the growth and dissemination of infectious illnesses. Fish are anxious and more prone to illness in crowded, comparatively artificial environments. Furthermore, the water environment and restricted water flow promote the spread of infections among densely populated areas.3
Fish health is significantly impacted by skin mucus, a crucial part of the innate immune system, and acts as the initial chemical and physical barrier against infections. Three distinct cell types- goblet, sacciform, and club cells- secrete it. The surface of the gills and all exterior surfaces are covered in goblet cells, which contain glycoproteins and create mucus granules. Club cells primarily exude proteinaceous substances, while sacciform cells combine their secretions with goblet cells. In addition to possessing several antimicrobial components such as proteins, lysozyme, immunoglobulin, and lectins, skin mucus also continuously produces and sheds, thwarting pathogen attacks. The viscosity, thickness, and glycoprotein (mucin) concentration of skin mucus, one of its main constituents, vary greatly depending on the species.4 Mucins are high molecular weight glycoproteins that give mucus its rheological and viscoelastic characteristics. Lysozyme, glycosaminoglycans, immunoglobulins, complements, carbonic anhydrase, lectins, and calmodulin are additional substances in fish mucus.5 The ability of fish skin mucus to fight off bacteria that can change the fish’s mucosal microbiome and raise their susceptibility to various diseases has drawn considerable attention in recent years. Mucus’s antibacterial properties make it a promising target for creating novel therapeutic medicines and new commercial agents to treat human infections.6
The freshwater fish Tilapia (Oreochromis spp.) is a member of the Cichlidae family. Three significant genera- Oreochromis, Sarotherodon, and Tilapia- make up the group. Originally from Africa, they were brought to the Philippines and many other tropical, subtropical, and temperate parts of the world in the second half of the 20th century. There are several qualities that make tilapia appealing to tanks. They can withstand a wide variety of salinities and like water temperatures between 29 °C and 31 °C. Unlike other aquaculture species, they can withstand the handling and crowding needed in a tank-based operation, as well as high ammonia concentrations and little dissolved oxygen. Compared to other aquaculture species, they are more resilient to bacterial, fungal, and viral infections as well as through the secreted mucus they are capable of inhibiting the spread of Vibrio and other pathogenic bacteria.7
There is a limitation in detailed knowledge about the antimicrobial abilities of many freshwater fishes like tilapia, which can be of high value in medicine, cosmetics, etc. Every species has its habits and habitat, lives in watery environments, and eats different foods. These factors may affect the quantity of mucus secreted and its constituents within or between species and aid in supplying a range of immune responses and components.
In this study, the aqueous extract of tilapia fish mucus is screened for their antimicrobial activity against gram-negative and gram-positive bacterial strains and fungal strains and also to understand the biological composition of the mucus using FT-IR, XRD, and SEM analysis.
Collection of mucus from fish
Tilapia fishes (Oreochromis niloticus) of size approximately 30 cm long and weighing 500 grams were collected from the Fisheries Research and Information Centre, Hebbal. They were fed in the aquarium maintained in the R&D block at Christ University. Daily monitoring was done to choose the fish that produced the thickest mucus and remove the dead ones from the aquarium. They were fed regularly in the monitoring period (at least 7 days) with commercial feed to provide good nutrition. After 7 days of feeding and keeping in incubation, followed by 24 hours of starvation, mucus from the tilapia was extracted. The extraction was done carefully using a sterile scalpel in the anteroposterior direction from the ventral side of the fish to avoid getting any urinogenital contaminants from the fish. After collection, fish were carefully placed back into the fish tank, and the extracted mucus was stored in a falcon tube. This extraction took about 3 months, and the pooled mucus was made to undergo centrifugation at high speed (10,000 rpm) for 10 minutes, separating out the pellet and keeping the supernatant containing protein-like soluble bioactive components.8 This was made to undergo lyophilization to get a powdered mucus sample and was stored at -20 °C for further use. Freeze drying was performed using LABOCENE SCANVAC COOLSAFE at -196 °C.
