ISSN: 0973-7510
E-ISSN: 2581-690X
Fruits of Platycladus orientalis growing wild in Arar region, Saudi Arabia was investigated for its antibacterial activity against Staphylococcus saprophyticus. A bacterium associate with serious urinary tract infections (UTI) and cystitis in young human females. GC-MS analysis discloses the presence of some bioactive compounds, such as cupressene, podocarpa-8,11,13-triene, kauran-16-ol, kerruginol, glycoside compounds, cymarin and Periplocymarin which could be attributed to the antibacterial efficacy of that plant product. The extract showed remarkable antibacterial activity against Staphylococcus saprophyticus, the disc diffusion test recorded 16.0±1.0mm zone of inhibition, with relative percentage inhibition (RPI) of Platycladus orientalis extract was 66.6% compared with the chloramphenicol. The MIC and MBC values were 12.5 mg/ml and 50 mg/ml, respectively. The MBC/MIC ratio was 4, indicating that the extract has a good bacteriostatic effect. Therefore, the fruits of Platycladus orientalis could be used in the formulation of antibacterial drugs against Staphylococcus saprophyticus associated with UTI infections.
Platycladus orientalis, GC-MS, antibacterial, Staphylococcus saprophyticus.
Medicinal plants have been used since times immemorial and the accumulated knowledge in herbal formulations were passed down throughout ancient civilizations until reach recent history where drug discoveries led to the isolation of numerous medications from medicinal plants such as morphine, quinine, digitoxin, cocaine, codeine and many more1. With the turn of the twentieth century, the discovery of antibiotics has given humans victory over pathogenic microbes. The discovered antibiotics were initially natural product secretions, isolated form fungi, bacteria and environmental microorganisms. Several decades later, the molecular genetic system of microbes developed resistance against these antibiotics, which led to the need to find urgent alternatives, under the absence of any newly discovered antibiotics in the last 30 years2. The growing problem of antibiotics resistance has led to raising the interest of medicinal plant investigations particularly those with ethnopharmacological heritage to uncover new antimicrobial agents3. Platycladus orientalis Linn. (Synonymous: Thuja orientalis) is belong to family Cupressaceae, it is a coniferous shrub with evergreen and scale-like leaves; It is used in traditional medicine in the treatment of bronchitis, cystitis, enuresis, psoriasis, amenorrhea, uterine carcinomas and rheumatism4. The essential oils obtained from Platycladus orientalis contains b-thujone and b-thujone which showed toxic effects on ruminant animals5. However, the ethanol extract of Platycladus orientalis is non-toxic at low concentration (£ 0.6 mg/ml) and it suppressed the growth of human lung cancer cell line6. Staphylococcus saprophyticus is a Gram-positive coccus, a leading cause of urinary tract infections (UTI) and cystitis in young human females with virulent strains resistant to many UTI antibiotics such as fosfomycin trometamol and cefixime7. The present investigation is aimed to study the efficacy of ethyl acetate extract of Platycladus orientalis against referenced strain of Staphylococcus saprophyticus.
Plant Material
The fruits of Platycladus orientalis were collected from Arar region, Northern area, Saudi Arabia in March 2018. An authenticated sample was deposited in Faculty of science girl section. Fruits were cut in to small pieces, dried in incubator at 40°C for up to a week and ground to a fine powder.
Plant extraction
The air-dried powdered fruit (100 g) of Platycladus orientalis were subjected to successive extraction by percolation in ethyl acetate for a week. Then, the percolate was filtered and the solvent was evaporated by rotary evaporator under reduced pressure, until dryness. The extract (18.00 g) was dissolved in a suitable amount of distilled H2O–MeOH (95:5 v/v) then the extract was kept for GC-MS analysis.
GC-MS analysis
The compounds were analyzed using a Thermo GC-Trace ultra system (Thermo Co. USA), they were separated on 30m X 0.25 mm X 0.25µm Elite-5MS column (Thermo Scientific GC Column). The column temperature was increased from 40oC to 220oC at a rate of 4oC/ min; injector temperature is 250oC; injection volume is 1µl; helium carrier gas flow rate is 20ml/min; transfer temperature is 280°C. MS parameters were as follows: EI mode, with ionization voltage 70 ev, ion source temperature, 180oC; scan range, 50-600 Da. The peaks were tentatively identified based on library search using NIST and Wiley Registry 8 Edition.
