ISSN: 0973-7510
E-ISSN: 2581-690X
Garlic contains various components, such as allicin, ajoenes, and allyl sulfides, which are organosulfur compounds. It exhibits antimicrobial activities in all forms, including garlic powder, garlic extract, and garlic oil. Additionally, it exerts anti-inflammatory, antibacterial, antibiofilm, and antifungal effects. The drastic increase in drug-resistant organisms worldwide, especially methicillin-resistant Staphylococcus aureus (MRSA) strains, has led to treatment failure in various infectious diseases. To overcome this issue, we aimed to assess the antibacterial activity of garlic extract against MRSA and explore its action mechanism against penicillin-binding proteins (PBPs) in this study. Mueller–Hinton agar was used for the agar well diffusion test. Moreover, bactericidal activity was determined using the Mueller–Hinton agar and spot tests. Molecular docking was performed using AUTODOCK to evaluate the PBP-binding ability of allicin. A wide zone of inhibition was observed for MRSA, and molecular docking revealed a strong interaction between PBP3 of S. aureus and allicin. Overall, our findings revealed the antibacterial activity of garlic extract against MRSA, a prevalent gram-positive coccus causing healthcare-associated infections. In addition to its affordability, the beneficial effects of garlic suggest its therapeutic potential for MRSA treatment in combination with cefoxitin.
Allicin, Antibacterial Activity, Staphylococcus aureus, Cefoxitin
Garlic is widely used as a medicinal herb owing to its organosulfur content. Approximately 3000 publications have reported its antibacterial activity.1 Garlic is consumed in various forms, such as powder, oil, roasted, and boiled forms. It is used as a traditional medicine worldwide owing its various health benefits, including antimicrobial activities, protection against food poisoning, and digestion-improving effects.2 Garlic (Allium sativum) is a natural spice and flavoring agent that prevents infections. The key phytochemicals in garlic are organosulfur compounds, such as allicin, ajoenes, and allyl sulfides. Garlic organosulfur compounds exhibit various antibacterial activities, including bactericidal and antibiofilm activities, against many bacteria, including multidrug-resistant (MDR) strains. Many studies have demonstrated its antibacterial effects against Mycobacterium tuberculosis and Listeria sp.2 but only a few have conducted in silico analyses of its association with penicillin-binding proteins (PBPs).
The World Health Organization lists allicin as an active ingredient with various targets. Allicin also inhibits DNA synthesis.3 It is electrophilic and reacts with cysteine, glutathione, and coenzyme A to inhibit protein synthesis.2 In addition to its antibacterial activity, garlic oil exhibits antifungal activity against Candida albicans.4 Kyo et al. reported that garlic exhibits antihistaminic activity and inhibits airway allergic responses.1 Treatment of infectious diseases is challenging owing to the prevalence of MDR and methicillin-resistant Staphylococcus aureus (MRSA) strains worldwide.5 S. aureus is a gram-positive bacterium.
Gram-positive bacteria have cell walls containing teichoic acid, which maintains cell homeostasis, division, and structure.6 Peptidoglycan is another important component of the bacterial cell wall, which is essential for bacterial shape and survival. Teichoic acid also plays an important role in virulence. Transpeptidases are important structural domains for bacterial survival. S. aureus causes various infections, such as superficial skin infections, urinary tract infections, sepsis, meningitis, cellulitis, food poisoning, endocarditis, and brain abscesses.7,8
Generally, infections caused by community-acquired S. aureus are self-limiting when properly treated with antibiotics.9 In contrast, hospital-acquired S. aureus are among the most common organisms causing healthcare-associated infections. Increased drug resistance of S. aureus led to the development of MRSA strains.10 MRSA infections are major life-threatening clinical problems that increase the morbidity and mortality of infected patients. S. aureus contains a PBP system encoding four proteins: PBP1, PBP2, PBP3, and PBP4.11 Specifically, PBP2a is resistant to β-lactam antibiotics, including methicillin, leading to the development of MRSA strains.12 MecA is responsible for drug resistance in these strains.13 Here, we aimed to determine the potential of garlic extract to overcome MRSA drug resistance. Additionally, we evaluated the in vitro susceptibility of MRSA strains to the garlic extract.
Here, a prospective study was conducted for three months (February–April, 2023) at the Saveetha Medical College and Hospital after obtaining approval from its Institutional Review Board. The experimental procedures used in this study are described below.