Preparation of aqueous skin mucus extract
The lyophilized mucus sample was thoroughly mixed with sterilized Phosphate buffered saline (PBS) in a 2.67 mg/ml ratio to prepare the aqueous extract. 16 mg of lyophilized mucus sample was weighed and mixed with 6 ml of sterile PBS. It was appropriately homogenized using a magnetic stirrer for 20 minutes. The homogenate was used for antimicrobial sensitivity tests, and the rest were stored at -20 °C for further use.9
Another extract was prepared in a 1:1 (w/v) ratio of lyophilized mucus sample with sterile distilled water for performing the antifungal efficacy and was stored at -20 °C for further analysis.
Microbial strains used
Two Gram-positive bacterial strains, Staphylococcus aureus (MTCC 87) and Bacillus cereus (MTCC 430), and two Gram-negative bacterial strains, Klebsiella pneumoniae (MTCC 109) and Pseudomonas aeruginosa (MTCC 424) were used for the antibacterial assay performed in the experimental study. All the strains used in the experimental study were obtained from the Microbial Type Culture and Gene Bank, Chandigarh, India. According to Eder et al., every bacterial strain was cultivated using microbiological safety procedures and circumstances. In nutritional broth (0.5% peptone, 0.5% NaCl, 0.3% beef extract, distilled water, pH 6.8 at 28 °C), each microbial strain was cultivated at 37 °C.10
Anti-bacterial assay
The antibacterial effect of the fish mucus extract prepared in PBS (2.67 mg/ml) against the selected bacterial strains was performed by the Agar well diffusion method, according to Valgas et al.10 Bacterial strains were sub-cultured in nutrient broth for 24 hours, and the OD at 600 nm was checked. Petri plates containing 25 ml nutrient agar medium were seeded with the 24-hour sub-cultured bacterial strains. Later, wells were made using a sterile micropipette tip and aqueous fish mucus extracts at 100 µl and 150 µl into the wells. As a positive control, streptomycin discs of 25 mcg concentration were used, and the solvent (PBS) was used as the negative control into the well. After that, the plates were incubated for 24 hours at 37 °C. The diameter of the zone of inhibition (ZOI) that developed around the well was measured to assess the bactericidal impact. The diameter of the zone of inhibition was measured in terms of centimeters (cm).11
Statistical analysis
The mucus of tilapia fish species and antibiotics was examined for significant variation using a one-way analysis of variance (ANOVA) and Duncan’s multiple range test. The significant difference between ZOIs derived from fish species aqueous extracts and the various bacterial strains under investigation was assessed using the student’s t-test. A probability value of P < 0.05 was used to determine statistical significance. SPSS Version 11.5 for Windows was used to perform all statistics.3
Anti-fungal assay
Candida albicans cell suspension was prepared and grown on media, and cultures were incubated for 48 hrs at 35 °C. The cell suspensions of all the cultures were adjusted to 2×106 cells/ml. The fungal strain was inoculated on Potato dextrose agar media (PDA) (90 mm), and wells were created using sterile micropipette tips. The test compound of 30 µl concentration was added to the well. Fluconazole standard discs (25 mcg) were used as the positive control, whereas sterile water (30 µl) was added as the negative control. The treated plates with Candida albicans were incubated at 35 °C for 48 hours. The treated plates were observed for the zone of inhibition around the wells.11
Fourier transform-infrared spectral analysis
