ISSN: 0973-7510
E-ISSN: 2581-690X
The Sygygium polyantum L. leaf has been used as spice as well as traditional herbal medicine. The methanolic extracts of S. polyanthum L. was tested for antibacterial and sporicidal activities against vegetative cells and spores of Bacillus pumilus ATCC14884 and B. megaterium ATCC14581. The disc diffusion assay (DDA), minimum inhibition concentration (MIC), minimal bacterial concentration (MBC) and sporicidal activities of the extract on Bacillus sp. were analyzed using Clinical and Laboratory Standard Institutes (CLSI) methods. The effect of the extract on spores was visualized using scanning electron microscope (SEM). The results show that the inhibition zone of the extract on B. pumilus and B. megaterium was 12.16 ± 0.57 mm. The extract has been able to inhibit the growth of B. pumilus and B. megaterium vegetative cells with MICs of 0.08- and 0.04 mg/ml, respectively. Moreover, the B. pumilus and B. megaterium can be killed by MBCs of 1.25- and 0.64 mg/ml, respectively. One presence of extract can kill 100% of all Bacillus sp. spores during 1 h exposure time. SEM observation showed that the spores were destroyed by the extract. These results suggested that S. polyanthum L. extract can be developed as antibacterial and antispore against Bacillus sp.
Antibacterial activity, Bacillus pumilus, B. megaterium, Sporicidal activity, Sygygium polyanthum L. extract
Gram-positive bacteria, such as Bacillus and Clostridium sp. respond to adverse environmental stresses by forming a dormant structure known as endospore (simply termed as spore) through the process of sporulation (Leggett et al., 2012). Spores are able to survive the harsh external conditions, such as nutrient starvation or desiccation, and germinate after the favourable growth conditions returned (Tan and Ramamurthi, 2013). Bacterial spores’ resilient and highly resistant characteristic poses problems to the food industries (Leggett et al., 2012). Germination of spores into vegetative cells under favourable conditions is frequently associated with food spoilage and foodborne diseases (Barker et al., 2005).
Bacillus spores are highly resistant to various chemical disinfectants. In addition, there are limitations to several chemical sporicidal agents used to eradicate Bacillus spores, such as formaldehyde and glutaraldehyde which are toxic and require special precaution for use (Kida et al., 2004). On the other hand, thermal processing is a relatively inexpensive and effective method of producing food safe from undesirable microorganisms and enzymatic reactions. However, the setbacks of thermal processing include reduction in the nutrient content and the organoleptic qualities are affected (Cho et al., 2008). Therefore, the development of effective, safe and stable sporicidal agents is gaining more attentions (Kida et al., 2004).
Medicinal plants are used widely in the food industry as spices for flavours and fragrances, and some of them contain phytochemical compounds that exhibit antimicrobial activity against a wide spectrum of foodborne bacteria. This led to suggestions that they could be used as natural food preservatives (Cho et al., 2008). The need to develop natural preservatives with potential sporicidal ability or natural sporicidal agent which able to reduce the populations of Bacillus spores in rice or starchy foods has prompted the study in determining the sporicidal activity of tropical medicinal plants.
The leaves of S. polyanthum L. which is also known as “daun salam” in Indonesia are commonly used in dishes as spice in culinary such as “nasi liwet” due to its aroma besides the sour taste and also as ingredient in the Indonesian traditional medicine ‘‘Jamu’’ (Kato et al., 2013). S. polyanthum L. is effective against ulcers, hypertension, diabetes, hyperuricemia, diarrheal, gastritis, skin diseases and inflammation (Ismail et al., 2013). In addition to its ability to neutralise residual alcohol, this plant also has diuretic and analgesic effects (Sumono and Wulan, 2008). Research conducted by Sumono and Wulan (2008) reported that the young shoots of S. polyanthum L. are consumed as a fresh salad (ulam), whereas the mature leaves were regularly added as a flavor enhancer in Malaysian cuisine. Fresh and dried aromatic leaves of S. polyanthum L. are useful in cooking for their scent, color and flavor. It is also often used as flavoring spice for meat, fish, and vegetable dishes, or in rice (De Guzman and Siemonsma, 1999). The aim of this study was to evaluate the antibacterial and sporicidal activities of S. polyanthum L. against B. pumilus and B. megaterium vegetative cells and spores.