Antibiotic susceptibility testing
The antibiotics susceptibility profile of Staphylococcus saprophyticus ATCC 43867 was performed using Kirby-Bauer disk diffusion method following the guideline of Clinical and Laboratory Standards Institute (CLSI)8. Briefly, an overnight culture from Staphylococcus saprophyticus isolate was adjusted to be equivalent to 0.5 McFarland. 100µl of the adjusted suspension was streaked over sterile Mueller-Hinton agar. Then, standard antibiotic rings (Mastring-STM MOH50/2 and MOH50/3) were aseptically loaded in to streaked plates and incubated for up to 18h at 37o C. The zone of inhibition around the antibiotic discs were measured and recorded as resistant or sensitive according the manufacturer’s instructions that are based on the CLSI guidelines.
Antibacterial activity assay
The antibacterial activity of Platycladus orientalis ethyl acetate extract against Staphylococcus saprophyticus was initially determined using disc diffusion method9, with minor modifications. The extract was re-constituted in dimethyl sulfoxide (DMSO) to get concentration 300mg/ml., DMSO has no effect on the bacterial growth. The bacterial suspension was adjusted to 0.5 McFarland standard (1×108 CFU/ml). 100µl of the adjusted suspension was spread over a sterile Mueller-Hinton Petri-dishes and left for a while. 6 mm paper discs (made from Whatman No. 1) were impregnated with about 20µL of the extracts and placed on the inoculated Mueller Hinton agar Petri-dish, a disc saturated with about 20µL Chloramphenicol (5mg/ml) was also placed on the plate to serve as control. The Petri-dish was incubated at 37°C for overnight and the diameters of the inhibition zones were measured.
Minimum inhibitory concentration (MIC)
The MIC of the extract was evaluated by tube dilution method10. The various two-folds dilutions of the extract (6.25, 12.5, 25, 50, 100 and 200 mg/ml) were made in tubes containing nutrient broth. Equal volume of the extract and nutrient broth were mixed in each test tube. 0.1ml of adjusted inoculum of Staphylococcus saprophyticus (1׳ 108 CFU/ml) was added to each tube. All tubes were incubated for 18 hours at 37oC. Two control tubes were also maintained,one was positive control contains antibiotic instead of extract and the other one is a negative control contains bacterial inoculum, physiological saline and broth medium. MIC was considered as the lowest concentration of the extract showing no visible growth (no turbidity) when compared with the control tubes.
Minimum bactericidal concentration (MBC)
MBC was determined by sub-culturing the MIC test dilutions11. Briefly, 50µl of the incubated test tubes (from the MIC assay) was pipetted and transferred to sterile nutrient agar plates, incubated overnight at 37oC and inspected for visible growth on the agar. The lowest concentration of MIC which exhibited no visible bacterial growth on agar medium was taken as MBC.
Relative percentage inhibition (RPI)
The relative percentage inhibition (RPI)12 of 5mg/ml chloramphenicol (10 mg/disc) 300mg/ml Platycladus orientalis extract (20µL/disc) with respect to Staphylococcus saprophyticus was calculated using the following formula:
Where: (X) is the total area of inhibition of the herbal extract, (Y) is the total area of inhibition of the solvent and (Z) is total area of inhibition of the antibiotic. The total area of the inhibition zone was calculated based on the following equation:
Area = Π X r2
where: (r) is the radius of the zone of inhibition, Π = 3.14
Medicinal herbs were the primary source of drugs since ancient times and till now. In the current investigation, the GC-MS analysis of ethyl acetate extract of Platycladus orientalis fruits revealed the presence of some important bioactive compounds, such as Ferruginol (5.5%), Thunbergol (3.2%), Periplocymarin (2.5%), Cymarin (2,3%), Kauran-16-ol (1.4%), Isosteviol methyl ester (1.2%), Podocarpa-8,11,13-triene (0.62%), Methyl abietate (0.45%), Cupressene (0.23%) and Keto-Dihydro-gendunin (0.23%), (Table 1). Similar study on the fruit oil of Platycladus orientalis (Syn. Thuja orientalis) was conducted in Nigeria, the GC-MS revealed the presence of 2-carene (21.1 %), 3-carene (15.8 %), b-myrcene (8.0 %), terpinen-4-ol (7.8 %), cedrol (7.8 %). D-limonene (5.5 %), b-caryophyllene (5.2 %), g-terpinene (4.7 %) and b-humulene (4.2 %)13. Variations in the chemical constituents of the same plant species could be attributed to variations in the environmental conditions between different geographical locations.
