ISSN: 0973-7510
E-ISSN: 2581-690X
Green synthesis of metal nanoparticles (NPs) from plant extracts has attracted significant interest in modern medicine. Therefore, this study prepared an aqueous extract of Sophora flavescens roots, which are used in folk medicine to treat several diseases, including bacterial infections. In addition, silver NPs (AgNPs) were synthesized from root extract using the green synthesis method. The NPs were diagnosed using modern methods. Additionally, the antibacterial activity of the root aqueous extract and AgNPs aqueous preparation (at concentrations of 7% and 9%, respectively) was examined against selected isolates of multidrug-resistant Pseudomonas aeruginosa and Staphylococcus aureus. The results indicated that both the plant extract and NP preparations inhibited pathogenic bacterial isolates.
Antibacterial Activity, Green Synthesis, Multidrug-resistance Bacteria, Silver Nanoparticles, Sophora flavescens
Humanity seems to be moving in the direction of what is called the ‘‘‘post-antibiotic era”’. This implies that it is possible for a simple bacterial species to become increasingly fatal to humans. Thus, scientists have described resistance to antibiotics as “the silent tsunami facing modern medicine”. Recent projections state that the absence of prompt intervention for multidrug-resistant (MDR) pathogens will result in an annual of 10 million people by 2050, a number that resembles the current rate caused by cancer (Naskar1; Zaboon & Al-Hayanni2).
Nanotechnology is a promising solution that can replace antibiotics, through the use of nanoparticles (NPs) sized between 10 – 1000 nm (Mohanraj & Chen3). Nanotechnology has been recently developed to obtain new preparations of NPs that widely differ in shape and size while having a wide spectrum of properties against bacteria. The fact that NPs can directly kill bacteria and carry other antibacterial agents makes them more promising for overcoming MDR (Gupta
et al4).
NP can directly attack the cell wall of a pathogen, without penetrating into the cell. This renders the majority of the mechanisms followed by MDR bacteria to avoid non-functional treatment agents, reducing the possibility of developing resistance to antibiotics. Such observations have focused on the development of state-of-the-art NP-derived materials that show bactericidal activity against bacteria (Wang et al5). NPs possess a wide range of properties that can be used to kill both gram-positive and gram-negative bacteria (Ramalingam et al6).
As a result of the increased applications of NPs in medical practices, the amount of research conducted to comprehend the mechanisms followed by these particles to combat bacteria has notably increased. One prominent example is the ability of these particles to alter bacterial metabolism, providing a remarkable advantage in the treatment of bacterial infections (Chatzimitakos & Stalikas7).
Sophora flavescens, also called ‘Kushen’ in China, is a plant that is prominently grown in China, Russia, Korea, Japan, and India. The roots of this plant have been used in traditional Chinese medicine as a common treatment for cancer, inflammation, eczema, pruritus, dysentery, trichomoniasis, and pyogenic skin infections (Li
et al8).
Therefore, in this study, we synthesized AgNPs and evaluated their antibacterial activity. In this context, Sophora flavescens aqueous root extract was used to synthesize (AgNPs). The antibacterial activity of the AgNPs was screened against two Gram-negative and Gram-positive bacterial strains.
Bacterial Isolates
Two isolates of Pseudomonas aeruginosa (Gram-negative) and Staphylococcus aureus (Gram-positive) were obtained from clinical samples and supplied by the Biology Department, College of Science for Women, University of Baghdad. Confirmatory diagnosis was made using the Vitek-2 system, in addition to determining their sensitivity to antibiotics.
Antibiotics Susceptibility Test
The antibiotic susceptibility test of the bacterial isolates to various classes of antibiotics (Ceftazidime, Amikacin, Meropenem, Tobramycin, Gentamicin, Levofloxacin, Cefepime for P. aeruginosa, and Tetracycline, Ciprofloxacin, Clindamycin, Erythromycin, Gentamicin, Vancomycin, and Trimethoprim for S. aureus) was conducted using the disk diffusion method (Kirby-Bauer method), as described by Bauer et al9 and based on the Clinical Laboratory Standard Institute recommendations (CLSI10). The sensitivity of bacterial isolates to a particular antibiotic was determined by measuring the diameter of the inhibition zone. Reference tables were used to classify the isolates as sensitive (S), intermediate (I), or resistant (R) to the aforementioned antibiotic agents.