Fourier-transform infrared (FTIR) spectroscopy was used to analyze the lyophilized fish mucus sample and determine which functional groups were present. Infrared analysis was performed by a Nicolet Nexus 670 Fourier transform infrared spectrometer in a wavelength range from 4000 to 400 cm-1. For analysis, the freeze-dried mucus extract was put in the FT-IR spectrophotometer’s sample chamber. The spectrum, which spans the usual range for identifying several functional groups including alcohols, amides, carboxyl groups, and others, was recorded using a wavelength range of 500 to 4000 cm-1. To keep the material stable, the analysis was carried out at a regulated temperature of 25 °C. The resultant FTIR spectra helped characterize the chemical components of the fish mucus by providing comprehensive information on its molecular structure and functional group composition.12
X-ray diffraction assay
The structural characteristics of the lyophilized fish mucus were examined using X-ray diffraction (XRD), specifically to investigate the crystalline or ordered structures, like proteins, that were present. The X-Ray Diffraction (XRD) patterns were recorded on PANalytical X-ray diffractometer equipped with a scintillation counter detector with Cu Kα radiation(λ = 0.15418 nm). operated at 50 kV and 100 mA. The experiments performed in the diffraction angle range of 2 = 20-80°. This technique employed the diffraction pattern to locate crystalline areas inside the mucus and examine its structural organization. Using the Debye-Scherrer formula, d = Kl/b cos θ, the mucus particle’s size is calculated using this method’s data. K is the Scherrer constant, l is the X-ray wavelength, d is the particle size in nanometres (nm), and b is the full width at half maximum of the diffraction peak and the diffraction angle, shown by θ, is half of the Bragg angle that corresponds to the material’s lattice plane.13
Scanning electron microscopy analysis
Using a scanning electron microscope in conjunction with EDX, the morphology and elemental analysis of lyophilized tilapia fish skin mucus samples were examined. The surface morphological characterization was performed by SEM (Joel-JSM 6701-F), whereas the EDX analysis was performed on a Hitachi SU6600 FE-SEM equipped with an EDX attachment. Gold was sputter-coated onto the prepared, lyophilized fish skin mucus samples.14
Anti-bacterial assay
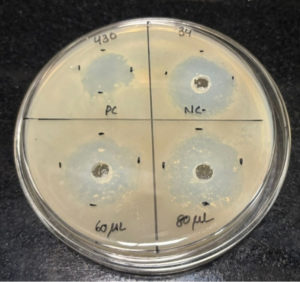
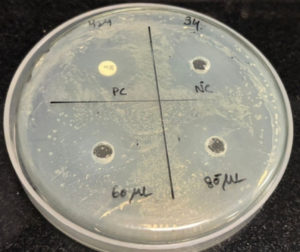
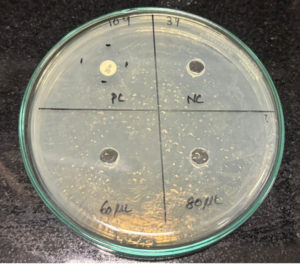
After performing the antibacterial assay, the plates were incubated at 37 °C overnight, and the next day, the zone of inhibition was measured and noted. Streptomycin discs (25 mcg) were placed as the positive control, and phosphate-buffered saline (PBS) was placed as the negative control, which has antibacterial properties. The zones were compared with both positive control and negative control. The measurements showed that the zone of inhibition of the extracts was much higher than the negative control, indicating that the mucus extract shows anti-bacterial properties. The Table 1 below shows the ZOI of the extract to selected bacterial strains. From the below Table 1, we got the results as the mucus extract showed zone of inhibition of 3 cm in 60 µL and zone of Inhibition of 3.2 cm at 80 μL concentration against Bacillus cereus as shown in Figure 1. Similarly, the extracts at 60 µL showed an inhibition of 2.6 cm and 2.8 cm for 80 µL concentration against the bacterial strain Pseudomonas aeruginosa as shown in Figure 2. Also, the extract showed a zone of inhibition of 2.5 cm for 2.5 cm for 60 μL concentration and 2.7 cm for 80 μL concentration, which showed anti-bacterial properties against the bacterial strain Staphylococcus aureus as shown in Figure 3. However, there was no zone of inhibition for both concentrations of mucus extracts against the bacterial strain Klebsiella pneumoniae as shown in Figure 4. This can mean that the tilapia fish mucus does not possess anti-bacterial properties against Klebsiella pneumoniae.
