ISSN: 0973-7510
E-ISSN: 2581-690X
This study provides a comprehensive evaluation of Ziziphus jujuba honey, commonly referred to as sidr honey (SH), collected from five distinct botanical origins in Morocco: SHE from Errachidia (Daraa-Tafilalet), SHZ from Zaggota (Rabat-Sale-Kenitra), SHA from Ain Chair (Oriental), SHS from Saka (Oriental), and SHK from Khenifra (Beni Mellal-Khenifra). The research investigates the honey’s antibacterial properties against a range of gram-positive and gram-negative bacterial strains. Furthermore, key physicochemical parameters were analyzed according to international honey standards, including pH, electrical conductivity, Brix scale, refractive index, color, hydrosoluble protein, and total sugar content. Besides, bioactive compounds of SH honey samples, namely Total Flavonoid Content (TFC), and Total Phenolic Content (TPC) were measured. The antioxidant capacity was assessed through Total Antioxidant Capacity (TAC) measurements and the DPPH assay. The results demonstrated significant variations in the biochemical and physical properties of the SH samples. SHZ had the highest protein content at 209.59 ± 0.91 mg Eq BSA/100 g and TFC of 77.10 ± 1.21 mg QE/100 g, besides a strong antioxidant activity with a DPPH IC50 value of 10.53 ± 0.01 mg/ml and a TAC value of 50.23 ± 0.60 AAE/100 g. Antibacterial assays revealed that SHZ displayed the most potent antibacterial effects, particularly targeting gram-negative bacteria, with an MIC of 0.024 w/w% for S. aureus and 0.049 w/w% for P. aeruginosa, both with corresponding MBC values. In contrast, SHS demonstrated weaker antibacterial activity, with MIC values reaching 12.5 w/w% for several strains. In addition, the analysis of the honey samples indicates substantial relationships among physicochemical parameters, bioactive components, antioxidant and antibacterial activities. These results demonstrate that SH exhibits notable antibacterial and antioxidant properties, underscoring its potential as a natural health product. However, further studies are essential to explore the precise mechanisms driving these bioactive effects and their broader implications for health and nutrition.
Antibacterial, Antioxidant, Sidr Honey, Ziziphus jujuba Honey
Considerable research efforts have been devoted to investigating the therapeutic properties of natural compounds, which have been utilized for medicinal purposes throughout history and are generally regarded as having low toxicity.1 Their potential benefits have attracted significant interest in various fields, including anti-inflammatory, antimicrobial, antioxidant, and anticancer research.1 Honey, one of the main substances created by honeybees, is a prominent example of such natural products. Civilizations such as the Greeks, Romans, and Arab-Islamic societies have incorporated honey into their medicinal practices, influenced by cultural beliefs and historical accounts.1-4 Honey holds significant esteem in the Arab-Islamic medical tradition for its health-promoting qualities and frequent application in wound care, as recorded by renowned medieval scholars.5 Different types of honey, some supported by scientific research, are regularly employed as natural remedies for promoting health and managing various conditions. Studies have demonstrated that honey and its components exhibit antibacterial, anti-inflammatory, antioxidant, antiproliferative, antimetastatic, and anticancer properties.2
Among honey’s traditional uses, its antimicrobial effects stand out prominently. Antimicrobial resistance poses a major global public health threat, primarily due to the high morbidity, severe complications, and mortality rates linked to multidrug-resistant bacteria, compounded by the limited availability of effective treatments.6 The increasing prevalence of antibiotic resistance has heightened interest in alternative antimicrobial agents, particularly those derived from natural sources.1 Among these, honey has emerged as a key candidate due to its unique composition and therapeutic properties. Honey serves not only as an energy source but also contains a variety of bioactive compounds that contribute to its medicinal attributes.2,3 Research has shown that honey exhibits broad-spectrum antibacterial activity, attributed to its high sugar content, low pH, and the presence of phytochemicals such as flavonoids and phenolic acids.7,8
SH honey, known as jujube or Sider honey, is native to Asia and parts of North Africa, including Morocco, where its honey is harvested. This honey is believed to possess significant antibacterial and antioxidant properties, making it a valuable subject for scientific investigation.4 SH is particularly noteworthy for its health benefits, as it not only combats pathogenic bacteria but also enhances the body’s defenses counter oxidative stress.4,9 Antioxidants are crucial for mitigating cellular harm caused by free radicals, and incorporating honey into dietary regimens may promote overall vitality and wellness.4,9 However, despite increasing interest, there is a lack of detailed comparative studies that assess Moroccan SH from different geographic regions in terms of both its biological activities and chemical profiles.
