ISSN: 0973-7510
E-ISSN: 2581-690X
Vaginal infections are common female disease conditions that account for the prevalence of gynecological disorders which facilitate the increasing antimicrobial resistance and failure of prevalent treatment choices. In this study, the antibacterial activity of cell free supernatants (CFS) of probiotic Lactobacillus obtained from ogi (fermented maize) was evaluated against bacterial pathogens associated with vaginal infections. Bacterial pathogens isolated from high vaginal (n=22) and endocervical swabs (n=18) were bio-typed and assayed for hemolytic activity, biofilm production, antibacterial susceptibility pattern, and the CFS antagonistic activity. The occurrence of the vaginal bacterial pathogens was 33.0% for Streptococcus spp. and 31.0% for Staphylococcus aureus, with more than 70% resistance rates to amoxicillin, cefotaxime, imipenem/cilastatin, nalidixic acid, nitrofurantoin, cefuroxime, ceftriaxone sulbactam, ampiclox, cefixime and levofloxacin. More than 30% of the isolates produced biofilms. Of the four identified probiotic strains, only CFS from L. plantarum and L. acidophilus exhibited observable antagonistic reaction, with L. plantarum showing higher antibacterial activity against Staphylococcus condimenti, and L. acidophilus against Klebsiella pneumoniae. With the results of this study revealing the antibacterial activity of probiotic Lactobacillus CFS against vaginal bacterial pathogens, probiotic Lactobacillus can be suggested for use as prophylactic and bioprotective agents in the therapeutic management of vaginal bacterial infections and preservation of the vaginal microbiota.
Probiotics, Ogi, Lactobacillus, Staphylococcus, Klebsiella, Vaginal Pathogens
Diverse bacterial flora in the female genitalia are reported to have a significant impact on vaginal health and flora.1 Vaginal infections are one of the most common female dysfunctions with severe morbid circumstances that account for the prevalence of gynecological disorders.2 The prevalence of bacterial vaginosis (BV) exhibits significant variation across different countries, with reported rates ranging from 20% to 60%.3 The presence of vaginal discharge accompanied by symptoms such as pruritus, erythema, and occasional dyspareunia affecting the vaginal and vulval regions arise from the impacts of bacterial pathogens.4 In spite of the reported less fatality of vaginal infections, the huge economic loss and treatment downtime are of special concern.5 Several risk factors predispose adult females mostly at reproductive age to floral imbalance that leads to high level vaginal morbidity. These risk factors include ethnicity, genetics, antibiotic use, hygiene, sexual activity, infections, hormones, lifestyle and food.6 The reports of Chee et al.7 highlight that changes in the microflora of the vagina have the potential to modify immune responses and promote the proliferation of pathogens. This facilitates the development of several diseases that promote major aerobic vaginitis such as Escherichia coli, Streptococcus, Pseudomonas spp. and Staphylococcus aureus reported most frequently in patients with aerobic vaginitis.8
Metronidazole or clindamycin are typically the preferred therapeutic agents to treat infections of the vaginal region.9 The reports of Serwecinska10 opine that excessive and uncontrolled administration of antibiotics can result in the emergence of antibiotic resistance, and consequently increasing secondary infections in patients. However, Prabhurajeshwar & Chandrakanth,11 had suggested probiotics as essential natural, safe and less toxic antimicrobial options to be utilized as alternative therapy for the management of bacterial-related vaginal infections, due mainly to the reports of increasing resistance and failure of antimicrobial treatment choices. Lactobacillus species, generally regarded as safe and frequently utilised as probiotics, are the largest genus among the lactic acid bacteria (LAB).12,13 More so, they possess the potential to improve vaginal infection conditions due to their ability to generate antibacterial agents.14 Literature has highlighted two distinct approaches for the administration of probiotic lactobacilli – oral and intravaginal.15,16 Orally administered probiotic lactobacilli undergo a sequential transit through the oral cavity, stomach, intestines, and colon, before ultimately reaching the vaginal region through cutaneous contact in the perineal area. The delivery of probiotics to the vaginal region typically occurs within a timeframe of approximately seven days.17 The intravaginal administration of probiotic lactobacilli can be facilitated through the utilisation of external equipment (an applicator). Thereafter, the effects of lactobacillus strains become evident within a period of 2-3 days.18 The composition of lactic acid, antimicrobial peptides and bacteriocins in probiotics cell free supernatants (CFS) are potent components enforcing the antagonistic outcomes on pathogenic bacteria,19 thereby inhibiting their replication and maintaining the vaginal ecosystem. Therefore, the study is aimed at evaluating the antibacterial activity of probiotic Lactobacillus against bacterial pathogens associated with vaginal infections obtained at the Federal Medical Center, Abeokuta, Nigeria.