Table (1):
Results of anti-bacterial test
Bacterial strains |
Positive control (ZOI in cm) |
Negative control (ZOI in cm) |
60 µl (mucus extract) (ZOI in cm) |
80 µl (mucus extract) (ZOI in cm) |
|---|---|---|---|---|
MTCC 430 |
2.5 ± 0.1 |
1.8 ± 0.1 |
3 ± 0.1 |
3.2 ± 0.1 |
MTCC 424 |
2.3 ± 0.1 |
2.0 ± 0.1 |
2.6 ± 0.1 |
2.8 ± 0.1 |
MTCC 109 |
1.6 ± 0.1 |
0 |
0 |
0 |
MTCC 87 |
2.1 ± 0.1 |
1.9 ± 0.1 |
2.5 ± 0.1 |
2.7 ± 0.1 |
Statistical analysis
The significant difference between ZOIs derived from tilapia fish aqueous extracts and the various bacterial strains under investigation was assessed using the student’s t-test. A probability value of P < 0.05 was used to determine statistical significance. The results confirmed a significant difference between the ZOI of the tilapia mucus sample of different concentrations obtained against various bacterial strains.
Anti-fungal assay
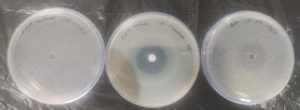
The zone of inhibition observed against the mucus sample’s fungal strain was 1.6 cm, and the standard fluconazole disc was 1.5 cm as shown in Table 2 and Figure 5. Therefore, we can confirm that the sample has shown inhibitory activity against Candida albicans in well diffusion assay.15 Tilapia fish skin mucus exhibits significant antifungal activity against Candida albicans, with efficacy slightly exceeding that of the tested concentration of Fluconazole under these experimental conditions. This suggests that fish skin mucus could be a potential source of bioactive compounds with antifungal properties. Further investigation is warranted to identify the active components and optimize their use in antifungal applications.
Table (2):
Results of anti-fungal test
Test Compounds |
Conc. Per ml |
Zone of Inhibition (cm) |
|---|---|---|
Control |
30 µl |
Nil |
Fluconazole disc |
25 mcg |
1.5 |
Sample |
30 µl |
1.6 |
Fourier transform-infrared spectral analysis
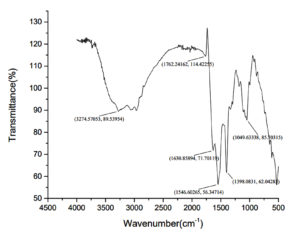
From Table 3 and Figure 6, we can interpret that the FTIR analysis of the fish mucus extract reveals the presence of various functional groups that are indicative of its biochemical composition. The broad O-H stretch observed at 3274.57 cm-1 suggests hydroxyl groups, likely derived from polysaccharides or phenolic 32 compounds, contributing to the mucus’s hydrophilic and adhesive properties. The C=O stretch at 1762.24 cm-1 indicates the presence of carbonyl groups, likely from esters or carboxylic acids, that could be associated with derivatives of fatty acids or lipid constituents in the mucus.16 The Amide I band at 1630.85 cm-1 and the Amide II band at 1546.60 cm-1 are strong indicators of the proteins, which are the major constituents of the mucus and provide structural and functional integrity. The C-H bending at 1398.08 cm-1 represents the existence of alkanes, which could be derived from lipids or fatty acid chains, thereby giving hydrophobic characteristics to the mucus. Finally, the C-O stretch at 1049.63 cm-1 indicates the existence of carbohydrates, which significantly contribute to the mucus’s protective, antimicrobial, and adhesive properties.17 Overall, the FTIR spectrum confirms that the fish mucus extract is a complex mixture of polysaccharides, proteins, lipids, and phenolic compounds, contributing to its biological functions, defense and environmental interaction.