Preparation of Sophora flavescens Root Aqueous Extract
In this study, the roots of S. flavescens were purchased from local markets in Baghdad. The plant samples were identified by a botanist in the Department of Biology/College of Science for Women. An aqueous extract of S. flavescens roots was prepared according to the method described by Al-Hayanni & El-Shora11. To obtain an extract solution from the roots, 60 g of dried powder was macerated with 600 ml of distilled water for 3 h, followed by filtration with Whatman No.1 filter paper and evaporation with a rotary evaporator (N-1200B, EYELA) under reduced pressure.
Preparation of AgNO3 Solution
In 100 ml of deionized water (DW), 16.98 g of silver nitrate was dissolved. Then, 1 ml of the prepared AgNO3 solution was added to another 1000 ml of DW to obtain a 1 mM solution (Alnuaimi et al12).
Green Synthesis of Silver Nanoparticles using Sophora flavescens Root Aqueous Extract
Five milliliters of S. flavescens root aqueous extract were sprayed into 95 ml of AgNO3 solution (1 mM). The solution was subjected to ultra-sonication (flow rate = 0.2 ml/min, ultrasonic power = 100 W, frequency = 42 kHz, time = 20 min)followed by mixing with a magnetic stirrer (800 rpm, 25°C, 30 min)and storage in dark bottles for 24 h. To obtain a clear supernatant, the mixture was centrifuged (10 min, 10000 rpm, 4°C), resulting in a colloid preparation which was kept in dark vials. The color change of the solution was monitored for five days (Alnuaimi et al12).
Characterization and Examination of Silver Nanoparticles
After obtaining a stable color change, the solution was centrifuged for 10 min at 5000 rpm to remove any precipitate. The supernatant obtained was then centrifuged for 30 min at 12000 rpm. To eliminate any residual plant extract, the pellet was washed with deionized DW. The final pellet was resuspended in DW before being used for characterization.
Spectrophotometric Analysis
To measure the absorption maxima of the synthesized AgNPs, spectral scanning (200-800 nm) was performed using a UV-visible spectrophotometer (Shimadzu) (Anandalakshmi et al13).
Zeta Potential
A zeta potential analyzing device was used to assess the stability of the synthesized AgNPs (Zeta sizer Nano-ZS90), with voltages ranging from − 160 to +160 mV, and the data were plotted (Raja et al14).
Atomic Force Microscopy (AFM)
A thin film sample of NPs (100 μL) was placed on a glass slide and left to dry for 5 min. The slides were examined using AFM (Elangovan et al15).
Fourier Transform Infrared Spectrometer (FT-IR)
An FT-IR analyzer was utilized to evaluate the solution of AgNPs and plant extract (500 – 4000 cm-1 region, 8 cm-1 resolution) using an FT-IR spectrometer (Shimadzu). The sample containing the synthesized AgNPs (1 mg) was incorporated with KBr (300 mg) to for a hydraulic pellet, which was then pressed and analyzed using FTIR spectroscopy. Peaks were observed for the functional groups in the synthesized particles (Anandalakshmi et al13).
Atomic Absorption Spectroscopy (AAS)
The concentration of Ag ions in the solution was determined using atomic absorption spectroscopy (GBC 932 AA) over time. These measurements can be used to infer the conversion of Ag+ to Ag0. The sample was withdrawn and centrifuged at 14,000–15,000 rpm during the course of the reaction. Unreacted silver nitrate (Ag+) in the supernatant solution should be much smaller than that of Ag0. Thus, the pellets contained AgNPs (Ag0). The amount of Ag+ ions in the supernatant solution was then determined using AAS. Because silver precipitation is highly sensitive to the presence of Cl–, DW was used in this procedure (Choudhary et al16).