Ethical Approval
Ethical approval was obtained from the Covenant Health Research Ethics Committee (CHREC), with each study participant providing written informed consent that was kept confidential.
Selection of Subjects and sample collection
The exclusion criteria applied in the selection of the participants include pregnant and postpartum women, individuals with immunosuppressive conditions, women that have reached menopausal stage and subjects with recent history of antibiotic use within the last three (3) weeks. Vaginal swab samples including high vaginal (n=22) and endocervical (n=18) samples were collected from individuals already diagnosed for vaginosis, aged between 20-45 years, and attending the out- and in-patients’ clinics of the Federal Medical Center, Abeokuta, Nigeria; for therapeutic management.
Bio-typing, hemolytic pattern and biofilm production assay
Aliquots of the collected swab samples were inoculated on Blood and MacConkey Agars, and incubated for 24 h at 37°C. The isolates were characterized using the Analytical Profile Index for Staphylococci (API 20S) and the Analytical Profile Index for Enterobacteriaceae (API 20E) (bioMérieux, Inc, Durham, USA). The hemolytic activity of the strains was tested by inoculation of pure strains onto 7% Blood Agar, and incubated for 24 h at 37°C, and the pattern of lysis interpreted according to Thakkar et al.20 Biofilm production of the strains was determined using the micro-bioassay method as described by Akinduti et al.21 in a sterile 96-well microtiter plates. Aliquots (200 µL) of freshly prepared 0.5 McFarland Nutrient Broth containing the bacterial isolates were dispensed into each well of the microtiter plate and incubated for 24 h at 37°C. After incubation, the microtiter plate was rinsed twice with water to discard non-adherent cells, before introduction of 50 µL of crystal violet into all the wells and allowed to stand for 2 minutes. Following another round of rinsing, 100 µl of ethanol was added to each stained well, before evaluating the absorbance of the developed color intensity using a UV spectrophotometer at 630nm. The level of biofilm production was determined according to Stepanovic et al.22
Antibiotics Susceptibility Assay of Vaginal Bacterial Pathogens
Susceptibility of each bacterial strain to different antibiotics was evaluated using agar diffusion method according to the method of Dragomirescu et al.23 These included amoxicillin (30 µg), cefotaxime (25 µg), cefixime (5 µg), imipenem/cilastatin (10/10 µg), nalidixic acid (30 µg), ofloxacin (5 µg), gentamycin (10 µg), nitrofurantoin (300 µg), cefuroxime (30 µg), ceftriaxone sulbactam (45 µg), ampiclox (10 µg) and levofloxacin (5 µg). Each bacterial isolate was diluted to 0.5 McFarland standard, before evenly spreading on Muller Hinton agar plate to make a lawn culture using a sterile swab stick. The plates were allowed to dry before placing the antibiotics impregnated disks, and incubating at 37 °C for 24 h. Thereafter, the areas of inhibition were estimated values interpreted according to the CLSI, 2020 guidelines.24
Isolation and Characterisation of Lactobacillus
Aliquots of serially diluted ogi (1g) were inoculated on De Man, Rogosa and Sharpe (MRS) agar and incubated for 48 h at 37°C under anaerobic condition.25 The isolated strains were further characterized using preliminary test (such as catalase), carbohydrate fermentation test, and Analytical Profile Index for Lactobacilli (API 50 CHL).26
Probiotic Assay