Table (1):
GC-MS analysis of ethyl acetate fraction of fruit of Platycladus orientalis
No. |
compounds |
Chemical structure |
Percentage (%) |
|---|---|---|---|
1 |
Cymarin |
2.3 |
|
2 |
Periplocymarin |
2.5 |
|
3 |
Cupressene |
0.23 |
|
4 |
Podocarpa-8,11,13-triene |
C17H24 |
0.62 |
5 |
Keto-Dihydro-gendunin |
0.23 |
|
6 |
Methyl abietate |
0.45 |
|
7 |
Kauran-16-ol |
1.4 |
|
8 |
Isosteviol methyl ester |
1.2 |
|
9 |
Ferruginol |
5.5 |
|
10 |
Thunbergol |
3.2 |
The antibiotic profile of the investigated strain of Staphylococcus saprophyticus showed that it was resistant to 7 among 16 tested antibiotics, in an average of resistance equals 47.75% (Table 2). This reflects the urgent need for developing new antibacterial drugs. Staphylococcus saprophyticus is a second most common cause of urinary tract infections, particularly in young women and it is showing growing resistance to antibiotics and requires urgent intervention to curb this serious health phenomenon14.
Table (2):
Antibiotic profile of Staphylococcus saprophyticus*
Sensitive |
Resistant |
Antibiotic |
|---|---|---|
+ |
– |
C |
– |
+ |
CAZ |
+ |
– |
IMI |
+ |
– |
CIP |
+ |
– |
PRL |
+ |
– |
T |
+ |
– |
AK |
– |
+ |
ATM |
– |
+ |
AUG |
– |
+ |
CTX |
– |
+ |
OFX |
– |
+ |
KF |
+ |
– |
TS |
+ |
– |
GM |
+ |
– |
TN |
– |
+ |
AP |
* + = Present, – =Absent, C=Chloramphenicol 25µg, CAZ=Ceftazidime 10 µg, IMI= Imipenem 10 µg, CIP= Ciprofloxacin 1µg, PRL= Piperacillin 30µg, T= Tetracycline 30µg, AK= Amikacin 30µg, ATM= Aztreonam 30µg, AUG=Augmentin 30µg, CTX= Cefotaxime 5µg, OFX= Ofloxacin 5µg, KF=Cephalothin 5µg, TS=Cotrimoxazole 25µg, GM=Gentamicin 10µg, TN=Tobramycin 10µg, AP=Ampicillin 10µg.