The chemical makeup of honey changes widely depending on geographic factors like local plants, climate, and farming practices, which affect its composition and effectiveness.8,10 Key components typically include sugars, acids, enzymes, and a wide range of phenolic molecules that shape both the flavor and health benefits of honey.8,10 Research has shown that Moroccan honeys, particularly those sourced from indigenous plants, may have unique phytochemical profiles that enhance their bioactivity.8,10 Despite this, limited data exist specifically for Moroccan. However, despite increasing interest, there is a lack of detailed comparative studies that assess Moroccan SH from different geographic regions in terms of both its biological activities and chemical profiles, and even fewer studies provide a systematic analysis across multiple regions of Morocco. To address this gap, the present study hypothesizes that Moroccan SH exhibits region-specific variations in its antibacterial and antioxidant properties, which correlate with differences in its chemical and physicochemical composition. This hypothesis is driven by the known influence of environmental and botanical factors on honey quality, and the urgent need for natural therapeutic alternatives to fight microbial resistance.
Understanding these regional variations can shed light on the therapeutic potential of honey and its applications in healthcare. This study aims to conduct a comprehensive analysis of Ziziphus jujuba honey from five Moroccan regions, contributing valuable insights to the fields of food science and natural medicine. By investigating its antibacterial and antioxidant activities, along with its chemical and physicochemical properties, this research will highlight the health-promoting properties of this honey and encourage the sustainable use of local resources. Additionally, the findings could facilitate the development of new natural products and strategies to combat microbial resistance, in line with global health initiatives aimed at reducing reliance on synthetic antibiotics.
SH samples were collected from five regions in Morocco between 2021 and 2022 (Table 1). The classification of honey types was based on criteria including geographical origin, melissopalynological analysis, and harvest time. Following collection, the samples were sealed in cans for preservation and stored under ambient, moisture-free conditions. They remained unopened until required for experimental procedures, which took place within three months of collection. All subsequent experiments were performed independently in triplicate, across three separate experimental runs.
Table (1):
Ziziphus jujuba honey (Sider honey) varieties: harvest year and location
Code |
Year of harvest |
Region |
Altitude |
Latitude |
|---|---|---|---|---|
SHE |
2021 |
Errachidia (Daraa-Tafilalet) |
1039 m |
31.9314° N, 4.4266° W |
SHZ |
2021 |
Zaggota (Rabat-Salé-Kenitra) |
390 m |
34.1681° N, 5.5250° W |
SHA |
2021 |
Ain Chair (Oriental) |
976 m |
32.2048° N, 2.5192° W |
SHS |
2021 |
Saka (Oriental) |
730 m |
34.6186° N, 3.4170° W |
SHK |
2022 |
Khenifra (Beni Mellal-Khenifra) |
827 m |
32.9349° N, 5.6616° W |
Protein content, total sugar content, pH, Brix index, refractive index, and electrical conductivity were evaluated following the standardized protocols outlined by the International Honey Commission (IHC) in their Harmonized Methods.11
Total Polyphenols Content (TPC) was measured using the Folin-Ciocalteu method, following established protocols in the literature.12
The total flavonoid content (TFC) was measured by combining 100 µL of SH with 5% sodium nitrite and 150 µL of 10% AlCl3. After 5 minutes, 200 µL of 1 M NaOH (1%) was added to the mixture. The optical density of the resulting solution was then recorded at 510 nm. The flavonoid content was expressed as milligrams of quercetin equivalent per 100 grams of SH (mg QE/100 g).