Microtiter plates with 96 wells were used for the phenol, bile and acid tolerance assays. The selected LAB isolates were grown under anaerobic conditions in MRS Broth at 37°C overnight and 100 µl of diluted broth culture was added to bile salt solutions (0.1%, 0.3%, 0.7% and 1.0%), phenol solutions (0.1%, 0.3%, 0.4% and 1.0%), cholesterol (200mg), MRS broths of varying pH (2, 3, 4 and 5.5), respectively. A 100 µl of sterile MRS broth (as negative) and the selected LAB were also prepared. The absorbance of each solution was read at 630 nm at 0 and 6 h to determine the relative growth rate.27
Antibiotics Susceptibility Assay of Lactobacillus
Antibiotic susceptibility of the selected Lactobacillus with probiotic properties were evaluated with agar diffusion technique using various classes of antibiotics that include ceftazidime (30 µg), ciprofloxacin (5 µg), cefuroxime (30 µg), nitrofurantoin (300 µg), gentamicin (10 µg), augmentin (30 µg), cefixime (5 µg) and ofloxacin (5 µg). Each Lactobacillus strain was diluted to 0.5 McFarland standard, inoculated on to MRS agar plates and allowed to dry, before placing the antibiotics-impregnated disks. The plates were incubated at 37°C for 24 h under anaerobic conditions and the observed zones of inhibition estimated and interpreted according to the CLSI, 2020 guidelines.24
Antibacterial Activity of the CFS
Pure cultures of Lactobacillus were transferred into MRS broth and incubated at 37°C. After 48 hours incubation, the broth culture was centrifuged at 3,000 rpm for 15 minutes and filtered using a 0.22 µm sterile filter. The CFS was collected into sterile container for antibacterial analysis against the isolated vaginal bacteria pathogens using agar well diffusion technique reported by Reuben et al.28 Overnight broth culture of the bacteria pathogen was adjusted to 0.5 McFarland and smoothly spread on nutrient agar plate. A sterile cork borer was used to make wells of 6 mm diameter and loaded with 50 µl of the CFS. To ensure a positive control, standard antibiotic, streptomycin (10 µg/ml) was added to one of the wells, then the plates were incubated for 24 h at 37°C. After incubation, the areas of inhibition were quantified and interpreted as resistant (0- 7 mm); susceptible (>10mm), as described by Kamble et al.29
Statistical Analysis
Significance of the antimicrobial activity of the probiotic LAB against the isolated vaginal pathogens was determined using chi-square and level of significance of the antimicrobial activity of the CFS was determined using ANOVA at p-value < 0.05. The statistical analyses were performed using SPSS 22.0 (SPSS Inc., Chicago, IL, USA).
Prevalence rate of bacteria pathogen associated with vagina infection
The vaginal swab cultures obtained from the women diagnosed (n=40) with vaginal bacterial infection, revealed a higher rate of Streptococcus spp. (33%), S. aureus (31%) and E. coli (13%) and very low rate of other strains including Klebsiella spp. (11%), Pseudomonas spp. (6%) and Enterobacter spp. (6%) as presented in Figure 1.
Antibiotics susceptibility pattern and biofilm production
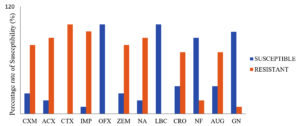
The isolates exhibited 75% resistance rates to amoxicillin, cefotaxime, imipenem/cilastatin, nalidixic acid, nitrofurantoin, cefuroxime, ceftriaxone sulbactam, ampiclox, cefixime and levofloxacin were recorded among the vaginal pathogens while significant susceptibility to levofloxacin and ofloxacin was observed (Figure 2). E. coli, S. aureus and Enterobacter spp. of more than 30% showed high biofilm production, as shown in Table 1.