Table (3):
Wavenumber and its corresponding functional group
Wavenumber range (cm-1) |
Functional group |
|---|---|
3274.57 |
O-H stretching (alcohol, phenol) |
1762.24 |
C=O stretching (esters, carbonyl groups) |
1630.85 |
N-H bending (primary amines) |
1546.60 |
Amide II band (proteins) |
1398.08 |
C-H bending (alkanes) |
1049.63 |
C-O stretching (carbohydrates) |
X-ray diffraction assay
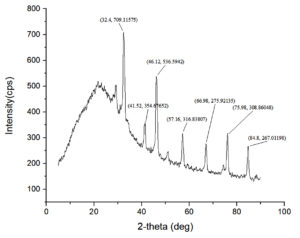
The XRD analysis on tilapia fish mucus resulted in a combination of crystalline and amorphous structures, which is typical in the complex biochemical composition of the sample as shown in Figure 7. The most significant peak at 32.4° with an intensity of 709 cps indicates a highly ordered crystalline structure or a biomolecular arrangement strongly contributing to the diffraction pattern. Another prominent peak at 46.12° with an intensity of 536 cps shows another crystalline phase, which can be due to a specific biomolecular constituent of the mucus. Other diffraction peaks at 41.52°, 57.16°, 66.98°, 75.98°, and 84.8° with intensities ranging from 267 to 354 cps, further prove the existence of multiple crystalline regions in the mucus. The baseline of the lower-angle regions is broad, particularly between 10° and 30°, indicating the presence of an amorphous phase. The amorphous nature is probably due to the disordered arrangement of biomolecules like polysaccharides and proteins that form hydrated and gel-like structures typical of fish mucus.
The sharp peaks corresponding to crystalline regions suggest the presence of organized molecular structures. These could include mineral components, such as calcium phosphate or hydroxyapatite, which may exist in trace amounts and contribute to structural stability. Structural proteins such as mucins might form 34 crystalline aggregates, further emphasizing their role in maintaining the integrity of the mucus.18 The disordered polysaccharides and other biomolecules are responsible for the amorphous regions that are reflected as indicated by the broad hump, providing such components to be adhesive or viscous in nature for the mucus to carry out its protective function.19
The XRD results highlight the dual nature of tilapia fish mucus, combining crystalline regions for structural stability and amorphous regions for flexibility and adhesion. This combination underscores the biological role of fish mucus as a multifunctional material, providing defense, hydration, and interaction with the external environment. The presence of both structural proteins and polysaccharides further reflects its complex composition and functional adaptability.
Scanning electron microscopy analysis
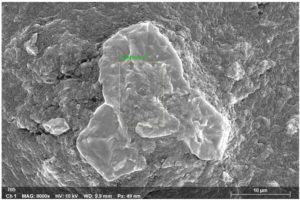
Scanning Electron Microscopy (SEM) analysis provides detailed insights into fish mucus’s surface morphology and structural properties. Based on the SEM image and accompanying EDS spectrum, here is a general interpretation of the SEM analysis of tilapia fish mucus:
The SEM analysis of tilapia fish mucus is shown in Figure 8 above at 8000x magnification. It included specific information such as the high voltage of 10 kV and a working distance of 9.9 mm with a pixel size of 49 nm. The results showed layered and agglomerated. This rough and irregular texture indicates the composition’s complexity, typical of biological mucus containing proteins, lipids, and polysaccharides. The dense and irregular morphology indicates that the mucus is a physical barrier to protect the fish against microbial invasion and environmental stress.20
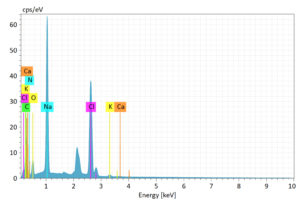
The EDS mapping results obtained in Figure 9 revealed the elemental mapping of the tilapia fish mucus. The major elements indicated are carbon, oxygen, and nitrogen. Various trace elements such as sodium, chlorine, potassium, and calcium were obtained from the peaks recorded from the EDS spectrum. Light elements such as C, O, and N are represented by peaks at lower energy levels (<2 keV). Salts are represented by medium energy peaks, such as those at about 2.6 keV for Na and 3 keV for Cl. Higher peaks for K and Ca indicate mineral content around 3.7-4 KeV.