Free radical scavenging activity (DPPH assay) 1,1-diphenyl-2-picrylhydrazyl, (SIGMA, St. Louis, MO, USA) was applied to evaluate the radical scavenging ability, following the method described in the literature.13
The total antioxidant capacity (TAC) was determined following the method outlined by Prieto, Pineda, and Aguilar.12
Antibacterial activity was assessed using the following bacterial strains: P. aeruginosa (strain 27853), S. aureus (strain BAA-1026), E. coli (strain 25922), S. pneumoniae (strain 49619), K. quasipneumoniae (strain 700603), H. influenzae (strain 49247), and B. subtilis (strain 6633), all sourced from the American Type Culture Collection (ATCC), Manassas, VA, USA. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of the honey samples were determined using 96-well, flat-bottom microtiter plates, in accordance with established methods.8,13
Statistical analysis
The error limits, represented by the error bars, indicate the standard deviations of the averages. Numerical results are presented with precision up to the least significant digit. Statistical significance between different samples was assessed using Student’s t-test for unpaired samples, with a significance threshold set at p < 0.05.
Hydrosoluble protein content, total sugar content, pH, Brix index, Refractive index, electrical conductivity, and color.
The examination of honey samples from various geographical regions revealed notable differences in their biochemical and physical characteristics (Table 2). The hydrosoluble protein content ranged from 88.64 mg BSA Eq/100 g of honey in the Ain Chair honey (MHA) to 209.59 mg BSA Eq/100 g in the Zaggota honey (MHZ), with a mean value of 138.14 mg BSA Eq/100 g across all samples. Notably, Zaggota honey demonstrated the greatest protein content, whereas Ain Chair honey exhibited the least.
Table (2):
Hydrosoluble protein levels, total sugar levels, pH, brix index, refractive index, electrical conductivity, and color of the sider honey samples
Honey |
Hydrosoluble protein |
Total sugars |
pH |
Brix scale index |
Refractive |
Electrical conductivity |
Color |
|---|---|---|---|---|---|---|---|
SHE |
122.17 ± 0.12 |
125.9 ± 0.3 |
4.87 ± 0.12 |
84.15 ± 0.07 |
14.5 ± 0.00 |
40.2 ± 2 |
Light Amber |
SHZ |
209.59 ± 0.91 |
217.1 ± 0.11 |
4.15 ± 0.02 |
81 ± 0.00 |
17.5 ± 0.07 |
60.9 ± 3.1 |
Dark Amber |
SHA |
88.64 ± 0.91 |
118.5 ± 0.2 |
4.47 ± 0.01 |
82.4 ± 0.14 |
16 ± 0.00 |
80.8 ± 2.9 |
Dark Amber |
SHS |
154.62 ± 0.15 |
220 ± 0.9 |
3.83 ± 0.06 |
82.05 ± 0.064 |
16.4 ± 0.00 |
81.1 ± 2.89 |
Light Amber |
SHK |
135 ± 0.3 |
200 ± 0.82 |
4.62 ± 0.05 |
81.9 ± 0.1 |
15 ± 0.1 |
70.9 ± 2.1 |
Light Amber |
Hydrosoluble Protein (mg Eq BSA/100 g of honey), Total sugar (mg Eq Glu/100 g of honey), Brix scale (%), and Conductivity (µs).
Total carbohydrate levels were highest in Saka honey (MHS) at 220 mg Glu Eq/100 g, followed closely by Zaggota honey (MHZ) at 217.1 mg Glu Eq/100 g. The lowest carbohydrate content was observed in Ain Chair honey (MHA) with 118.5 mg Glu Eq/100 g, indicating a broad range of carbohydrate composition among the samples.
The pH values varied significantly, with Saka honey (MHS) showing the lowest pH of 3.83, while Zaggota honey (MHZ) exhibited the highest pH of 4.15. These pH values suggest a trend towards acidity in certain honey samples, particularly Saka.
Brix scale measurements indicated a range from 81.0% in Zaggota honey (MHZ) to 84.15% in Errachidia honey (MHE). This variation reflects differences in sugar concentration, with higher Brix values correlating to greater sugar content.