Table (1):
Rate of biofilm formation among the strains
Bacteria Isolate |
Biofilm Production n/N (%) |
|---|---|
Staphylococcus aureus |
6/17(35) |
Escherichia coli |
3/8 (37.5) |
Enterobacter cloacae |
1/3 (33) |
Pseudomonas aeruginosa |
0/6 (0) |
Klebsiella pneumoniae |
0/6 (0) |
Streptococcus pneumoniae |
1/18 (6) |
Figure 2. Susceptibility pattern of the vaginal bacterial strains to various classes of antibiotics
KEY: CXM- Cefuroxime, ACX- Ampiclox, CTX- Cefotaxime, IMP- Imipenem/Cilastatin, OFX- Ofloxacin, ZEM- Cefixime, NA- Nalidixic Acid, LBC- Levofloxacin, CRO- Ceftriaxone Sulbactam, NF- Nitrofurantoin, AUG- Amoxicilin Clavulanate, GN- Gentamycin
Probiotic Assay on Lactobacillus
Among the nine Lactobacillus isolates isolated from the fermented ogi samples, only four isolates PL1, PL4, PL8 and PL9 showed more than 80% tolerance to acid and bile solution at pH 2 and 3, 0.3% bile concentration and 0.4% of phenol (Table 2). Further assessments revealed that the four selected strains of Lactobacillus exhibited no hemolytic activity, although susceptible to all the antibiotics tested (Table 3).
Table (2):
Probiotic survival assay
| Acid tolerance | Bile tolerance | Cholesterol | Phenol |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isolate Codes | pH2 | pH3 | pH4 | pH5.5 | 0.1% | 0.3% | 0.7% | 1.0% | 200mg | 0.1% | 0.3% | 0.4 | 1.0 |
| PL1 | 151.65 | 129.90 | 142.61 | 111.19 | 91.83 | 86.58 | 79.15 | 73.19 | 86.48 | 99.04 | 98.34 | 94.53 | 84.85 |
| PL2 | 78.96 | 79.83 | 89.69 | 108.92 | 86.65 | 72.00 | 71.65 | 63.32 | 85.39 | 86.34 | 77.89 | 74.06 | 70.38 |
| PL3 | 75.73 | 73.55 | 92.67 | 99.88 | 80.55 | 76.48 | 69.14 | 71.04 | 92.08 | 72.14 | 59.89 | 51.94 | 50.74 |
| PL4 | 102.89 | 97.47 | 101.59 | 95.66 | 87.83 | 82.66 | 78.55 | 71.87 | 85.28 | 94.44 | 93.59 | 94.43 | 91.91 |
| PL5 | 77.12 | 78.51 | 80.06 | 81.56 | 85.53 | 76.38 | 70.78 | 67.99 | 89.05 | 98.75 | 86.29 | 79.59 | 66.57 |
| PL6 | 79.01 | 79.73 | 85.54 | 106.39 | 90.61 | 79.87 | 71.18 | 65.25 | 95.24 | 51.97 | 71.61 | 62.86 | 49.38 |
| PL7 | 78.85 | 79.38 | 89.98 | 115.88 | 88.97 | 74.87 | 58.28 | 48.81 | 91.69 | 80.19 | 70.28 | 78.39 | 70.88 |
| PL8 | 100.19 | 99.36 | 100 | 92.11 | 90.76 | 84.27 | 75.55 | 70.92 | 99.42 | 89.74 | 98.23 | 93.65 | 81.94 |
| PL9 | 116.89 | 118.22 | 115.91 | 109.08 | 89.29 | 84.24 | 78.87 | 72.78 | 89.82 | 98.24 | 99.14 | 92.58 | 86.40 |
Table (3):
Antibiotics susceptibility of selected probiotics
| Isolates |
||||
|---|---|---|---|---|
| Antibiotics | L. fermentum | L brevis | L. plantarum | L. acidophilus |
| Ceftazidime | S | S | S | S |
| Ciprofloxacin | S | S | S | S |
| Cefuroxime | S | S | S | S |
| Nitrofurantoin | S | S | S | S |
| Gentamicin | S | S | S | S |
| Augmentin | S | S | S | S |
| Cefixime | S | S | S | S |
| Ofloxacin | S | S | S | S |
Key: R for resistance; S for susceptible
CFS Antibacterial Activity
The cell free supernatants of the selected probiotics strains assessed for antibacterial activity against isolated bacteria pathogens obtained from vaginal infections as highlighted in Table 4, showed varying zones of inhibition. Results show that CFS from Lactobacillus plantarum CFS exhibited good antibacterial activity against Staphylococcus condimenti (15mm), with Lactobacillus acidophilus showing significant zone of inhibition (12mm) against Klebsiella pneumoniae; while L. brevis and L. fermentum exhibited a diverse range of antibacterial activity against the vaginal pathogens. L. plantarum and L. acidophilus exhibited the most significant zone of inhibition compared to L. brevis and L. fermentum.