Extraction of allicin
Fresh garlic was purchased from the local market. Then, garlic cloves (20 g) were peeled, mixed with distilled water, and ground using a sterile motor and pestle. The extract was centrifuged at 5000 rpm for 10 min. Then, the supernatant was collected and filtered for future use. The obtained extract was 100% concentrated. Here, a previously described procedure was used.4
Bacterial isolation
Clinical samples (pus) were collected and cultured on nutrient and mannitol salt agar. Various biochemical tests, including gram staining, catalase, coagulase, mannitol motility, and Christen’s urease tests, were performed using the suitable controls.3 Moreover, antimicrobial susceptibility tests were performed using penicillin, gentamycin, cotrimoxazole, clindamycin, erythromycin, ciprofloxacin, vancomycin, and linezolid. Cefoxitin discs were used for MRSA screening.
16S rRNA for microbial identification
Gene sequencing was performed to confirm the MRSA isolates. DNA was isolated from the culture using a spin column-based manual extraction procedure.14 Its quality was evaluated on 1.0% agarose gel, and a single band of large molecular weight DNA was observed. The 16S rRNA gene fragment was amplified using the 16S rRNA-F and 16S rRNA-R primers. A single discrete PCR amplicon band of 1500 bp was observed on the agarose gel. Then, PCR amplicons were purified to remove any contaminants. Forward and reverse DNA sequencing of the PCR amplicons were performed using the 16S rRNA-F and 16S rRNA-R primers, respectively, with the BDT v3.1 Cycle sequencing Kit on the ABI 3730xl Genetic Analyzer. The consensus sequence of 16S rRNA was generated using the forward and reverse sequence data with the Aligner software. Then, 16S rRNA gene sequence was used to perform BLAST on the ‘nr’ NCBI GenBank database. Based on the maximum identity score, first 10 sequences were selected and aligned using the multiple alignment software program, ClustalW.15 Distance matrices and phylogenetic trees were constructed using MEGA 10.
Bactericidal activity
Bactericidal activity was assessed by determined the minimum inhibitory concentration (MIC). The culture and aqueous garlic extracts were prepared. Various dilutions of aqueous garlic extract (16, 8, 4, 2, 1, 0.5, 0.25, and 0.125 µL) were used. Then, 1 µL of the MRSA inoculum was added and incubated for 24 h at 37 °C. Then, the microtiter plate was taken, and spots were made for each dilution on the nutrient agar plate for 24 h at 37°C.16
Figure 1 shows the bactericidal activity of allicin against MRSA.
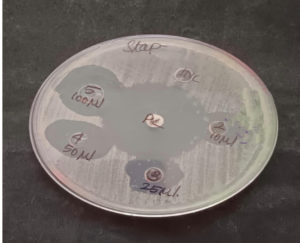
Antibacterial effects of garlic on S. aureus assessed using the agar well diffusion method
MRSA inoculum was added to a nutrient broth and incubated overnight. Then, the culture was swabbed onto the Mueller–Hinton agar plate, and a cylindrical bore was made using a syringe puncture. The aqueous garlic extract was pipetted into the cylindrical bore using a micropipette (10, 25, 50, and 100 µL) along with the positive and negative controls, incubated for 24 h at 37 °C, and the zone of inhibition was observed. The presence of a zone of inhibition indicates that the drug is effective.17
Figure 2 shows the antibacterial effects of garlic assessed using the agar well diffusion method.
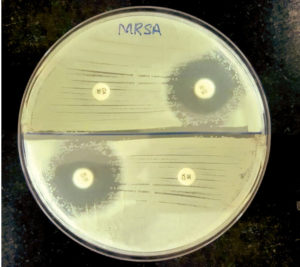
Infusion of garlic extract into the commercial cefoxitin disc
As MRSA was susceptible to the garlic extract, we further assessed the activity of A. sativum using commercially available cefoxitin discs. MRSA inoculum was swabbed onto the Mueller–Hinton agar plate.18 Then, commercial cefoxitin was placed on the Muller–Hinton agar plate, and 50 μL of garlic extract was added to the cefoxitin disc. The plate was incubated for 24 h at 37°C.
Molecular docking
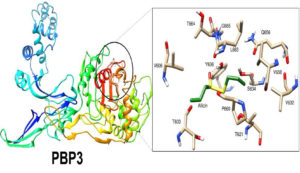
Molecular docking was conducted against PBP3, as previously described, using the AUTODOCK software.5,19 Briefly, ligand allicin (PubCMEM ID: 65036) was prepared and directly downloaded as a Mol 2 file. The downloaded ligand file was minimized and converted into the PDBQT format using Open Babel. AUTODOCK uses gradient calculations and Monte Carlo strategies to search for and rank the docking poses based on a scoring function.20 This approach facilitates the exploration of similar conformational spaces.21,22 The converted ligands, along with PBP3, were selected from Wizard. A grid was defined around the active site residues (N450, Q524, T621, S448, T603, P660, and E623) based on the crystallographic structure, and the coordinates were set.23,24 Subsequently, the allicin ligand was allowed to dock with its respective protein targets, and the results were obtained.25 The structures with the lowest intermolecular energies were selected for analysis. Non-bonded interactions involving hydrogen bonds and van der Waals forces were analyzed using the DIMPLOT server.26 Finally, an interaction image was prepared using the chimera software.