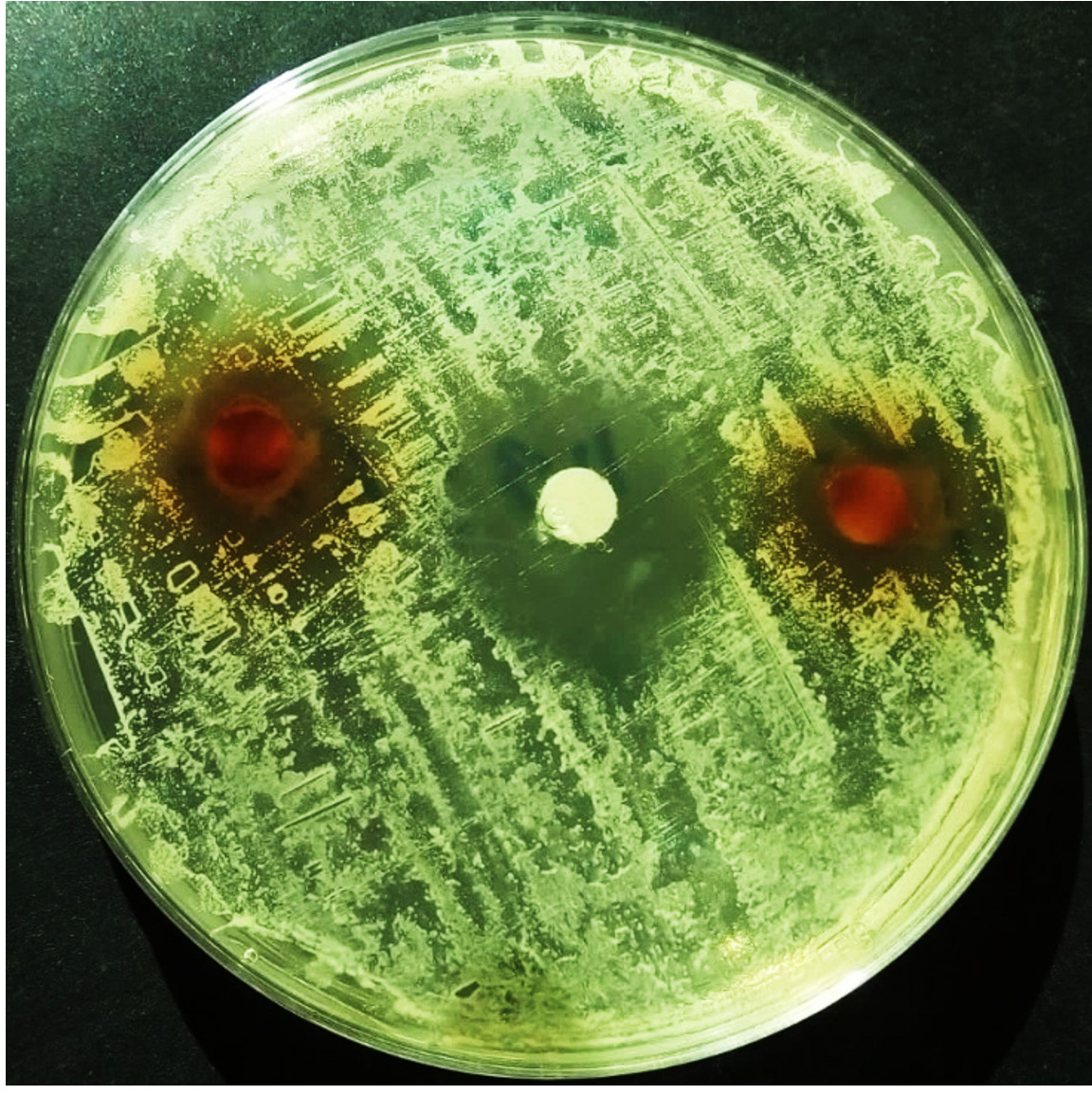
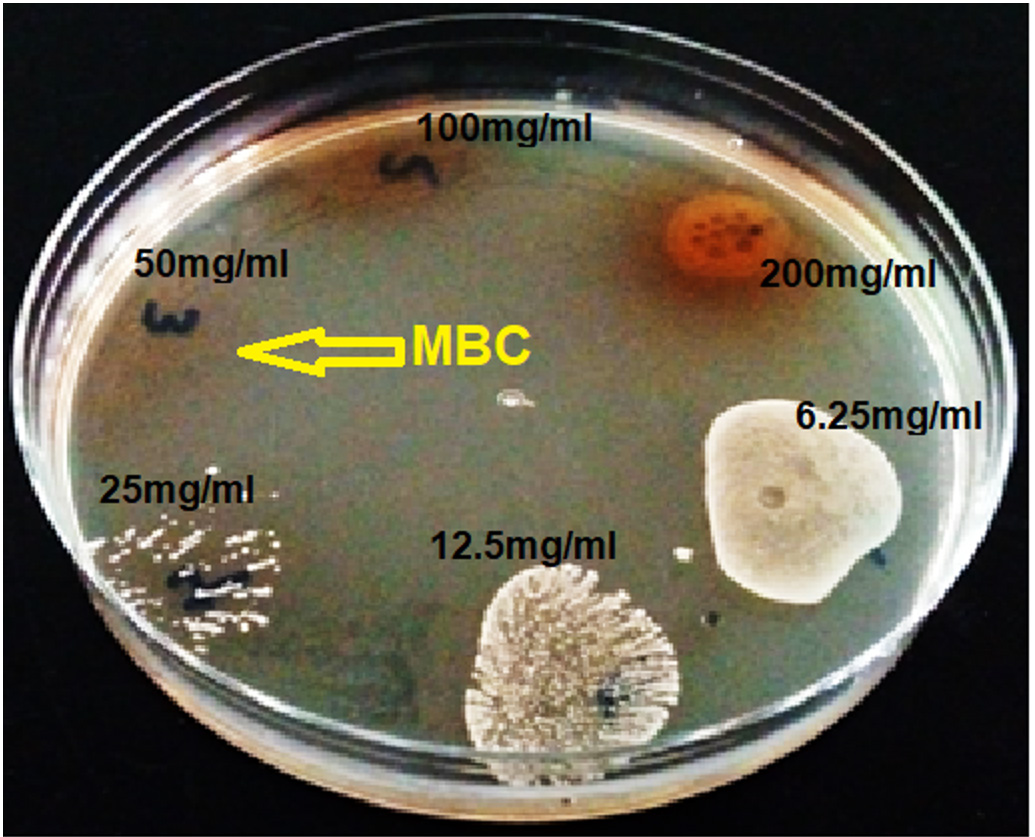
Result of the antibacterial activity of the ethyl acetate fraction of fruit of Platycladus orientalis against Staphylococcus saprophyticus is represented in (Table 3). Which showed that this plant extract has remarkable antibacterial activity, with mean zone of inhibition 16.0±1.0, in comparison with the standard drug (chloram-phenicol) that recorded 24.5±0.5 mm zone of inhibition (Fig. 1 and 2). The RPI, MIC and MBC values supported the findings of the disc diffusion test, which are tabulated in (Table 4), The Relative percentage inhibition (RPI) was calculated and it was found to be 66.6%, which means that the ethyl acetate fraction of fruit of Platycladus orientalis has comparable anti-bacterial efficacy against Staphylococcus saprophyticus. The MIC value was 12.5 mg/ml, whereas the MBC value was 50 mg/ml (Fig. 3). Moreover, the MBC/MIC ration is 4, which means that the extract has a bacteriostatic effect on Staphylococcus saprophyticus. It is known that, the plant extract is considered to be bactericidal when the ratio of MBC/MIC £4, while it is considered bacteriostatic when MBC/MIC ³415. It was reported that, the essential oil from seed coats of Platycladus orientalis (Syn. Thuja orientalis) has shown good to moderate activity against all six tested bacteria, including Salmonella typhi which was the most susceptible bacteria16. Also, the essential oil extracted from fresh