Plant materials
Dried leaves of S. polyanthum L. was purchased from Herbal Markets (Pasar Baru, Bandung) in Indonesia. The samples were deposited in the Laboratory of Natural Products at Institute of Bioscience (IBS) in the Universiti Putra Malaysia (UPM). Dried leaves were ground using a heavy duty blender (Waring, USA) until fine powder was formed. The powder of S. polyanthum L. was kept in a sealed polyethylene plastic bag and stored in a refrigerator (4°C) until required.
Plants extraction preparation
Extracts were prepared using the maceration method as described previously by (Rukayadi et al., 2008). The dried leaves were ground to a powder using an electric miller, and 100 g of the powder was mixed with 400 ml of 99.8% (v/v) methanol and incubated at room temperature for 48 hours. The plant extracts were filtered with Whatman ûlter paper size No. 1, and concentrated with a rotary vacuum evaporator at 40oC. Firstly, the crude extract was dissolved in 100% dimethylsulfoxide (DMSO) at a concentration of 100 mg/ml (10%), and the solution was then further diluted by 1:10 (v/v) in distilled water to obtain a 10 mg/ml (1%) stock solution. The final concentration of DMSO was 10% in the stock solution. The 10% of DMSO did not kill the bacteria used.
Bacillus strain and spore preparation
Bacillus pumilus ATCC14884 and Bacillus megaterium ATCC14581 were obtained from American Type Collection Culture (Rockville, MD, USA). Both B. pumilus and B. subtilis were cultured, grown and maintained statically in nutrient broth (NB) (Difco, Sparks, MD, USA) or NB supplemented with 1.5% (w/v) agar (NA). B. pumilus and B. megaterium spores were prepared according to the method described previously by Kida et al. (2003) and Rukayadi et al. (2009), with modification. B. pumilus and B. megaterium were grown on NA at 30°C for over 1 week. The spores and vegetative cells were harvested and suspended in sterile 0.85% NaCl solution. Heat shock at 65°C was applied to the suspension for 30 min to kill vegetative cells. Spores were harvested by centrifugation and washed four times with the original volume of sterile 0.85% NaCl solution by centrifugation (13,000 × g for 30 min at 4°C). A 1 mL portion of the spore suspension containing approximately 108 spores/ml was stored in a 1.5 ml plastic cryopreservation tube at -18°C until further use.
Disc diffusion assay
The methanolic S. polyanthum L. leaves extract was tested for antimicrobial activity using the disc diffusion method as described by the Clinical and Laboratory Standards Institute (CLSI, 2012). The B. pumilus and B. megaterium were streaked on Mueller Hinton Agara (MHA) (Difco, Spark, USA) plates with a sterile cotton swab. Sterile filter paper discs of 6 mm diameter were placed on top of the agar, and 20 µl of the 10 mg/ml (w/v). S. polyanthum L. extract was loaded onto the paper discs. A 0.1 mg/ml chlorhexidine (CHX) was used as a positive control in the assay. Finally, the plates were incubated at 30°C for 24 hours. The presence of a clear zone indicated bacterial growth inhibition, and its diameter was measured in mm.
Determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
The determination of MIC and MBC were done according to the methods described by CLSI (2012). The MIC and MBC of S. polyanthum L. extract against the vegetative cells of B. pumilus and B. megaterium were determined in a 96 Wells microtiter plate using two-fold standard broth microdilution methods with an inoculum of approximately 106 CFU/ml. Briefly, 100 µl of S. polyanthum L. extract stock solution (10 mg/ml = 10,000 µg/ml) was mixed and diluted two-fold with the test organism in Mueller Hinton broth (MBH) (Difco, Spark, USA) (100 µl). Column 12 of the microtiter plate contained the highest concentration of the extract (5,000 µg/ml), while column 3 contained the lowest concentration (19.50 µg/ml). Column 2 served as the positive growth control for all the samples (MHB and inoculum), and column 1 as negative growth control (only MHB, no inoculum and antibacterial agent), respectively.
The micro titer plate was then incubated aerobically at 30°C for 24 hours. The MIC was defined as the lowest concentration of antibacterial agent that resulted in the complete inhibition of visible growth. The MBC was determined for each bacterial species as outlined for MIC, by removing the media from each well showing no visible growth, and further sub-cultured onto MHA plates. The plates were then incubated at 30°C for 24 hours until visible growth was seen in the control plates. Similarly, MBC was defined as the equivalent concentration required killing the microorganism completely. Additionally, both MIC and MBC tests were done in duplicate (n = 3 × 2).