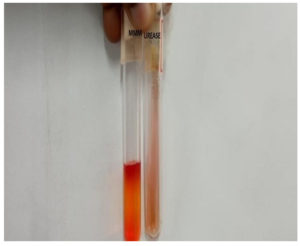
Here, of the 250 exudate samples collected, 183 showed uniformly stained gram-positive cocci arranged in pairs and clusters. Moreover, 158 samples formed golden-yellow pigmented colonies on artificial non-selective culture media. Of the 158 samples, 63 tested positive for MRSA. Subsequently, one isolate was selected and processed for biochemical reactions. In the tests, urea was hydrolyzed and mannitol was fermented. Catalase test produced effervescence, and the coagulase test was positive. Figure 3 and 4 show the biochemical test results for S. aureus. Table 1 presents the antibiogram pattern of the S. aureus isolate. As the isolate was resistant to cefoxitin, it was identified as MRSA according to the Central Laboratory Standard Institute 2022 guidelines.
Table (1):
Shows the antimicrobial susceptibility pattern of Staphylococcus aureus
Name of the drug |
Resistant/susceptible/intermediate |
|---|---|
Penicillin |
Resistant |
Gentamycin |
Resistant |
Cefoxitin |
Resistant |
Cotrimoxazole |
Intermediate |
Erythromycin |
Resistant |
Ciprofloxacin |
Susceptible |
Clindamycin |
Resistant |
Linezolid |
Susceptible |
Vancomycin |
Susceptible |
Next, the isolate was subjected to
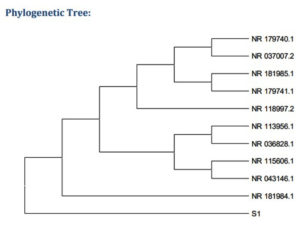
16S rRNA sequencing. It showed a high similarity based on nucleotide homology and phylogenetic analyses. Table 2 lists the sequences with the most prominent alignments. Figure 5 shows the phylogenetic tree.
Table (2):
Represents the sequence producing significant alignments
Description |
Max Score |
Total Score |
Query Cover |
E value |
Per. Ident |
Accession |
|---|---|---|---|---|---|---|
Staphylococcus aureus strain NBRC 100910 |
2673 |
2673 |
100 |
0.0 |
99.32 |
NR_113956.1 |
Staphylococcus aureus strain ATCC 12600 |
2667 |
2667 |
100 |
0.0 |
99.32 |
NR_115606.1 |
Staphylococcus argenteus strain DSM 28299 |
2669 |
2669 |
99 |
0.0 |
99.26 |
NR_181984.1 |
Staphylococcus aureus strain MVF-7 |
2662 |
2662 |
100 |
0.0 |
99.25 |
NR_036828.1 |
Staphylococcus argenteus strain DSM 28299 |
2314 |
2314 |
86 |
0.0 |
99.22 |
NR_179740.1 |
Staphylococcus aureus strain S33 R |
2662 |
2662 |
100 |
0.0 |
99.19 |
NR_037007.2 |
Staphylococcus schweitzeri strain DSM 28300 |
2662 |
2662 |
100 |
0.0 |
99.19 |
NR_181985.1 |
Staphylococcus aureus strain ATCC 12600 |
2656 |
2656 |
100 |
0.0 |
99.13 |
NR_118997.2 |
Staphylococcus schweitzeri strain DSM 28300 |
2303 |
2303 |
86 |
0.0 |
99.06 |
NR_179741.1 |
Staphylococcus simiae CCM 7213 = CCUG 51256 |
2639 |
2639 |
100 |
0.0 |
99.92 |
NR_043146.1 |
Notably, the aqueous garlic extract exhibited effective antibacterial activity even at the minimal dilution (1 μL). This bactericidal activity may be due to the organosulfur compounds in garlic that inhibit microbial growth. The zone of inhibition for the agar dilution was larger at the highest concentration. The zones of inhibition in the agar well diffusion tests were 18 mm with 100 µL, 15 mm with 50 µL, 12 mm with 25 µL, and 5 mm with 10 µL of the extract. Additionally, synergistic activity was observed between the garlic extract and cefoxitin disc. This combination formed a 24-mm zone of inhibition, whereas no zone of inhibition was observed with the cefoxitin disc alone. Figure 6 shows the activity against A. sativum with the commercial cefoxitin disc. Docking revealed that allicin exhibited strong interactions with PBP3, with a favorable docking score of –3.09 and energy of –30.384 Kcal/mol. A hydrogen bond was formed between the oxygen atom of allicin and Y636 of PBP3, with a contact distance of 2.58 Å. Additionally, many hydrophobic interactions were observed between allicin and T603, V606, T664, G665, L663, S634, Q656, V658, V632, P660 and T621 residues of PBP3. The stability of the PBP3–allicin complex is attributed to these interactions. Figure 7 shows the PBP3–allicin molecular complex, with the zoomed image showing the hydrogen bond and hydrophobic interactions between them.