Refractive index values also varied across samples, with Zaggota honey (MHZ) recording the highest at 17.5, indicating a denser composition compared to the other samples. Electrical conductivity ranged from 40.2 mS in Errachidia honey (MHE) to 81.1 mS in Saka honey (MHS), suggesting differences in ionic content and overall mineral composition.
In terms of color, the honey samples ranged from light amber in Errachidia (MHE), Saka (MHS), and Khenifra (MHK) to dark amber in Zaggota (MHZ) and Ain Chair (MHA). The variation in color correlates with the different floral sources and geographic regions of the honeys.
Overall, these results demonstrate significant diversity in the chemical and physical characteristics of honey based on its geographical source, highlighting the potential for these parameters to serve as indicators of honey quality and provenance.
Total polyphenols, flavonoids, and antioxidant capacity
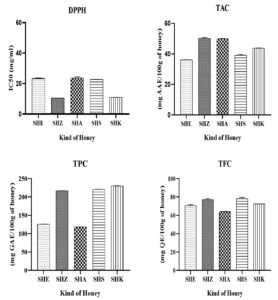
The results reveal significant variation in the antioxidant properties of the tested Sider honey samples (Figure 1). SHZ and SHK consistently demonstrated strong antioxidant activity, characterized by low DPPH IC50 values and high TFC and TPC. In contrast, SHE exhibited the weakest antioxidant capacity. These findings highlight the potential of Sider honey as a natural source of antioxidants, with implications for its use in health and nutrition. Additional research is needed to investigate the specific mechanisms underlying the antioxidant properties of these honey samples.
Figure 1. Antioxidant properties and bioactive compounds of honeys
SHE: Sider Honey Errachidia. SHZ: Sider Honey Zeggota. SHA: Sider Honey Ain Chair. SHS: Sider Honey Saka. SHK: Sider Honey Khenifra
DPPH IC50
The DPPH IC50 values represent the concentration of honey needed to achieve 50% inhibition of free radicals. SHZ exhibited the greatest antioxidant activity with the least IC50 value of 10.33 ± 0.01 mg/ml, followed closely by SHK (11 ± 0.01 mg/ml). In contrast, SHA demonstrated the least antioxidant capacity with a significantly higher IC50 value of 23.31 ± 0.49 mg/ml (Figure 1).
Total Flavonoid Content (TFC)
The TFC was highest in SHS at 78.11 ± 1.5 mg QE/100 g, indicating a robust flavonoid presence that may contribute to its antioxidant effects. SHZ also had a high TFC of 77.10 mg QE /100 g, correlating with its strong antioxidant capacity. Conversely, SHA had the lowest TFC (64.11 mg QE/100 g) (Figure 1).
Total Phenolic Content (TPC)
SHK showed the highest TPC at 230 ± 0.5mg GAE/100 g, which is significantly higher than the other samples. This indicates a abundant presence of phenolic compounds, which are well-known for their antioxidant properties. SHS and SHZ also exhibited notable TPC values (220.7 ± 0.3 mg GAE/100 g and 217.1 ± 0.2 mg GAE/100 g, respectively), while SHE had a comparatively lower TPC of 125.9 mg GAE/100 g.
Total Antioxidant Capacity (TAC)
TAC values indicated the overall ability of the honey to act as an antioxidant, with SHZ having the highest TAC (50.23 g AAE/100 g). SHA (49.88 ± 0.2 g AAE/100 g) and SHK (43.82 g AAE/100 g) also displayed respectable TAC values, while SHE had the lowest TAC at 36.08 ± 0.03 g AAE/100 g (Figure 1).
The antibacterial properties
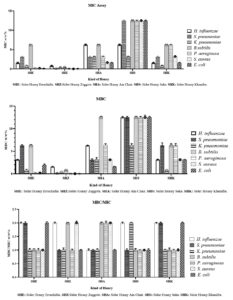
The antibacterial efficacy of five Moroccan SH samples-SHE, SHZ, SHA, SHS, and SHK-was evaluated against a range of bacterial strains, such as H. influenzae, S. pneumoniae, K. pneumoniae, B. subtilis, P. aeruginosa, S. aureus, and E. coli. The MIC and MBC values were established for each honey type against the corresponding bacterial strains. Overall, the results show that the honey samples impacted both gram-positive bacteria (S. pneumoniae, B. subtilis, S. aureus) and gram-negative bacteria (H. influenzae, K. pneumoniae, P. aeruginosa, and E. coli). However, gram-negative bacteria were generally more susceptible, especially P. aeruginosa and E. coli (Figure 2).