Worldwide, continued usage of antibiotics has led to the emergence of several resistant bacterial strains. Therefore, a search for synthetic antibiotic substitutes is urgently needed. Fish live in a harsh environment with several problems. The bacteria significantly impact the health of the fish. They can escape such an environment by producing certain substances on their dermal layer called mucus. Water and gel-forming macromolecules, such as mucin and other glycoproteins, comprise most of the epidermal mucus generated by epidermal goblets or mucus cells. This layer is important in playing various defense roles, such as in osmoregulation and providing an immune system for the fish.21 Various small specialized peptides derived from various animal and plant origins play a role in mucus as antimicrobial peptides. These possess protective functions against various bacteria, yeast, and fungal species and act as a biological barrier between the fish and the potential threats in the surrounding environment. Numerous organisms exhibit antimicrobial properties; however, most antibacterial agents derived from marine sources lack sufficient activity compared to conventional antimicrobials from microorganisms.22
In the present study, the aqueous mucus extract of Oreochromis niloticus has been studied for its antimicrobial activity and characteristics, such as its functional groups, crystalline, amorphous nature, etc. From the results obtained by performing the agar well diffusion assay, it is well understood that the fish mucus of Nile tilapia shows antibacterial activity against bacteria, Bacillus cereus, Pseudomonas aeruginosa, and Staphylococcus aureus, except Klebsiella pneumoniae, for which it did not exhibit any. The highest inhibitory effect was observed against Bacillus cereus, as indicated by a larger zone of inhibition, suggesting a strong antibacterial potential. In contrast, Staphylococcus aureus exhibited the lowest inhibition, implying a degree of resistance or reduced sensitivity to the extract. Also, as per Figure 5, the aqueous extract shows antifungal properties against Candida albicans, a most potent fungal species, by a zone of inhibition of 1.6 cm owing to the various antimicrobial peptides, lysozymes, and other antimicrobial components. This study establishes that these agents in the fish mucus can be a potential source of therapeutic drugs against various microbes. While the study provides valuable insights, it is limited by the lack of chemical characterization of the active compounds. Future studies should employ chromatographic and spectroscopic techniques to identify the specific bioactive molecules. Additionally, testing a broader range of microorganisms, including multidrug-resistant strains, would enhance the applicability of these findings. Esteban and Tiralongo et al. highlighted the antimicrobial potential of fish mucus, particularly due to bioactive molecules like lysozymes, lectins, antimicrobial peptides (AMPs), and proteases. The FTIR results showing the presence of amide, hydroxyl, and carbonyl groups support the existence of such molecules.23
The FTIR spectrum revealed the functional groups present in the sample, confirming its chemical composition. Key peaks corresponded to bonds such as O-H (hydroxyl), C=O (carbonyl), and C-H (alkyl or aromatic groups), which may contribute to the antibacterial activity. The presence of these functional groups supports the hypothesis that the material contains bioactive molecules with antimicrobial potential.24 The XRD graph provided information on the crystalline nature of the material. Distinct peaks at specific 2θ angles indicate the presence of crystalline phases, while any broad peaks suggest amorphous content. The reports from Chinnadurai et al. and Cordero et al. describing structured protein-polysaccharide complexes in fish mucus contribute to the observation of amorphous and amorphous regions in the XRD, depicting an idea of its functional stability and antimicrobial action. The crystallinity and phase composition of the sample may influence its physical and biological properties, including antibacterial efficacy.25 Thus, the tilapia’s mucus contains bioactive substances that can stop the growth of microorganisms and acts as a natural defense against infections. By developing natural and efficient methods to fight bacterial and fungal infections in aquaculture, an understanding of the antimicrobial qualities of tilapia mucus might lessen the need for antibiotics. Sustainable fish farming with little chemical inputs can result from leveraging fish’s inherent defensive systems to support better stocks. The promising antimicrobial properties of Nile tilapia warrant the development of advanced delivery systems or formulations to maximize stability, bioavailability, and efficacy. Research has revealed that Nile tilapia mucus has several antimicrobial substances such as lysozyme, piscidin, and beta-defensins. Future work can concentrate on purifying these substances in their pure state, determining their maximum purity and concentration, and defining their precise mechanisms of action. Isolated chemicals can be unstable under specific conditions or not absorbed by the body well. Future formulations can solve this by adding stabilizing additives, applying controlled release mechanisms, or applying nanotechnology to deliver them more effectively to target tissue or cells. Research can also target the development of formulations for human health, e.g., wound healing products or antimicrobial medical device coatings, or aquaculture use, e.g., disease prevention or treatment of tilapia aquaculture.