Garlic is widely used as a medicinal herb. Many studies have reported the antibacterial activities of garlic. Daka et al. revealed that no antibacterial effects of garlic are observed at concentrations lower than the MIC.9 This may be due to the differentiation of the strains or garlic species.10 Durairaj et al. reported that gram-positive Bacillus spp. are more sensitive than other organisms to garlic. Our results are consistent with those of Atheer, who revealed a zone of inhibition of 23 nm with 100 mg/mL of garlic, which is the highest reported concentration. In addition to its effect on MRSA, Natasya-Ain et al. revealed the antibacterial effects of 10% methanol garlic extract (MBC: 1 mg/mL) on Vibrio alginolyticus and V. harveyi.22 Moreover, Durairaj et al. revealed a zone of inhibition of 33 nm with 100% concentration of the aqueous garlic extract.10 Many studies have shown the antibacterial effects of garlic extract on various microorganisms, such as Salmonella typhi, Pseudomonas aeruginosa, Proteus mirabilis, and Escherichia coli. Cirković et al. revealed the synergistic effects of the ethanol garlic extract with various drugs using synergy tests.8 Owing to the high prevalence of MRSA, drug development for resistant organisms has drastically increased. Here, we found that the garlic extract overcame the drug resistance of such microbes. Garlic extract consists of various components, such as allicin, ajoenes, and allyl sulfides, which exert various effects, such as antimicrobial, anti-inflammatory, and antihistaminic effects.
Several studies have shown the antimicrobial activities of the garlic extract. Bhatwalkar et al. reported that garlic contains organosulfur compounds exhibiting various antibacterial activities, such as antibiofilm, bactericidal, antitoxin, and anti-quorum sensing activities, against many bacteria, including MDR strains.2
Kyo et al. reported that the aged garlic extract reduces the ear inflammation in allergic mice.1 Additionally, it exerts inhibitory effects on sarcoma 180 and carcinoma cell lines. Sharifi-Rad et al. evaluated the effects of a combination of allicin and silver nanoparticles against MRSA skin infections.22 Here, our study demonstrated the antibacterial activity of garlic extract against MRSA.
In addition to this study, many studies are focusing on agents targeting MRSA to combat its increasing prevalence worldwide. Daka et al. revealed the antibacterial effects of garlic extract against S. aureus.9 In this study, molecular docking revealed the strong interactions between allicin and PBP3 of S. aureus.
In conclusion, this study demonstrated the antibacterial activities of garlic extract against MRSA in vitro. Our findings revealed the synergistic activities of the garlic extract with cefoxitin; however, further animal studies are necessary to validate these results and overcome the drug resistance and prevalence of MRSA. Furthermore, a database of useful organosulfur compounds, other than allicin, that can be used in combination with drugs should be created. Future studies should also assess the efficacy of such drug–extract combinations against superficial pyogenic infections in animals.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Institutional Review Board, Saveetha Institute of Medical and Technical Sciences, Thandalam, India, with approval number SMC/IEC/2022/05/004.
- Kyo E, Uda N, Kasuga S, Itakura Y. Immunomodulatory effects of aged garlic extract. American Society for Nutritional Sciences. 2001;131(3s):1075S-9S.
Crossref - Bhatwalkar SB, Mondal R, Krishna SBN, Adam JK, Govender P, Anupam R. Antibacterial Properties of Organosulfur Compounds of Garlic (Allium sativum). Frontiers in Microbiology. 2021; 12:613077.
- Atheer K. Antibacterial activity of garlic extract (ALLIUM SATIVUM) against Staphylococcus aureus in vitro. Journal of Bio-Science. 2014;3:346-348.