Antibacterial Activity of Sophora flavescens Root Aqueous Extract and Aqueous AgNPs
The study of the antibacterial activity of the S. flavescens root aqueous extract and the aqueous AgNPs against the antibiotic-resistant P. aeruginosa and S. aureus was conducted by transferring 0.1 ml of standardized inoculum (1.5 ×108 CFU/mL, 0.5 McFarland’s standard) of each bacterial isolate and 0.1 mL of each concentration (7% and 9%) prepared from the extract and the aqueous AgNPs to sterile test tubes. After incubation at 37°C for 15 min, the mixture was poured onto sterile Petri dishes containing Muller-Hinton agar (MHA) and spread using a spreader. All the dishes were incubated at 37°C for 24 h. The antibacterial activity was observed by counting the bacterial colonies and comparing them with that of the control. Three dishes (replicates) were used for each concentration to reduce errors that might have resulted from conducting the experiment (Balouiri et al17).
Statistical Analysis
The Statistical Analysis System (SAS18) program was used to detect the effects of the different factors on the study parameters. The least significant difference (LSD) test (analysis of Variance -ANOVA) was used to compare the means in this study.
Green Synthesis of Silver nanoparticles using Sophora flavescens root aqueous extract
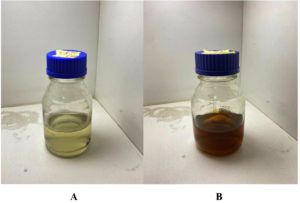
In the current study, S. flavescens roots aqueous extract was used to synthesize AgNPs. The reaction mixture exhibited a color change that was monitored by visual observation. Following the addition of 1mM silver nitrate solution to the root extract of S. flavescens, the solution initially exhibited a yellowish color. Color intensity exhibited a gradual increase to dark brown as incubation time was raised (Kalimuthu et al19), as shown in Figure 1.
Figure 1. Color change during the bio-reduction of AgNO3 into AgNPs using S. flavescens roots aqueous extract, (A): S. flavescens roots aqueous extract, (B): AgNPs
Spectrophotometric analysis
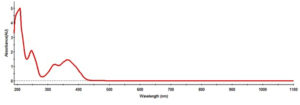
The UV-vis spectral patterns of the AgNPs prepared from the aqueous extract of S. flavescens roots are shown in Figure 2. A broad absorption band appeared at 380 nm, indicating that the NPs were poly-disperse. Surface plasmon resonance (SPR) revealed that the NPs had an absorption band that reflected an almost identical or symmetrical shape. These results suggested that spherical NPs were formed, without a high degree of aggregation (Samimi et al20).
Figure 2. The UV-Vis absorption spectrum of the synthesized Silver nanoparticles of S. flavescens root aqueous extract
The bioreduction of Ag+ to Ag0 was verified by measuring the sample after dilution with a constant volume of deionized water to avoid a high value of absorbance (Kalimuthu et al19) DW was used as blank.
Zeta potential
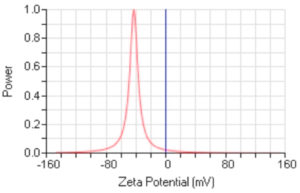
Using the green method for the synthesis of AgNPs revealed stable AgNPs, as confirmed by the zeta potential value of −42.75 mV (Figure 3). Zeta potential analysis is dependent on the manner in which the NPs move under the effect of an electric field. Such movement shows a dependence on the particle’s surface charge and the local environment (Retamal Marín et al21).
AFM analysis
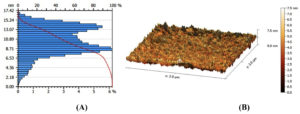
The findings related to the morphological aspects of AgNO3 NPs coated with powder prepared from the aqueous solution of S. flavescens roots were validated by AFM analysis. These NPs demonstrated varying features of morphology and size; they were generally monodisperse and had a uniform size of approximately 11 nm (Figure 4).
Figure 4. AgNPs produced by the reaction of AgNO3 solution (1 mM) with S. flavescens roots aqueous extract as imaged using an AFM micrograph, (A): Column AFM diagram of the size range of AgNPs (B): Three-dimensional AFM image of AgNPs
FTIR spectrum analysis of AgNPs
Figure 5 depicts the results of the FTIR spectral analysis of AgNO3 NPs produced using the aqueous extract of S. flavescens roots. The FTIR spectra of S. flavescens root aqueous extract are shown in Figure 5 (A), whereas those of the AgNPs are shown in Figure 5 (B). The AgNPs expressed visible bands at 3419.79, 2926.01, 2854.65, 1739.79, 1629.85, 1382.96, 1074.35, 1049.28, 929.69, 829.39, 707.88, 597.93, 470.63 and 437.84 cm−1. The bands observed at 3419.79 cm−1 represent the existence of N–H stretching vibrations of the amine/phenol functional groups. The bands observed at 2926.01 and 2854.65 cm−1 suggested the binding of AgNPs with hydroxyl and carboxyl groups. The band at 1739.79cm−1 suggests the binding of AgNPs with the C=O band. The peaks observed at 1629.85 cm−1 correspond to the C=C stretch of the alkene functional groups. Therefore, the observed functional groups are believed to stabilize the synthesized AgNO3 NPs through their reduction and capping activities (Bin Bakri et al22).