Sporicidal assay
The prepared spores suspension was thawed and diluted 1:100 in 0.85% NaCl solution (pH 6.6), yielding an initial B. pumilus and B. megaterium spores suspension of each approximately 5.00 × 105 spores/ml. The stock extract (10%) was diluted in adjusted spores suspension, resulting in final concentrations of extract (0.10, 0.50 and 1.00%). A standard 25% commercially available glutaraldehyde solution (Merck Millipore, Darmstadt, Germany) was used as positive control in the determination of sporicidal activity. The glutaraldehyde was diluted 1:25 in distilled water to yield 1% (w/v) concentration for further testing. The pH of these test solutions was not changed by addition of extract or glutaraldehyde. One ml of each concentration was then exposed to 1 h incubation times in a water bath (30°C). A 100 µl aliquot was removed and transferred to microcentrifuge tubes, centrifuged (12, 000 × g at 4°C for 5 minutes) and rinsed twice with 0.9 ml of 0.85% NaCl solution (pH 6.6) to obtain bacterial-free spores and to avoid effect of vegetative cells residue. Pellets were suspended in 100 µl of 0.85% NaCl solution (pH 6.6), serially diluted and spread onto NA plates and incubated at 30°C for 24 hours or until the colonies were seen on the plates. Colonies that formed on the duplicate plates were counted and the mean of colony-forming unit (CFU/ml) was calculated. Differences were obtained by subtracting the Log10 CFU/ml values of the test solution from those of the control (no antimicrobial). The reduction of spore cells in CFU was expressed as sporicidal activity. The determination of sporicidal activity was done three times with duplicate per each experiment (n = 3 × 2).
Scanning Electron Microscopy (SEM) Analysis
B. pumilus spores were mixed with 1% (w/v) of methanolic S. polyanthum L. extract then incubated for 1 hour at 30°C. Spores were recovered by centrifugation and pellets were fixed with 4% buffered glutaraldehyde for 6 hours at 4°C, washed with 0.1 M sodium cacodylate buffer for 10 minutes and was repeated for 3 times. The spore pellets was then post fixed with 1% osmium tetroxide for 2 hours at 4°C, washed again with 0.1 M sodium cacodylate buffer for 10 minutes and was repeated for 3 times. Then the pellets were dehydrated using 35, 50, 75 and 95% for 15 minutes each. Finally the pellets were dehydrated using 100% acetone (Merck Millipore, Darmstadt, Germany) for 15 minutes and were repeated for 3 times. Cell suspension was transferred into a specimen basket, made from aluminium foil coated with albumin, and put in critical dryer for 0.5 hour. The specimen was mounted on a stub and the sputter was coated with gold. The morphology of the spores was observed and images were obtained using SEM instrument (JSM 6400, JEOL Ltd., Tokyo, Japan).
Statistical Analysis
Mean of data obtained (n = 3 × 2) were calculated using Microsoft Excel 2010 for Windows. Data were then analysed using the Analysis of Variance (One-way ANOVA) procedure of the Minitab ® Version 16. 2. 4 for Windows (Minitab Inc.). When significance was indicated, means were separated using Tukey test (±=0.05).
The antimicrobial properties of plants have been recognized for a long time. In many cases, however, mechanisms are not well understood (García-alvarado et al., 2008). In this study, the results showed the potency of S. polyanthum L. extracts to inhibit and kill the vegetative cells of B. pumilus and B. megaterium as well as to kill the their spores. Table 1 shows the results of disc diffusion assay of S. polyanthum L. on vegetative cells of B. pumilus and B. megaterium. Visible clear zone indicates inhibition of bacterial growth. The diameter of inhibition zone against B. pumilus and B. megaterium was 12.66 ± 0.57 mm. Lau et al. (2014) reported that methanolic extract of S. polyanthum L. leaves can inhibit the growth of B. cereus and B. subtilis with visible clear zone of 8.0 and 7.5 mm, respectively. The results suggested that S. polyanthum L. extract was more susceptible on B. pumilus and B. megaterium. Moreover, Prabhakaran et al. (2011) reported that ethanol and water root and bark extracts of S. cumini inhibited bacterial growth of B. pumilus, B. cereus, B. megaterium and B. subtilis with inhibition zones ranged between 11mm and 14 mm.
Table (1):
Inhibition zone diameter of S. polyanthum L. extracts against B. pumilus and B. megaterium.