Investigating the antibacterial qualities of tilapia fish mucus provides insightful information and possible uses in various industries, including medicine and aquaculture. The tilapia’s mucus contains bioactive substances that can stop the growth of microorganisms and act as a natural defense against infections. By developing natural and efficient methods to fight bacterial and fungal infections in aquaculture, an understanding of the antimicrobial qualities of tilapia mucus might lessen the need for antibiotics. Sustainable fish farming with little chemical inputs can result from leveraging fish’s inherent defensive systems to support better stocks. Antimicrobial peptides (AMPs), enzymes, and glycoproteins are among the bioactive compounds found in tilapia mucus that may be turned into novel medications to treat infections resistant to antibiotics. The mucus is a possible source of broad-spectrum antibacterial chemicals because it shows effectiveness against various pathogens. The potential of tilapia mucus extracts as natural antibacterial agents in food preservation could be investigated to increase the shelf life of fish and other perishable goods. To stop infections and encourage healing, tilapia mucus components such as antimicrobial peptides could be used in skin care products and wound dressings. Researching the antibacterial qualities of tilapia fish mucus has two advantages: it advances our understanding of innate immunity and opens practical applications in aquaculture, medicine, and food safety. Using these natural defenses could promote healthier aquaculture methods and provide long-term answers to urgent global issues like food preservation and antibiotic resistance.
ACKNOWLEDGMENTS
The authors would like to thank CHRIST (Deemed to be University) for providing the necessary support and guidance to carry out the research work.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
MG conceptualized the study. MG, SK and AM applied methodology. MP collected resources and performed supervision. SSA, JM and GM helped in providing the resources, software support and data validation. SK and AM performed formal analysis. SSA, JM and GM wrote, reviewed and edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
ETHICS STATEMENT
Not applicable.
- Diaz-Puertas R, Adamek M, Mallavia R, Falco A. Fish Skin Mucus Extracts: An Underexplored Source of Antimicrobial Agents. Mar Drugs. 2023;21(6):350.
Crossref - Falco A, Adamek M, Pereiro P, et al. The Immune System of Marine Organisms as Source for Drugs against Infectious Diseases. Mar Drugs. 2022;20(6):363.
Crossref - Kumari S, Tyor AK, Bhatnagar A. Evaluation of the antibacterial activity of skin mucus of three carp species. Int Aquat Res. 2019;11(3):225-239.
Crossref - Tiralongo F, Messina G, Lombardo BM, Longhitano L, Li Volti G, Tibullo D. Skin Mucus of Marine Fish as a Source for the Development of Antimicrobial Agents. Front Mar Sci. 2020;7:541853.
Crossref - Dash S, Das SK, Samal J, Thatoi HN. Epidermal mucus, a major determinant in fish health: a review. Iran J Vet Res. 218;19(2):72-81.
- Llewellyn MS, Leadbeater S, Garcia C, et al. Parasitism perturbs the mucosal microbiome of Atlantic Salmon. Sci. Rep. 2017;7(1):43465.
Crossref - Vajargah MF. A review of the physiology and biology of Nile tilapia (Oreochromis niloticus). J Aquac Mar Biol. 2021;10(5):244-246.
Crossref - Al-Rasheed A, Handool KO, Garba B, et al. Crude extracts of epidermal mucus and epidermis of climbing perch Anabas testudineus and its antibacterial and hemolytic activities. Egypt J Aquat Res. 2018;44(2):125-129.
Crossref - Puray JJS, Villaber RAP. Extraction, characterization, and vascular response of proteins from catfish (Clarias batrachus L.) mucus. Food Chem Adv. 2023;3:100444.