Figure 5. FTIR spectra results
(A): FTIR of S. flavescens root aqueous extract, (B): The FTIR of AgNPs
Atomic Absorption Spectroscopy (AAS)
Following the stabilization of the changes occurring in the color of AgNO3 NPs solutions of various concentrations, the concentration of Ag+ ions in their supernatants was determined using an AAS device. The conversion of Ag+ to Ag0 is represented by the rate of reduction in the concentration of Ag+ ions (Choudhary et al16).
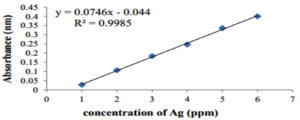
Different concentrations of AgNO3 (1, 2, 3, 4, 5, and 6 mM) were prepared in DW and a standard curve was obtained (Figure 6). The concentrations were found to be 73 and 58 ppm for the AgNPs and S. flavescens root aqueous extract, respectively.
Figure 6. The standard curve made of AgNO3 dilutions for the determination of Silver ion concentration
The antibiotics susceptibility patterns
According to the antibiotic susceptibility result (Table 1), the two bacterial isolates exhibited MDR patterns. The isolate of P. aeruginosa was resistant to all the tested antibiotics, whereas the isolate of S. aureus was sensitive to only two antibiotics (Gentamicin and Trimethoprim).
Table (1):
The results of antibiotics susceptibility test for Pseudomonas aeruginosa and Staphylococcus aureus isolates by Kirby Bauer method.
Antibiotics |
P. aeruginosa |
Antibiotics |
S. aureus |
|---|---|---|---|
Ceftazidime |
R |
Tetracycline |
R |
Amikacin |
R |
Ciprofloxacin, |
R |
Meropenem |
R |
Clindamycin |
R |
Tobramycin |
R |
Erythromycin |
R |
Gentamicin |
R |
Gentamicin |
S |
Levofloxacin |
R |
Vancomycin |
R |
Cefepime |
R |
Trimethoprim |
S |
The antibacterial activity
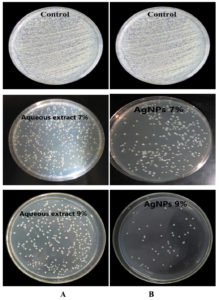
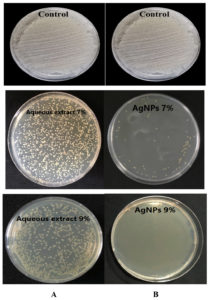
The current study showed that S. flavescens roots aqueous extract and AgNPs have antibacterial effects against MDR P. aeruginosa and S. aureus isolates at concentrations of 7% and 9%; respectively. The AgNPs were found to have a higher antibacterial than that of the aqueous extract activity, as shown in Table 2, and Figures 7 and 8.
Table (2):
The antibacterial activity of the Sophora flavescens roots aqueous extract and the aqueous AgNPs against Pseudomonas aeruginosa and Staphylococcus aureus.
| The antibacterial activity of the Sophora flavescens roots aqueous extract | |||
|---|---|---|---|
| Concentration of extract (% ) | Pseudomonas aeruginosa | Staphylococcus aureus | |
| Control | 1.5 ×108 a | 1.5 ×108 a | |
| 7% | 1.84×103 b | 1.4×106 b | |
| 9% | 2.96 ×102 c | 4.11×102 c | |
| LSD value | 20.81 * | 17.63 * | |
| The antibacterial activity of the aqueous AgNPs | |||
| Concentration of AgNPs (% ) | Pseudomonas aeruginosa | Staphylococcus aureus | |
| Control | 1.5 ×108 a | 1.5 ×108 a | |
| 7% | 1.74×102 b | 7.8×101 b | |
| 9% | 4.8 ×101 c | Non c | |
| LSD value | 38.16 * | 44.68 * | |
Means having with the different letters in same column differed significantly.