Bacterial species |
Inhibition zone diameter (mm) |
|---|---|
Bacillus pumilus ATCC14884 Positive control (0.05% Chlorhexidine) Negative control (10% DMSO) |
12.16 ± 0.57 12.00 ± 0.00 na |
Bacillus megaterium ATCC14581 Positive control (0.05% Chlorhexidine) Negative control (10% DMSO) |
12.16 ± 0.57 11.50 ± 0.05 na |
na: No inhibition zone
Disc diffusion method is just semi qualitative method as a preliminary check for antibacterial activity (Burt, 2004); however, the antibacterial activity of plant extract may be more accurately evaluated using minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) values (Jun et al., 2013). The MICs and MBs values of S. polyanthum L. extract against B. pumilus and B. megaterium is presented in Table 2. The MIC was defined as the lowest concentration of antimicrobial agent that resulted in the complete inhibition of visible growth, while MBC is the corresponding concentrations required to kill the microorganisms completely (Rukayadi et al., 2009). Meaning that 0.08- and 0.04 mg/ml of extract can inhibit the growth of B. pumilus and B. megaterium vegetative cells, respectively. The extract can kill completely the vegetative cells of B. pumilus and B. megaterium with concentration of extract of 1.25- and 0.63 mg/ml. Lau et al. (2014) reported that the methanolic S. polyanthum L. extract the growth vegetative cells of B. cereus ATCC33019 and B. subtilis ACTT6633 with MIC of 0.31- and 0.63 mg/ml, respectively. Moreover, those bacteria can be killed by the extract with concentration of 2.50 mg/ml. These results were also support that the methanolic S. polyanthum L. extract was more powerful to inhibit and to kill B. pumilus and B. megaterium.
Table (2):
MIC and MBC values of S. polyanthum L. extracts against B. pumilus and B. megaterium.
Tested bacteria |
MIC (mg/ml) |
MBC (mg/ml) |
|---|---|---|
Bacillus pumilus ATCC14884 |
0.08 |
1.25 |
Bacillus megaterium ATCC14581 |
0.04 |
0.63 |
The sporicidal activity of S. polyanthum L. extract was tested at different concentrations of 0.1, 0.5 and 1.0% for 1 h. Glutaraldehyde was reported to have sporicidal activity against spore forming bacteria and used as positive control in this study, even though it is not allowed in food application (Russell, 1990). The reduction in the viability of B. pumilus and B. megaterium spores at different concentrations for 1 h of incubation were presented in Table 3. The initial inoculum of B. pumilus and B. megaterium spores was approximately 5 × 105 spores/ml. The S. polyanthum L. extract at 0.5% concentration showed potential sporicidal activity with a sharp reduction in the number of B. pumilus and B. megaterium spores for more than 3 Log units (99.99%). However, the complete killing of B. pumilus and B. megaterium spores was achieved after treated with S. polyanthum L. extract at 1% concentration for 1 h of incubation.
Table (3):
Sporicidal activity of S. polyanthum L. against spores of B. pumilus and B. megaterium.
| Concentration of extract or glutaraldehyde (%) | CFU/ml of spores / glutaraldehyde or S. polyanthum L. extract / bacterial strain | |||
|---|---|---|---|---|
| Glutaraldehyde | S. polyanthum L. extract |
|||
| B. pumilus ATCC14884 |
B. megaterium ATCC14581 |
B. pumilus ATCC14884 |
B. megaterium ATCC14581 |
|
| 0.0 | a5.53 ± 0.05 | a5.48 ± 0.11 | a5.31 ± 0.05 | a5.31 ± 0.05 |
| 0.1 | c3.10 ± 0.07 | b4.19 ± 0.07 | b4.12 ± 0.07 | b4.12 ± 0.07 |
| 0.5 | d0.00 ± 0.00 | c3.78 ± 0.08 | c3.75 ± 0.05 | c3.75 ± 0.05 |
| 1.0 | d0.00 ± 0.00 | d0.00 ± 0.00 | d0.00 ± 0.00 | d0.00 ± 0.00 |
a,b,c: Significant different (α = 0.05)
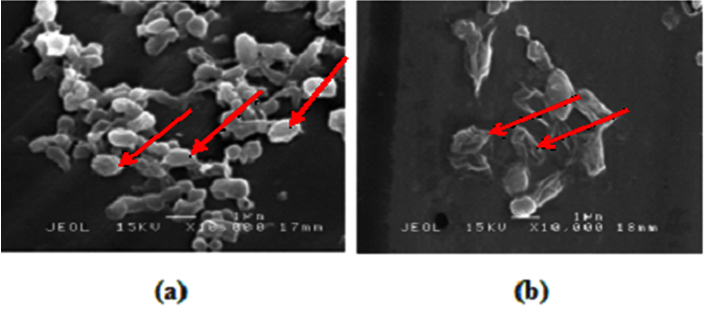
The reports that plant extract has sporicidal activity are still limited. Torilis japonica extract showed activity against spores of B. subtilis (Cho et al., 2008), while the essential oils of cardamom, tea tree, and juniper leaf was also found to be effective against spores of B. subtilis (Lawrence and Palombo, 2009). Lau et al. (2014) reported that methanolic S. polyanthum L. extract has sporicidal activity against spore of B. cereus and B. sublitis; 2.50% of the extract completely killed the spores of those bacteria after 1 h of incubation. In this study, the extract completely killed the spores of B. pumilus and B. megaterium with concentration of 1.0% after 1 h of incubation. These results suggested that the sporicidal activity of methanolic S. polyanthum L. against spores of B. pumilus and B. megaterium was relatively stronger than that against the spores of B. cereus and B. subtilis. Moreover, scanning electron microscope observation showed that 1% of the extract for 1 h destroyed the spores of B. pumilus (Figure 1).