- W Tesfaye, H Kuma, D Kassahun, A Beza. Anti-bacterial activity of garlic extract against human pathogenic bacteria. Journal of Pharmacovigilance. 2018;(6)-253
- Y Hisashi, F Kawai, E Obayashi, et al. Crystal Structures of Penicillin-Binding Protein 3 (PBP3) from Methicillin-Resistant Staphylococcus aureus in the Apo and Cefotaxime-Bound Forms. Journal of Molecular Biology, 2012;423 (3):351–64.
Crossref - Ruth H, Morris GM, Olson AJ, Goodsell DS. A Semiempirical Free Energy Force Field with Charge-Based Desolvation. Journal of Computational Chemistry, 2007;28(6):1145–52.
Crossref - Morris GM, David SG, Robert SH, et al. Automated Docking Using a Lamarckian Genetic Algorithm and an Empirical Binding Free Energy Function. Journal of Computational Chemistry, 1998;19(14):1639–62.
Crossref - Ivana C, Jovalekic M, Jegorovic B. In vitro antibacterial activity of garlic and synergism between garlic and antibacterial drugs. Arch Biol Sci. 2012;64:1369-1375.
Crossref - Daka D. Antibacterial effect of garlic (Allium sativum) on Staphyloccus aureus: An in vitro study. Afr J Biotechnol. 2011;10(4):666-669.
- Durairaj S, Srinivasan S, Lakshmana P. In vitro Antibacterial activity and stability of garlic extract at different pH and temperature. Electronic Journal of Biology. 2010;5(1):5-10.
- Huey R, Morris GM, Olson AJ, Goodsell DS. A Semi empirical Free Energy Force Field with Charge-Based Desolvation. J Comput Chem. 2007;28(6):1145-1152.
Crossref - Khashan A. Antibacterial activity of garlic extract (Allium sativum) against Staphylococcus aureus invitro. Journal of Bio-Science. 2014;3:346-348.
- Kimura M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111-120.
Crossref - Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGAX: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35(6):1547-1549.
Crossref - Koneman EW, Allen SD, Janda WM, Schreckenberger PC. Color Atlas and textbook of diagnostic microbiology. 6th edition. 1992:624-704.
- Mohamed SB, Adlan TA, Khalafalla NA, et al. Proteomics and Docking Study Targeting Penicillin-Binding Protein and Penicillin-Binding Protein2a of Methicillin-Resistant Staphylococcus aureus StrainSO-1977 Isolated from Sudan. Evol Bioinform Online. 2019;15:1-13.
Crossref - Monte J, Abreu AC, Borges A, Simoes LC, Simoes M. Antimicrobial activity of selected phytochemicals against Escherichia coli and Staphylococcus aureus and their biofilms. Pathogens.2014;18;3(2):473-498.
Crossref - Morris GM, Goodsell DS, Halliday RS, et al. Automated Docking Using a Lamarckian Genetic Algorithm and an Empirical Binding Free Energy Function.
J Comput Chem. 1998;19(14):1639-1662.
Crossref - Morris GM, Ruth H, William L, et al. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. Journal of Computational Chemistry, 30(16): 2785–91.
Crossref - Reiter J, Hübbers AM, Albrecht F, Leichert LIO, Slusarenko AJ. Allicin, a natural antimicrobial defence substance from garlic, inhibits DNA gyrase activity in bacteria. International Journal of Medical Microbiology; 2020;310(1):151359.
Crossref - Natasya-Ain R, Eirna-Liza N, Jasmin MY, Karim M. Journal of Environmental Biology, 2018; 39 808-812
Crossref - Sharifi-Rad J, Hoseini Alfatemi S, Sharifi Rad M, Iriti M. Antimicrobial synergic effect of Allicin and silver nanoparticles on skin infection caused by Methicillin- Resistant Staphylococcus aureus spp. Annals of Medical and Health Sciences Research; 2014; 4(6):863-868.
Crossref - Umamaheswari S. Antibacterial activity of Allium sativum on pathogenic bacterial strain. Global Veterinaria. 2010;4(5):519-522.
- Wacnik K, Rao VA, Chen X, et al. Penicillin-BindingProtein 1 (PBP1) of Staphylococcus aureus has multiple essential functions in cell division. mBio. 2022;13(4):e0066922.
Crossref - Wallock-Richards D, Doherty CJ, Doherty L, et al. Garlic Revisited: Antimicrobial activity of Allicin containing garlic extracts against Burkholderia cepacia Complex. PLoS ONE. 2014;9 (12).
Crossref - Laskowski RA, Mark B. LigPlot+: Multiple Ligand-Protein Interaction Diagrams for Drug Discovery. Journal of Chemical Information and Modeling, 2011 ;51(10): 2778–86.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.