* (P≤0.05).
Figure 7. (A): The antibacterial activity of the Sophora flavescens roots aqueous extract with concentrations of 7% and 9% against Pseudomonas aeruginosa, (B): The antibacterial activity of the 7% and 9% aqueous AgNPs against P. aeruginosa
Figure 8. (A): The antibacterial activity of the Sophora flavescens roots aqueous extract with concentrations of 7% and 9% against Staphylococcus aureus, (B): The antibacterial activity of the 7% and 9% aqueous AgNPs against S. aureus
In the current study, S. flavescens root aqueous extract had a bactericidal activity when used to treat gram-positive bacteria than for gram-negative bacteria. These results are similar to those of another study that revealed that S. flavescens root aqueous extract had an inhibitory effect on S. aureus but had no effect on P. aeruginosa (Yang et al23). The present results also agree with those of previous studies. For example, the same extract elicited remarkable antibacterial activity upon its application to treat Escherichia coli, Staphylococcus aureus, Streptococcus, Bacillus dysenteriae, Salmonella spp., and Bacillus proteus. In addition, the extract strongly inhibited the growth of methicillin-resistant S. aureus and methicillin-sensitive Staphylococcus epidermidis, while revealing a particularly potent killing activity against S. epidermis (Yang et al24).
This may be due to the structural difference in the bacterial wall, as gram-positive bacteria lack a layer of outer membranes, which makes the permeability of materials entering the cell greater compared to that in gram-negative bacteria, whose inner wall has an internal barrier represented by a common lipopolysaccharide with multiple proteins, has the ability to prevent the passage of many harmful substances into the cell (Nader et al25; Al-Mossawei et al26). This reason may also be due to the higher resistance of P. aeruginosa compared to S. aureus to antibiotics used in the current study.
S. flavescens is known for the production of a large spectrum of secondary metabolites, a number of which have gained remarkable interest because of their wide range of biological activities and novel structures. Before 2015, researchers were able to isolate and identify approximately 200 compounds from S. flavescens. These compounds were divided into four main groups: flavonoids (15 flavonols, 41 flavanones, 13 flavanonols, 27 isoflavones, 3 isoflavones, 2 homo isoflavones,7chalcones, 2 biflavonoids, and14 pterocarpans), 47 alkaloids, 9 terpenoids, and 26 other compounds that are regarded as the main active components of this Chinese medicine
(He et al27).
Alkaloids and flavonoids are the main components of S. flavescens. These two groups have been reported to confer significant pleiotropic activities, such as anti-inflammatory, anti-bacterial, anti-cancer, and anti-arrhythmic (Ma et al28).
Alkaloids from this plant have the ability to produce the pro-inflammatory cytokine TNF-a. The goal of this production, which occurs through the regulation of the expression of BMP2, Runx2, and other proteins, to increase the activity of alkaline phosphatase to treat chronic osteomyelitis condition to increase from infection with S. aureus (Wang et al29).
Studies have demonstrated the medicinal properties of different types of alkaloids and flavonoids, extracted from the roots through various procedures. Recent research has shown the positive effects of these extracts against many diseases like different kinds of tumors, allergies, diabetes, hepatitis, dental caries, and various types of infections, owing to their anti-microbial and analgesic properties (Liaqat et al30).
Unfortunately that the pathways used by NPs to exert their bactericidal activity against bacteria are not completely clear. One proposal is that this activity depends on the production of reactive oxygen species (ROS), while other suggestions include non-oxidative mechanisms such as the release of metal ions that could lead to direct damage to the bacterial cell (Zhao
et al31). Another finding that has been reported in more recent reports is the possible interference between NPs and the synthesis pathways of bacterial DNA and proteins (Chuy et al32; Chakraborty et al33). These particles were also shown to be capable of regulating the expression of metabolic genes. Also of great importance is the effective inhibition caused by the NPs to the process of biofilm formation in bacteria (Smerkova et al34).