Figure 2. Demonstrates the antibacterial activity of SHE, SHZ, SHA, SHS, and SHK, showing the MIC and MBC values for these samples against different bacterial strains. The presented data represent the average results ± SD from three separate experiments carried out four replications
SHE exhibited MIC values of 1.562 w/w% for H. influenzae and 3.125 w/w% for S. pneumoniae, with MBC values of 3.125 w/w% and 6.25 w/w%, respectively. Against K. pneumoniae, SHE had an MIC of 0.78125 w/w% and an MBC of 0.78125 w/w%. For B. subtilis, both MIC and MBC were 6.25 w/w%. In terms of P. aeruginosa, SHE showed MIC and MBC values of 0.195 w/w%, and for S. aureus, both values were also 0.195 w/w%. For E. coli, the MIC was 0.097 w/w% with an MBC of 1.94 w/w%.
SHZ demonstrated the strongest antibacterial activity overall, with MIC values of 0.78125 w/w% for H. influenzae and 0.1953 w/w% for S. pneumoniae, with corresponding MBC values of 1.562 w/w% and 0.1953 w/w%. For K. pneumoniae, SHZ achieved both MIC and MBC at 0.39 w/w%. Against B. subtilis, the MIC was also 0.39 w/w%. SHZ excelled against P. aeruginosa with MIC and MBC both at 0.049 w/w%, and showed exceptional results against S. aureus (MIC: 0.024 w/w%, MBC: 0.048 w/w%) and E. coli (MIC and MBC: 0.024 w/w%).
SHA exhibited weaker antibacterial effects, with MIC values of 6.25 w/w% for H. influenzae and 3.125 w/w% for S. pneumoniae, both with MBCs equal to their MICs. For K. pneumoniae, both MIC and MBC were 3.125 w/w%, while for B. subtilis, MIC was 6.25 w/w% and MBC was 12.5 w/w%. Against P. aeruginosa, SHA had MIC of 3.215 w/w% and MBC of 6.43 w/w%. For S. aureus, the MIC was 1.5625 w/w% and MBC 3.125 w/w%. For E. coli, both MIC and MBC were 1.5625 w/w%.
SHS showed high MIC values of 6.25 w/w% for H. influenzae and 12.5 w/w% for S. pneumoniae, both with MBCs equal to their MICs. For K. pneumoniae, the MIC was 3.125 w/w% with an MBC of 6.25 w/w%. Against B. subtilis, both MIC and MBC were 12.5 w/w%. SHS exhibited poor antibacterial activity across the board for P. aeruginosa, S. aureus, and E. coli, with MIC and MBC values of 12.5 w/w% for all strains tested.
SHK had MIC values of 1.562 w/w% for H. influenzae and 3.125 w/w% for S. pneumoniae, both with MBCs equal to their MICs. For K. pneumoniae, SHK exhibited MIC and MBC values of 0.78125 w/w%. Against B. subtilis, both values were 6.25 w/w%. For P. aeruginosa, SHK showed MIC and MBC of 6.25 w/w%. For S. aureus, the MIC was 3.125 w/w% and MBC was 3.125 w/w%, while for E. coli, MIC was 1.5625 w/w% with an MBC of 3.125 w/w%.