Table (4):
Antibacterial pattern of CFS from Probiotic LAB
| API Identity | S. aureus | K. pneumoniae | P. aeruginosa | S. condimenti | E. cloacae | E. coli | P. values |
|---|---|---|---|---|---|---|---|
| Zones of inhibition (mm) | |||||||
| L. fermentum | 11 | 11 | 10 | 10 | 9 | 8.5 | 0.055 |
| L. brevis | 10 | 11 | 10 | 11 | 9 | 8.5 | 0.034 |
| L. plantarum | 10 | 11 | 10 | 15 | 11 | 8.5 | 0.02 |
| L. acidophilus | 9 | 12 | 11 | 11 | 9 | 11 | 0.02 |
The decrease in essential Lactobacilli residing in the vaginal region, alongside the possible increased presence of pathogenic bacteria represents a noteworthy determinant in the potential onset of vaginal infections. The vaginal microflora of individuals with vaginal infections is primarily inhabited by various pathogenic microorganisms that may include bacteria of the Streptococcus, Staphylococcus, E. coli, and Enterobacter genera. A vaginal microbiome that is in equilibrium has the capacity to present protection against vaginal infections. In this study, Lactobacillus fermentum, Lactobacillus plantarum, Lactobacillus brevis and Lactobacillus acidophilus showed susceptibility to all the tested antibiotics. This makes these strains safe because they do not possess resistant genes that could be transferred to the vagina pathogens.30 Also, the reports of Mohankumar et al.31 suggest that the restorative therapy of vaginal microbiota appears to be negatively affected by the susceptibility of Lactobacillus strains to antibiotics.
The current investigation reports that the Lactobacillus obtained from fermented ogi demonstrated probiotic characteristics and agrees with the reports of Olatunde et al.,32 who documented the isolation of probiotic Lactobacillus from waste products obtained during ogi manufacturing. More so, the Lactobacillus exhibited antibacterial efficacy against the pathogenic bacteria strains from patients diagnosed with vaginal infections. The results of this study aligns with numerous prior studies that have demonstrated the capacity of Lactobacillus to generate organic acids, hydrogen peroxide and bacteriocins among others.2 Since four Lactobacillus strains isolated from fermented ogi demonstrated probiotic properties, it could be inferred that fermented ogi possesses adequate probiotic LAB that could be harnessed for both antimicrobial activity and source, with high survival rates above 80% in the gastrointestinal tract (GIT). This feature could be a crucial factor in promoting the use of oral probiotics. In this context, it is imperative that probiotics possess non-pathogenic properties, exhibit resilience in the midst of unfavorable conditions present within the gastrointestinal tract and ultimately arrive at the intestine in an efficient/viable state.33
Furthermore, it was observed that the CFSs of the four Lactobacillus strains exhibited inhibitory effects on the vaginal pathogens, showing a varying spectrum of activity. Dasari et al.2 has previously demonstrated the antibacterial efficacy of the bacteriocin derived from the vaginal probiotic Lactobacillus against a range of pathogens that include Staphylococcus aureus, Pseudomonas aeruginosa, Enterobacter cloacae, Escherichia coli, K. pneumonia, and Streptococcus pneumonia isolated from individuals with cervicovaginal infections. More so, Andreeva et al.34 has reported that CFS from Lactobacillus strain exhibited antibacterial properties against isolated vaginal pathogen. In a study conducted by Faniran & Omemu,35 probiotic