The results of this study strengthen and support the use of S. flavescens in folk medicine to treat bacterial infections. They confirmed that this plant possesses a wide spectrum of inhibitory activities against important pathogenic bacteria responsible for many diseases that threaten human health, and a number of these species are known to be responsible for infections acquired in hospitals, including those with high resistance to antibiotics, in addition to their in food poisoning, foodborne diseases, and food spoilage. This plant plays an important role in the pharmaceutical and food industries, in addition to its potential role in the synthesis of nanoparticles that can be used to control pathogenic bacteria, thus giving it future pharmaceutical importance.
Based on the results of this study, we recommend further studies to separate and characterize the antimicrobial compounds present in S. flavescens. In addition, we recommend studying the effects of nanoparticles and S. flavescens extracts on other important pathogens such as fungi, viruses, and other pathogenic bacteria. Pharmaceutical companies and research institutes should be particularly interested in producing new medicines using this plant.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All listed authors have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
- Naskar A, Kim KS. Nanomaterials as delivery vehicles and components of new strategies to combat bacterial infections: Advantages and limitations. Microorganisms. 2019;7(9):356.
Crossref - Zaboon SM, Al-Hayanni HAS. Detection of virulence factor genes and antibiotic resistance of Enteropathogenic Escherichia coli (EPEC) isolated from children with diarrhea. Biochem. Cell. Arch. 2021,21:791-796.
- Mohanraj VJ, Chen Y. Nanoparticles-a review. Tropical journal of pharmaceutical research. 2006;5(1):561-73.
Crossref - Gupta A, Mumtaz S, Li CH, Hussain I, Rotello VM. Combatting antibiotic-resistant bacteria using nanomaterials. Chemical Society Reviews. 2019; 48(2):415-27.
Crossref - Wang L, Hu C, Shao L. The antimicrobial activity of nanoparticles: present situation and prospects for the future. International journal of nanomedicine. 2017;12:1227.
Crossref - Ramalingam B, Parandhaman T, Das SK. Antibacterial effects of biosynthesized silver nanoparticles on surface ultrastructure and nanomechanical properties of gram-negative bacteria viz. Escherichia coli and Pseudomonas aeruginosa. ACS applied materials & interfaces. 2016;8(7):4963-76.
Crossref - Chatzimitakos TG, Stalikas CD. Qualitative alterations of bacterial metabolome after exposure to metal nanoparticles with bactericidal properties: a comprehensive workflow based on 1H NMR, UHPLC-HRMS, and metabolic databases. Journal of proteome research. 2016, 15(9):3322-30.
Crossref - Li JC, Zhang ZJ, Liu D, Jiang MY, Li RT, Li HM. Quinolizidine alkaloids from the roots of Sophora flavescens. Natural Product Research. 2020;1-8.
- Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45(4):493–496.
Crossref - Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing. Twenty-first informational supplement. Wayne, PA, USA: CLSI.2020.
- Al-Hayanni HS, El-Shora H. Various Extracts of Some Medicinal Plants as Inhibitors for Beta-lactamase Activity. Baghdad Science Journal. 2021;18(1):0047.
Crossref - Alnuaimi MT, Hamdan NT, Abdalraheem E, Aljanabi ZZ. Biodegradation of malathion pesticide by silver bio-nanoparticles of Bacillus licheniformis extracts. Research on Crops. 2019;20.
Crossref - Anandalakshmi K, Venugobal J, Ramasamy V. Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Applied nanoscience. 2016;6(3):399-408.
Crossref - Raja S, Ramesh V, Thivaharan V. Green biosynthesis of silver nanoparticles using Calliandra haematocephala leaf extract, their antibacterial activity and hydrogen peroxide sensing capability. Arabian journal of chemistry. 2017;10(2):253-61.
Crossref - Elangovan K, Elumalai D, Anupriya S, Shenbhagaraman R, Kaleena PK, Murugesan K. Phyto mediated biogenic synthesis of silver nanoparticles using leaf extract of Andrographis echioidesand its bio-efficacy on anticancer and antibacterial activities. Journal of Photochemistry and Photobiology B: Biology. 2015;151:118-24.
Crossref - Choudhary MK, Kataria J, Cameotra SS, Singh J. A facile biomimetic preparation of highly stabilized silver nanoparticles derived from seed extract of Vigna radiata and evaluation of their antibacterial activity. Applied nanoscience. 2016;6 (1):105-11.