fruits of Thuja orientalis (Syn. Platycladus orientalis) recorded remarkable antibacterial effect against all the Gram-positive and Gram negative bacteria tested with MIC values ranging between 12.8 to 25.6 mg/ml17. A recent study stated that, Platycladus orientalis possessed a potential anti-bacterial activity against hospital-and community-acquired methicillin-resistant Staphylococcus aureus (MRSA) and it was recommended for the control of this pathogen18. However, no dataor study was conducted on the antibacterial effect of Platycladus orientalis on Staphylococcus saprophyticus, in particular, and as the best of the authors knowledge this is the first study on this issue. Accordingly, the current study recommends this plant as a potential antibacterial drug against Staphylococcus saprophyticus.
Table (3):
Antibacterial activity of ethyl acetate fraction of fruit of Platycladus orientalis against Staphylococcus saprophyticus
Tested compound |
Mean zone of inhibition (mm) |
|---|---|
TO (300 mg/ml) |
16.0±1.0 |
CH (2.5 mg/ml) |
24.5±0.5 |
Legend: TO= Platycladus orientalis (ethyl acetate fraction), CH= chloramphenicol, mean of two replicates, diameter of the paper disc =6 mm.
Table (4):
MIC and MBC value of fruits of Platycladus orientalis against Staphylococcus saprophyticus
| Test | Concentration of ethyl acetate fraction of Platycladus orientalis (mg/ml) | |||||
|---|---|---|---|---|---|---|
| 200 | 100 | 50 | 25 | 12.5 | 6.25 | |
| MIC | – | – | – | – | + | + |
| MBC | – | – | + | + | + | + |
– = No microbial growth, + = Presence of microbial growth.