LAB CFS demonstrated the ability to inhibit the proliferation of S. aureus, Proteus mirabilis, Escherichia coli, Bacillus cereus and K. pneumonia. The significant antimicrobial activity of the probiotic CFS revealed the potential of organic acids, hydrogen peroxide and bacteriocins in Lactobacillus-dominant environments to provide protection to the host against infections caused by pathogenic organisms. This is achieved through the synthesis of antimicrobial substances and short chain fatty acids, which serve to acidify the local microenvironment and maintain vaginal pH levels below 4.5.36 Some examples of the metabolites that have been shown to have a positive effect on vaginal health include bacteriocin, lactic acid, hydrogen peroxide and acetic acid among others.37-39
Researchers have found that hydrogen peroxide produced by Lactobacillus is beneficial against sexually transmitted diseases and bacterial vaginosis.40 The breakdown products of glycogen are used in the generation of lactic acid under anaerobic conditions, resulting in a decrease in pH of the vagina.17,41 This acidification acts as a physiological defense mechanism that can potentially boost the efficacy of other immunomodulatory and antibacterial capabilities, by penetrating cell membranes and producing osmotic stress, as well as by destabilizing the outer membrane of vaginal pathogenic Gram-negative organisms.42
This study demonstrated that CFS from probiotic Lactobacillus sourced from fermented ogi contains antimicrobial compounds that exhibit antibacterial activity against pathogens isolated from the vagina. Therefore, it could be plausible to utilize CFS from probiotic LAB as prophylactic and bioprotective agents for the management of vaginal bacterial infections, while also preserving the typical vaginal microbiota.
ACKNOWLEDGMENTS
The authors would like to acknowledge the management and Laboratory staff of the Federal Medical Center Abeokuta Ogun State, Nigeria, as well as the Covenant Centre for Research, Innovation and Discovery (CUCRID) for their support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Covenant Health Research Ethics Committee (CHREC), Covenant University, Ogun State, Nigeria, with reference number CU/HREC/EGN/206/23.
- Zhu B, Tao Z, Edupuganti L, Serrano MG, Buck GA. Roles of the Microbiota of the Female Reproductive Tract in Gynecological and Reproductive Health. Microbiol Mol Biol Rev. 2022;86(4):e00181-21.
Crossref - Dasari S, Shouri RND, Wudayagiri R, Valluru L. Antimicrobial activity of Lactobacillus against microbial flora of cervicovaginal infections. Asian Pac J Trop Dis. 2014;4(1):18-24.
Crossref - Coudray MS, Madhivanan P. Bacterial vaginosis-A brief synopsis of the literature. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2020;245:143-148.
Crossref - Loveless M, Myint O. Vulvovaginitis- presentation of more common problems in pediatric and adolescent gynecology. Best Pract Res Clin Obstet Gynaecol. 2018;48:14-27.
Crossref - Moradi S, Hasani MT, Darvish L, Roozbeh N. Evaluating Cervicovaginal Infections and Cervical Cancer in Women with Low Socioeconomic Levels. Iran J Public Health. 2017;46(6):867-868.
- Kaur H, Merchant M, Haque MM, Mande SS. Crosstalk Between Female Gonadal Hormones and Vaginal Microbiota Across Various Phases of Women’s Gynecological Lifecycle. Front Microbiol. 2020;11:551.
Crossref - Chee WJY, Chew SY, Than LTL. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microbial Cell Factories. 2020;19(1):203.