Correlation between physicochemical, bioactive compounds, antioxidant, and antibacterial activities of jujube honey samples
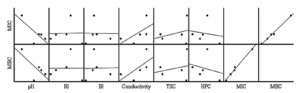
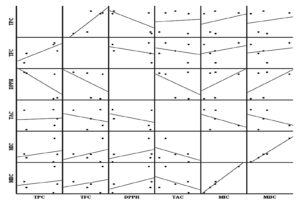
The analysis of the honey samples indicates substantial relationships among physicochemical parameters, bioactive components, antioxidant and antibacterial activities (Table 3, Figure 3 and 4). Total phenolic content (TPC) and total flavonoid content (TFC) are strongly correlated, both contributing to antioxidant capacity, as evidenced by their negative correlation with DPPH activity (r = -0.709, r = -0.418, respectively). Additionally, the DPPH assay, which measures free radical scavenging activity, has a negative correlation with TAC (r = -0.443), reflecting the relationship between antioxidant power and bioactive compounds. The TAC shows a negative correlation with MIC (r = -0.424) and MBC (r = -0.391), reflecting that the higher antioxidant activity of honey samples is associated with their strong antibacterial effect. Physicochemical parameters, such as pH, display a highly negative correlation with antibacterial activity (r = -0.753, r = -0.722 for MIC and MBC, respectively), indicating that lower pH may enhance microbial inhibition of honey. Besides, a negative correlation was detected between total protein content and MIC (r = -0.159) and MBC (r = -0.241), which suggests that honey proteins may play a supportive role in boosting their antibacterial properties.
Table (3):
Pearson correlation coefficients between the evaluated parameters and conducted tests
TPC |
TFC |
DPPH |
TAC |
pH |
Refractive index |
Brix index |
Conductivity |
Total sugar content |
Total protein content |
MIC |
MBC |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
TPC |
1 |
0.814 |
-0.709 |
0.066 |
-0.537 |
0.400 |
-0.723 |
0.305 |
0.973** |
0.710 |
0,267 |
0,188 |
TFC |
1 |
-0.418 |
-0.245 |
-0.642 |
0.441 |
-0.439 |
-0.044 |
0.895* |
0.856 |
0.199 |
0,205 |
|
DPPH |
1 |
-0.443 |
-0,095 |
-0,312 |
0,685 |
0,048 |
-0,627 |
-0,656 |
-0.443 |
0,526 |
||
TAC |
1 |
0 |
0,647 |
-0,714 |
0,430 |
0,075 |
0,180 |
-0,424 |
-0,391 |
|||
pH |
1 |
-0,650 |
0,514 |
-0,606 |
-0,671 |
-0,430 |
-0,753 |
-0,722 |
||||
Refractive index |
1 |
-0,810 |
0,406 |
0,552 |
0,676 |
0,021 |
0,002 |
|||||
Brix index |
1 |
-0,576 |
-0,745 |
-0,629 |
-0,012 |
0,018 |
||||||
Conductivity |
1 |
0,297 |
-0,153 |
0,605 |
0,636 |
|||||||
Total sugar content |
1 |
0,806 |
0,307 |
0,226 |
||||||||
Total protein content |
1 |
-0.159 |
-0,241 |
|||||||||
MIC |
1 |
0,995** |
||||||||||
MBC |
1 |
*: The correlation is significant at the 0.05 level. ** : The correlation is significant at the 0.01 level.
Figure 3. A scatterplot matrix representing the correlation between the physicochemical parameters of Sider Honey Samples (SHS) and their antibacterial effects (MIC and MBC)
RI: Refractive index, BI: Brix index, TSC: Total sugar content, HPS: Hydrosolubles protein content
This study investigates the antibacterial properties of five Moroccan Sider honey varieties (SHE, SHZ, SHA, SHS, and SHK) against various bacterial strains, along with their physicochemical and antioxidant characteristics. The results reveal distinct antibacterial activity patterns, suggesting potential correlations with their physicochemical and antioxidant activities.
Honey has long been recognized for its antibacterial properties, showing effectiveness against both gram-positive and gram-negative bacteria, with Gram-positive bacteria generally being more sensitive.14,15 Extensive laboratory research has explored honey’s biological properties, including its antibacterial, antifungal, antiviral, and antiprotozoal activities.16-18 This biological activity sets honey apart from other natural products, highlighting its importance in medical and therapeutic applications.19-21
Our study focused on the antibacterial effects of the five varieties of Moroccan Sider honey against both Gram-positive bacteria (S. pneumoniae, B. subtilis, S. aureus) and Gram-negative bacteria (H. influenzae, K. pneumoniae, P. aeruginosa, and E. coli). The findings revealed significant variations in antibacterial activity among the honey varieties, underscoring the potential of Moroccan Sider honey as a natural antimicrobial agent.