Crossref - Valgas C, de Souza SM, Smania EFA, Smania Jr. A. Screening methods to determine antibacterial activity of natural products. Braz J Microbiol. 2007;38(2):369-380.
Crossref - Peterson LR, Shanholtzer CJ. Tests for bactericidal effects of antimicrobial agents: technical performance and clinical relevance. Clin Microbiol Rev. 1992;5(4):420-432.
Crossref - Nirmala MJ, Dhas SP, Saikrishna N, Raj US, Sai PS, Nagarajan R. Green nanoemulsions: Components, formulation, techniques of characterization, and applications. Bio-Based Nanoemulsions for Agri-Food Applications. 2022:47-69.
Crossref - Ghafarifarsani H, Hoseinifar SH, Vijayaram S, et al. Comparative Effect of Chemical and Green Zinc Nanoparticles on the Growth, Hematology, Serum Biochemical, Antioxidant Parameters, and Immunity in Serum and Mucus of Goldfish, Carassius auratus (Linnaeus, 1758). Biol Trace Elem Res. 2024;202(3):1264-1278.
Crossref - Sridhar A, Manikandan DB, Palaniyappan S, Sekar RK, Ramasamy T. Correlation Between Three Freshwater Fish Skin Mucus Antiproliferative Effect and Its Elemental Composition Role in Bacterial Growth. Turk J Fish & Aquat Sci. 2021;21(5):233-244.
Crossref - Bergeron MG, Brusch JL, Barza M, Weinstein L. Bactericidal activity and pharmacology of cefazolin. Antimicrob Agents Chemother. 1973;4(4):396-401.
Crossref - Oluwole AJO, Ikhu-Omoregbe DI, Jideani VA. Physicochemical Properties of African Catfish Mucus and Its Effect on the Stability of Soya Milk Emulsions. Appl Sci. 2020;10(3):916.
Crossref - Yaswanthkumar S, Raja S, Kannan K, Nagasundar S. Investigation of functional groups in epidermal mucus of endemic Bagridae fishes of Western Ghats. Int J Fish Aquat Stud. 2022;10(1):112-115.
Crossref - Shanthi N, Murugesan S, Nithia SMJ, Kotteswari M, Shyamala Gowri S. UV-B Induced Changes to the Physiological and Phytochemical Parameters of Phyllanthus amarus Schum. Haya Saudi J Life Sci. 2020;5(12):265-273.
Crossref - Tasleem F, Tabassum R, Siddique AB, et al. Epidermal mucus as a potential biological and biochemical matrix for fish health analysis. GSC Adv Res Rev. 2024;19(3):246-262.
Crossref - Cordero H, Brinchmann MF, Cuesta A, Esteban MA. Chronic wounds alter the proteome profile in skin mucus of farmed gilthead seabream. BMC Genomics. 2017;18(1):939.
Crossref - Uthayakumar V, Ramasubramanian V, Senthilkumar D, Priyadarisini VB, Harikrishnan R. Biochemical characterization, antimicrobial and hemolytic studies on skin mucus of fresh water spiny eel Mastacembelus armatus. Asian Pac J Trop Biomed. 2012;2(2):S863-S869.
Crossref - Ellis AE. Immunity to bacteria in fish. Fish Shellfish Immunol. 1999;9(4):291-308.
Crossref - Akhavan-Bahabadi M, Shekarabi SPH, Yilmaz E. Fish epidermal mucus-derived antimicrobial peptides: Classification, structure, biological activities, and potential biotechnological applications. Ann Anim Sci. 2025.
Crossref - Baker MJ, Trevisan J, Bassan P, et al. Using Fourier transform IR spectroscopy to analyze biological materials. Nat Protoc. 2014;9(8):1771-1791.
Crossref - Chinnadurai G, Subramanian R, Ahamed M. Fish mucus mediated biosynthesis of copper oxide nanoparticles: spectral characterization, morphology and biological activity. Mater Res Express. 2020;7(12):125012.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.