Crossref - Kalia N, Singh J, Kaur M. Microbiota in vaginal health and pathogenesis of recurrent vulvovaginal infections: a critical review. Ann Clin Microbiol Antimicrob. 2020;19(1):5.
Crossref - Abou Chacra L, Fenollar F, Diop K. Bacterial Vaginosis: What Do We Currently Know? Front Cell Infect Microbiol. 2022;11:672429.
Crossref - Serwecinska L. Antimicrobials and Antibiotic-Resistant Bacteria: A Risk to the Environment and to Public Health. Water. 2020;12(12):3313.
Crossref - Prabhurajeshwar C, Chandrakanth K. Evaluation of antimicrobial properties and their substances against pathogenic bacteria in-vitro by probiotic Lactobacilli strains isolated from commercial yoghurt. Clin Nutr Exp. 2019;23:97-115.
Crossref - Yan Y, Li X, Guan H, et al. Microbial community and fermentation characteristic of Italian ryegrass silage prepared with corn stover and lactic acid bacteria. Bioresour Technol. 2019;279:166-173.
Crossref - Dean SN, Leary DH, Sullivan CJ, Oh E, Walper SA. Isolation and characterization of Lactobacillus-derived membrane vesicles. Sci Rep. 2019;9(1):877.
Crossref - Happel A-U, Kullin B, Gamieldien H, et al. Exploring potential of vaginal Lactobacillus isolates from South African women for enhancing treatment for bacterial vaginosis. PLOS Pathogens. 2020;16(6):e1008559.
Crossref - Foligne B, Daniel C, Pot B. Probiotics from research to market: the possibilities, risks and challenges. Curr Opin Microbiol. 2013;16(3):284-292.
Crossref - New FJ, Theivendrampillai S, Juliebo-Jones P, Somani B. Role of Probiotics for Recurrent UTIs in the Twenty-First Century: a Systematic Review of Literature. Curr Urol Rep. 2022;23(2):19-28.
Crossref - Borges S, Silva J, Teixeira P. The role of lactobacilli and probiotics in maintaining vaginal health. Arch Gynecol Obstet. 2014;289(3):479-489.
Crossref - Liu P, Lu Y, Li R, Chen X. Use of probiotic lactobacilli in the treatment of vaginal infections: In vitro and in vivo investigations. Front Cell Infect Microbiol. 2023;13:1153894.
Crossref - Nataraj BH, Ali SA, Behare PV, Yadav H. Postbiotics-parabiotics: the new horizons in microbial biotherapy and functional foods. Microb Cell Fact. 2020;19(1):168.
Crossref - Thakkar P, Modi HA, Prajapati JB. Isolation, characterization and safety assessment of lactic acid bacterial isolates from fermented food products. Int J Curr Microbiol Appl Sci. 2015;4(4):713-725.
- Akinduti AP, Ayodele O, Motayo BO, Obafemi YD, Isibor PO, Aboderin OW. Cluster analysis and geospatial mapping of antibiotic resistant Escherichia coli O157 in southwest Nigerian communities. One Health. 2022;15:100447.
Crossref - Stepanovic S, Vukovic D, Hola V, et al. Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS. 2007;115(8):891-899.
Crossref - Dragomirescu CC, Lixandru BE, Coldea IL, et al. Antimicrobial Susceptibility Testing for Corynebacterium Species Isolated from Clinical Samples in Romania. Antibiotics. 2020;9(1):31.
Crossref - Matuschek E, Longshaw C, Takemura M, Yamano Y, Kahlmeter G. Cefiderocol: EUCAST criteria for disc diffusion and broth microdilution for antimicrobial susceptibility testing. J Antimicrob Chemother. 2022;77(6):1662-1669.
Crossref - Omemu AM, Okafor UI, Obadina AO, Bankole MO, Adeyeye SAO. Microbiological assessment of maize ogi cofermented with pigeon pea. Food Sci Nutr. 2018;6(5):1238-1253.