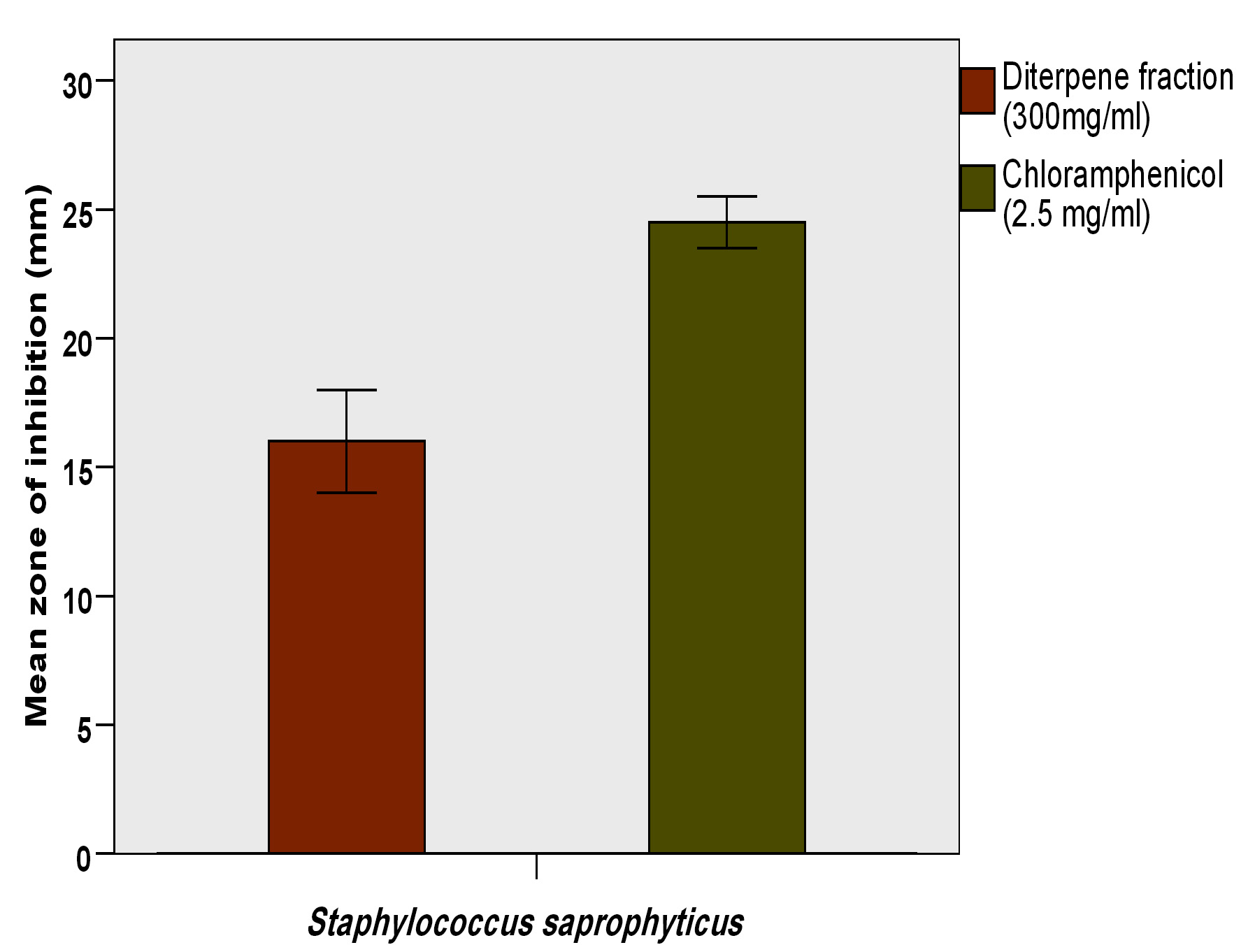
Fig. 1. Susceptibility of Staphylococcus saprophyticus to the extract of Platycladus orientalis compared with chloramphenicol
Based on the findings of the current investigation, Fruits of Platycladus orientalis could be used in the formulation of effective antibacterial drug to be used against UTI infections, in particular. However, compounds revealed by the GC-MS analysis should be isolated and studied individually and more future toxicological and pharmacological studies are recommended. Moreover, it is worthy for the pharmaceutical companies to pay great attention to the natural products and medicinal herbs, in order to innovate new effective antibacterial drugs.
Acknowledgements
None.
Conflict Of Interest
The authors declares that there is no conflict of interest.
Authors’ Contribution
All authors have made substantial, direct and intellectual contribution to the work and approved it for publication.
Funding
None.
Data Availability
All datasets generated or analyzed during this study are included in the manuscript.