Fig. 1. Effect of methanolic S. polyanthum L. extract on Bacillus pumilus ATCC14884; (a) no extract, (b) with 1% extract for 1 h
The sporicidal properties of medicinal plants are related to the phytochemical components present. Tassou et al. (1991) reported that oleuropein purified from olive extract inhibited both the germination and the subsequent outgrowth of spores of B. cereus. In addition, macelignan isolated from nutmeg exhibit inhibition activity towards the growth of vegetative cells and sporicidal activity against spores of B. cereus (Rukayadi et al., 2009). On the contrary, lichocalcone A isolated from the roots of licorice (Glycyrrhiza inflate) has antibacterial activity against vegetative cells of B. subtilis, but did not inhibit the germination B. subtilis spores (Tsukiyama et al., 2002). In reality, simple comparisons are difficult because of differences in tested bacteria and the concentrations used. In this study, the methanolic S. polyanthum L. extract was found to exhibit the germination B. pumilus and B. megaterium spores.
S. polyanthum L. leaf was found to contain essential oils such as simple phenols, phenolic acids, and lactones sekuisterfenoid, triterpenoids, saponins, flavonoids, and tannins (Davidson and Branen, 1993). In addition, Zhang et al. (2013) reported that leaves of S. polyanthum L. contain polyphenols and flavonoids, and that these bioactive compounds help the plant to resist against microorganisms, thereby providing evidence for anti-infectious properties. The high content of polyphenols and flavonoids indicates antimicrobial properties. These biological activities are related to the molecules structures; through their hydroxyl groups or phenolic rings, phenolic compounds have the capacity to link with proteins and bacterial membranes to form complexes (Cheikna et al., 2011). Thus, further research, identification and isolation the active compounds which responsible to sporicidal activity in the extract of S. polyanthum L. need to be analysed.
In conclusion, it is remarkable to note that the methanolic S. polyanthum L., extract confers significant antibacterial and sporicidal activity against vegetative cells and spores of spore-forming bacteria, B. pumilus and B. megaterium. Thus, S. polyanthum L. extract might be good to be developed as natural antibacterial and anti-spore.
ACKNOWLEDGMENTS
This research is supported by FRGS grant Yaya Rukayadi Project no: FRGS/2/2014/SG05/UPM/02/2
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
- Barker, G.C., Malakar, P.K., Peck, M.W. Germination and growth from spores: variability and uncertainty in the assessment of food borne hazards. Int J Food Microbiol, 2015; 100(13): 67-76.
- Burt, S. Essential oils: their antibacterial properties and potential applications in foods a review. Int J Food Microbiol, 2004; 94(3): 223-253.
- Cheikna, Z., Savadogo, A., Somda, M. K., Koudou, J., & Traore, A. S. In vitro evaluation of the antimicrobial and antioxidant properties of extracts from whole plant of Alternanthera pungens HB & K. and leaves of Combretum sericeum G. Don. Int J Phytomed, 2011; 3: 182.
- Cho, W.I., Choi, J.B., Lee, K., Chung, M.S., Pyun, Y.R. Antimicrobial activity of torilin isolated from Torilis japonica fruit against Bacillus subtilis. J Food Sci, 2008; 73(2): M37-M46.