Crossref - Adediran AB, Aforijiku S. Carbohydrate Fermentation Profile and Physiological Studies of Lactic Acid Bacteria from Native Raw Cow Milk. J Adv Microbiol. 2020;20(7):83-93.
Crossref - Zafar H, Ain NU, Alshammari A, et al. Lacticaseibacillus rhamnosus FM9 and Limosilactobacillus fermentum Y57 Are as Effective as Statins at Improving Blood Lipid Profile in High Cholesterol, High-Fat Diet Model in Male Wistar Rats. Nutrients. 2022;14(8):1654.
Crossref - Reuben RC, Roy PC, Sarkar SL, Alam R-U, Jahid IK. Isolation, characterization, and assessment of lactic acid bacteria toward their selection as poultry probiotics. BMC Microbiol. 2019;19(1):253.
Crossref - Kamble A, Naik S, Talathi M, Jadhav D, Pingale S, Kaul-Ghanekar R. Cervicovaginal microflora isolated from healthy women exhibited probiotic properties and antimicrobial activity against pathogens isolated from cervical cancer patients. Research Square. 2022.
Crossref - Georgieva R, Yocheva L, Tserovska L, et al. Antimicrobial activity and antibiotic susceptibility of Lactobacillus and Bifidobacterium spp. intended for use as starter and probiotic cultures. Biotechnol Biotechnolog Equip. 2015;29(1):84-91.
Crossref - Mohankumar B, Shandil RK, Narayanan S, Krishnan UM. Vaginosis: Advances in new therapeutic development and microbiome restoration. Microb Pathog. 2022;168:105606.
Crossref - Olatunde OO, Obadina AO, Omemu AM, Oyewole OB, Olugbile A, Olukomaiya OO. Screening and molecular identification of potential probiotic lactic acid bacteria in effluents generated during ogi production. Ann Microbiol. 2018;68(7):433-443.
Crossref - Dincer E, Kivanc M. In vitro evaluation of probiotic potential of Enterococcus faecium strains isolated from Turkish pastırma. Arch Microbiol. 2021;203(6):2831-2841.
Crossref - Andreeva P, Shterev A, Danova S. Antimicrobial activity of vaginal lactobacilli against Gardnerella vaginalis and pathogens. Int. J Adv Res Biol Sci. 2016;3(5):200-207.
- Faniran OW, Omemu AM. Assessment of the Antimicrobial Activity of Lactic Acid Bacteria Isolated from Two Fermented Maize Products – ogi and kunnu-zaki. Malays J Microbiol. 2011;7(3):124-128.
Crossref - Kyrgiou M, Mitra A, Moscicki A-B. Does the vaginal microbiota play a role in the development of cervical cancer? Transl Res. 2017;179:168-182.
Crossref - Ghartey JP, Smith BC, Chen Z,et al. Lactobacillus crispatus Dominant Vaginal Microbiome Is Associated with Inhibitory Activity of Female Genital Tract Secretions against Escherichia coli M. Desvaux (ed.). PLoS ONE. 2014;9(5):e96659.
Crossref - Kovachev S. Defence factors of vaginal lactobacilli. Crit Rev Microbiol. 2018;44(1):31-39.
Crossref - Rosca AS, Castro J, Sousa LGV, Cerca N. Gardnerella and vaginal health: the truth is out there. FEMS Microbiol Rev. 2020;44(1):73-105.
Crossref - Yarbrough VL, Winkle S, Herbst-Kralovetz MM. Antimicrobial peptides in the female reproductive tract: a critical component of the mucosal immune barrier with physiological and clinical implications. Hum Reprod Update. 2015;21(3):353-377.
Crossref - Gaspar C, Donders GG, Palmeira-de-Oliveira R, et al. Bacteriocin production of the probiotic Lactobacillus acidophilus KS400. AMB Express. 2018;8(1):153.
Crossref - Tachedjian G, Aldunate M, Bradshaw CS, Cone RA. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res Microbiol. 2017;168 (9-10):782-792.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.