Ethics Statement
This article does not contain any studies with human participants or animals performed by any of the authors.
- Balunas M.J., Kinghorn A.D. Drug discovery from medicinal plants. Life Sci., 2005; 78(5): 431-441.
- Durand G.A., Raoult D., Dubourg G. Antibiotic discovery: History, methods and perspectives, Int. J. Antimicro. Agents, 2018; doi: https://doi.org/10.1016/j.ijantimicag.2018.11.010
- Rםos J.L., Recio M.C. Medicinal plants and anti-microbial activity. J. Ethnopharma., 2005; 100: 80–84.
- Srivastava P., Kumar P., Singh D.K., Singh V.K. Biological Properties of ThujaOrientalis Linn. Advan. Life Sci., 2012; 2(2): 17-20.
- Chizzola R., Hochsteiner W., Hajek S. GC analysis of essential oils in the rumen fluid after incubation of Thujaorientalis twigs in the Rusitec system. Res. Vet. Sci., 2004; 76(1): 77-82.
- Breeta R.E., Jesubatham P.D., Grace V.M.B., Viswanathan S., Srividya S. Non-toxic and non-teratogenic extract of Thujaorientalis L. inhibited angiogenesis in zebra fish and suppressed the growth of human lung cancer cell line. Biomed. Pharmaco., 2018; 106: 699-706.
- Pailhoriטs H., Cassisa V., Chenouard R., Kempf M., Eveillard M, Lemariי C. Staphylococcus sapro-phyticus: Which beta-lactam?. Int. J. Infect. Dis., 2017; 65: 63-66.
- Oteo J., Campos J., Baquero F. Antibiotic resistance in 1962 invasive isolates of Escherichia coli in 27 Spanish hospitals participating in the European Antimicrobial Resistance Surveillance System. J. Antimicro.Chemothera., 2002; 50(6): 945-952.
- Su P.W., Yang C.H., Yang J.F., Su P.Y., Chuang L.Y. Antibacterial Activities and Antibacterial Mechanism of Polygonum cuspidatum Extracts against Nosocomial Drug-Resistant Pathogens. Molec., 2015; 20: 11119-11130.
- Akinyemi K.O., Oluwa O.K.,Omomigbehin E.O. Antimicrobial activity of crude extract of three medicinal plants used in South-West Nigeria folk medicine on some food borne bacterial pathogens. Afrin. J. Trad. Compl. Alter. Med., 2006; 3(4): 13-22.
- Doughari J.H. Antimicrobial Activity of Tamarindus indica Linn. Trop. J. Pharmaceut. Res., 2006; 5(2): 597-603.
- Abdallah E.M. Antibacterial activity of Hibiscus sabdariffa L. calyces against hospital isolates of multi-drug resistant Acinetobacter baumannii. J. Acu. Dis., 2016; 5(6): 512–516.
- Usman L.A., Agboola T.A., Abdul Waheed J.O., Ismaeel R.O., Ogundele V.A., Ibrahim A. Phytochemical profile of fruit and leaf essential oils of Thujaorientalis grown in North Central Nigeria. Nigerian J. Pure Appl. Sci., 2016; 29 (2): 2968-2976.
- Latham R.H., Running K., Stamm W.E. Urinary Tract Infections in Young Adult Women Caused by Staphylococcus saprophyticus. J. American Med. Asso., 1983; 250(22):3063-3066.
- Djeussi D.E., Noumedem J.A., Seukep J.A., Fankam A.G., Voukeng I.K., Tankeo S.B., et al. Antibacterial activities of selected edible plants extracts against multidrug-resistant Gram-negative bacteria. BMC Compl. Altern. Med., 2013; 13: 164.
- Jain R.K. and Garg S.C. Antimicrobial activity of the essential oil of Thujaorientalis L. Anc. Sci. Life, 1997; 16(3): 186–189.
- Shah W.A., Qadir M. Chemical composition, Antioxidant and Antibacterial activity of Thuja-orientalis essential oil. World J. Pharma. Sci., 2014; 2(1): 56-61.
- Chakraborty S., Afaq N., Singh N., Majumdar S. Antimicrobial activity of Cannabis sativa, Thujaorientalis and Psidium guajava leaf extracts against methicillin-resistant Staphylococcus aureus. J. Integr. Med., 2018; 16: 350-357.
© The Author(s) 2019. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.