- Clinical and Laboratory Standards Institute (CLSI). Quality Manual, 3rd edition. USA: CLSI, 2012.
- Davidson, P.M., Branen, A.L. Antimicrobials in Foods, 2nd ed. Marcel Dekker, Inc. N.Y., 2013.
- De Guzman, C. C., Siemonsma, J. S. Plant resources of South. East Asia, 2008; 13: 148-164.
- García-Alvarado, J. S., Verde-Star, M. J., Heredia, N. L. Traditional uses and scientific knowledge of medicinal plants from Mexico and Central America. J Herbs, Spices Med Plants, 2001; 8: 37-89.
- Ismail, A., Mohamed, M., Sulaiman, S.A., and Wan Ahmad, W.A.N. 2013. Autonomic nervous system mediates the hypotensive effects of aqueous and residual methanolic extracts of Syzygium polyanthum (Wight) Walp. var. polyanthuml eaves in anaesthetized rats. Evid-Bas Comp Alter Med, 2013; ID 716532.
- Jun, H., Kim, J., Bang, J., Kim, H., Beuchat, L.R., Ryu, J.-H. Combined effects of plant extracts in inhibiting the growth of Bacillus cereus in reconstituted infant rice cereal. Int J Food Microbiol, 2013; 160(3): 260-266.
- Kato, E., Nakagomi, R., Gunawan-Puteri, M.D.P.T., Kawabata, J. Identification of hydroxychavicol and its dimers, the lipase inhibitors contained in the Indonesian spice, Eugenia polyantha. Food Chem, 2013; 136(3 4): 1239-1242.
- Kida, N., Mochizuki, Y., Taguchi, F. An effective iodide formulation for killing Bacillus and Geobacillus spores over a wide temperature range. J App Microbiol, 2004: 97:402-409.
- Kida, N., Mochizuki, Y., Taguchi, F. An effective sporicidal reagent against Bacillus subtilis spores. Microbiol Immunol, 2003; 47: 279-283.
- Lawrence, H.A., Palombo, E.A. Activity of essential oils against Bacillus subtilis spores. J Microbiol Biotechnol, 2009; 19(12): 1590-1595.
- Lau, K. Y., Zainin, N. S., Abas, F., Rukayadi, Y. Antibacterial and sporicidal activity of Eugenia polyantha Wight against Bacillus cereus and Bacillus subtilis. Int J Curr Microbiol Appl Sci, 2014; 3: 499-510.
- Leggett, L.E, Mackean, G., Noseworthy, T.W. Current status of health technology reassessment of non-drug technologies: survey and key informant interviews. Health Res Policy Systems, 2012; 10:38.
- Russell, A.D. Bacterial spores and chemical sporicidal agents. Clin Microbiol Rev, 1990; 3(2): 99-119.
- Rukayadi, Y., Shim, J.-S., Hwang, J.-K. Screening of Thai medicinal plants for anticandidal activity. Mycoses, 2008; 51(4): 308-312.
- Rukayadi, Y., Lee, K., Han, S., Kim, S., Hwang, J.-K. Antibacterial and sporicidal activity of macelignan isolated from nutmeg (Myristica fragrans Houtt.) against Bacilluscereus. Food Sci Biotechnol, 2009: 18(5): 1301-1304.
- Sumono, A., Wulan, S.D.A The use of bay leaf (Eugenia polyantha Wight) in dentistry. Dent J. 2008; 41:147-50.
- Tan, I.S, Ramamurthi, K.S. Spore formation in Bacillus subtilis. Env Microbiol Reports, 2014; 6(3): 212–225.
- Tassou, C.C., Nychas, G.J., Board, R.G. Effect of phenolic compounds and oleuropein on the germination of Bacillus cereus T spores. Biotechnol Appl Biochem, 1991; 13(2): 231-237.
- Tsukiyama, R., Katsura, H., Tokuriki, N., and Kobayashi, M. Antibacterial activity of licochalcone A against spore forming bacteria. Ant Agents Chemother, 2002; 46(5): 1226-1230.
- Zhang, L., Ravipati, A. S., Koyyalamudi, S. R., Jeong, S. C., Reddy, N., Bartlett, J., Jiménez, E. Anti-fungal and anti-bacterial activities of ethanol extracts of selected traditional Chinese medicinal herbs. Asian Pac J Trop Med, 2013; 6: 673-681.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.