The MIC and MBC results indicated that SHZ exhibited the strongest antibacterial activity, particularly against S. pneumoniae and E. coli, with MIC values as low as 0.024% w/w. These low MIC values are biologically significant, as they indicate that SHZ honey can inhibit bacterial growth at very low concentrations, demonstrating a high degree of potency comparable to conventional antibiotics in some cases. This suggests a high potency likely linked to specific phytochemicals or enzymatic components unique to this variety. Conversely, SHS showed minimal antibacterial effectiveness, reflected in notably higher MIC and MBC values across all tested bacteria, which may indicate fewer bioactive compounds or a different chemical profile. The elevated MIC and MBC values for SHS suggest limited clinical applicability without further enhancement or combination with other agents.
The diverse antibacterial properties of the honey types underscore the impact of botanical origin and environmental factors on their composition. For instance, SHK and SHE demonstrated moderate antibacterial effects, especially against K. pneumoniae and P. aeruginosa, suggesting a diverse array of phytochemicals contributing to their efficacy. In contrast, SHA displayed significant resistance against B. subtilis and S. aureus, hinting at a possible synergistic effect of its components that merits further exploration.
These findings are consistent with existing literature on honey’s antibacterial effects, which demonstrate its efficacy against over 60 bacterial species. The activity varies based on the honey type. Numerous studies have also highlighted honey’s antibacterial and antibiofilm properties against a broad spectrum of gram-positive and gram-negative bacteria.19-21
The antibacterial mechanisms of honey are attributed to several factors, including hydrogen peroxide production, low pH, high sugar concentration, and the presence of phytochemicals such as flavonoids and phenolic acids.21-23 The varying concentrations of these components across different honey varieties could account for the differences in observed antibacterial activity. Notably, the IC50 values, reflecting the concentration required to inhibit 50% of bacterial growth, further support the high efficacy of certain honey types. In particular, the low IC50 values observed for SHZ emphasize its potential as a biologically potent antimicrobial agent, suitable for future development into therapeutic formulations. In particular, the efficacy of SHZ may be linked to higher concentrations of flavonoids, which are recognized for their antimicrobial properties.
The physicochemical characteristics of honey, including pH, moisture content, and sugar composition, are critical determinants of its antibacterial efficacy.14,24 For instance: Honey typically has a low pH, which can inhibit bacterial growth.14,24 Varieties with a lower pH may show enhanced antibacterial effects, as observed in the stronger activities of SHZ and SHE against multiple bacterial strains. Furthermore, high sugar content not only contributes to the osmotic effect, which can inhibit bacterial growth, but also supports hydrogen peroxide production through enzymatic reactions.25-28
To better understand these patterns, we conducted a correlation analysis between the antibacterial activity (MIC/MBC values) and physicochemical parameters. The results showed moderate to strong negative correlations between total sugar content and MIC values, particularly in SHZ and SHE, suggesting that higher sugar concentrations are associated with stronger antibacterial effects. Similarly, a negative correlation was observed between pH and antibacterial activity, supporting the idea that lower pH enhances antimicrobial effectiveness.
The correlations among the antibacterial effects, physicochemical properties, and antioxidant activity of Moroccan Sider honey suggest a complex interplay of factors that enhance its medicinal potential. Such multifactorial relationships highlight that antibacterial effectiveness cannot be attributed to a single component but rather to a synergistic combination of parameters including sugar content, acidity, and phenolic compounds.
Further research should focus on detailed analyses of the specific compounds present in each honey variety, as well as their interactions, to better understand how these properties can be optimized for therapeutic use. This understanding could lead to the development of effective natural alternatives in combating bacterial infections and harnessing the health benefits of honey.