Crossref - Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: A review. J Pharm Anal. 2016;6 (2):71-79.
Crossref - SAS, Statistical Analysis System, User’s Guide. Statistical. Version 9. 6th ed. SAS. Inst. Inc. Cary. N.C. USA. 2018.
- Kalimuthu K, Babu RS, Venkataraman D, Bilal M, Gurunathan S. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids and surfaces B: Biointerfaces. 2008;65(1):150-3.
Crossref - Samimi S, Maghsoudnia N, Eftekhari RB, Dorkoosh F. Lipid-based nanoparticles for drug delivery systems. Characterization and biology of nanomaterials for drug delivery. Micro and Nano Technologies, 2019;1: 47-76.
Crossref - Retamal Marín RR, Babick F, Hillemann L. Zeta potential measurements for non-spherical colloidal particles – Practical issues of characterisation of interfacial properties of nanoparticles. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2017;532: 516-521.
Crossref - Bin Bakri MK, Rahman MR, Khui PLN, Jayamani E, Khan A.5 -Use of sustainable polymers to make green composites. Advances in Sustainable Polymer Composites. Woodhead Publishing Series in Composites Science and Engineering. 2021;109-129.
Crossref - Yang JF, Yang CH, Wu CC, Chuang LY. Antioxidant and antimicrobial activities of the extracts from Sophora flavescens. Journal of Pharmacognosy and Phytochemistry. 2015;3(6).
- Yang J, Liu P, Wu XY. In vitro antibacterial activity of extracted Sophora flavescens against Staphylococcus epidermidis. Chin.J.Nosocomiol., 2007;17: 1357–1358.
- Nader MI, Ghanima KK, Ali SA, Azhar DA. Antibacterial activity of ginger extracts and its essential oil on some of pathogenic bacteria. Baghdad Science Journal. 2010;7(3):1159-65.
Crossref - Al-mossawei MT, Ali AH, Abid HS, Murad RH. Antimicrobial activity of ethanolic extracts of Raphanus sativus and Cyperus rotundus against some pathogenic bacteria and Candida albicans. Baghdad Science Journal. 2014:11(1).
- He X, Fang J, Huang L, Wang J, Huang X. Sophora flavescens Ait.: Traditional usage, phytochemistry and pharmacology of an important traditional Chinese medicine. Journal of Ethnopharmacology. 2015; 172:10-29.
Crossref - Ma H, Huang Q, Qu W, Li L, Wang M, Li S, Chu F. In vivo and in vitro anti-inflammatory effects of Sophora flavescens residues. Journal of ethnopharmacology. 2018;224:497-503.
Crossref - Wang X, Zheng R, Huang X, Mao Z, Wang N, Li H, Wen C, Shou D. Effects of alkaloids from Sophora flavescens on osteoblasts infected with Staphylococcus aureus and osteoclasts. Phytotherapy Research. 2018; 32(7):1354-63.
Crossref - Liaqat S, Durrani A, Sajjad F, et al. Sophora Flavescens in Dentistry: A Systematic Review: Sophora Flavescens in Dentistry. Pakistan BioMedical Journal. 2022;31:139-43
Crossref - Zhao R, Lv M, Li Y, et al. Stable Nanocomposite Based on PEGylated and Silver Nanoparticles Loaded Graphene Oxide for Long-Term Antibacterial Activity. ACS Applied Materials & Interfaces. 2017;9(18): 15328-15341.
Crossref - Chuy GP, Muraro PCL, Viana AR, et al. Green Nanoarchitectonics of Silver Nanoparticles for Antimicrobial Activity Against Resistant Pathogens. Journal of Inorganic and Organometallic Polymers and Materials. 2022;32 (4): 1213-1222.
Crossref - Chakraborty B, Kumar RS, Almansour AI, et al. Evaluation of antioxidant, antimicrobial and antiproliferative activity of silver nanoparticles derived from Galphimia glauca leaf extract. J. King Saud Univ Sci. 2021;33:101660.
Crossref - Smerkova K, Dolezelikova K, Bozdechova L, et al. Nanomaterials with active targeting as advanced antimicrobials. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2020 ;12(5):e1636.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.