In this study, we evaluated protein content, total sugar content, pH, Brix index, refractive index, and electrical conductivity, all of which are key indicators for quality control. The pH levels of the honey samples fell within the standard range and matched those documented for honey varieties from Palestine, Morocco, and Algeria.8,13,28
Honey’s acidity is derived from a range of organic acids, with gluconic acid being the most prevalent, along with formic, tartaric, maleic, citric, succinic, butyric, lactic, oxalic, and other aromatic acids. This acidity is essential for shaping honey’s flavor profile and contributes to its stability by preventing microbial spoilage and inhibiting the growth of microorganisms.15,16
Electrical conductivity indicates the presence of ionizable organic and inorganic substances, with values ideally remaining below a specified threshold to ensure quality. The electrical conductivity of the tested varieties was similar to that of other Palestinian honey varieties.28 Although sugar and protein content may vary between different honey batches, the main protein profiles generally remain consistent across various botanical and geographic origins. Overall, the total sugar and protein content in the honey were similar to those found in Palestinian, Algerian, and Moroccan samples.28,30,31
The antioxidant properties of honey are primarily attributed to the presence of phenolic compounds and flavonoids. These compounds can also exhibit direct antibacterial properties.26,27 Honey with higher antioxidant capacity often contains elevated levels of phenolic acids.1,26,27 The correlation between antioxidant activity and antibacterial effects suggests that honey varieties rich in these compounds, like SHZ, are likely to exhibit both high antioxidant and strong antibacterial properties. These compounds are known to enhance the immune response and can have direct antibacterial actions.
Varieties of honey demonstrating robust antioxidant activities may correlate with low MIC values, indicating effective antibacterial properties. The data obtained in this study show that the TPC values are consistent with those reported in various Palestinian honey samples.29 Similarly, the results are comparable to the TPC values observed in thyme honey from other regions of North Africa.29 The TFC levels are consistent with those documented for Palestinian, Moroccan, and Algerian honey varieties.29-31 Moreover, the TAC was found to be similar to values reported for other samples from the Mediterranean region.28-31 This comparison with regional studies strengthens the relevance of our findings and suggests that Moroccan Sider honey shares similar therapeutic properties with honey from other North African and Middle Eastern regions. A number of studies have shown a link between the total phenolic and flavonoid contents and antioxidant activities in vitro. The concentration of phenolic compounds can be affected by factors such as location, plant origin, specific types of phenolics, storage conditions, and processing techniques.32
The present study provides a comprehensive analysis of five Moroccan Sider honey samples, highlighting substantial variability in their physicochemical, antioxidant, and antibacterial profiles. This variability reflects the influence of floral and geographical origins on honey’s bioactive composition, offering a scientific basis for distinguishing honey types based on functional properties. Notably, Zaggota honey (SHZ) demonstrated superior antibacterial and antioxidant properties, while other samples like Ain Chair honey (SHA) and Saka honey (SHS) showed more moderate to minimal effects.
Our findings reveal strong correlations between antioxidant capacity (particularly TPC and TFC) and antibacterial efficacy, as well as significant links between physicochemical traits (such as pH and protein content) and bioactivity. This underscores a multi-factorial mechanism in honey’s antimicrobial and antioxidant action, likely mediated by synergistic interactions among phenolics, flavonoids, proteins, and acidity.
The robust antimicrobial activity of SHZ, notably against clinically relevant pathogens such as E. coli and S. pneumoniae, suggests a promising potential for therapeutic applications. These findings position certain varieties of Moroccan Sider honey as valuable candidates for development as natural antimicrobial agents or antioxidant-rich nutraceutical products. Given the global rise in antibiotic resistance, the exploration of natural alternatives like honey gains critical relevance. Moreover, the diversity in biological activities among the samples supports the concept of using honey’s biochemical profile as a biomarker for functional quality, which may inform quality assurance protocols and value-added applications in both food and medical industries.
Future research should aim to isolate and characterize the specific bioactive molecules responsible for the most potent effects observed, particularly in SHZ, and evaluate their modes of action using in vivo models. This will help establish standardized criteria for the therapeutic use of honey and reinforce its integration into evidence-based nutraceutical or clinical interventions.
ACKNOWLEDGMENTS
The authors would like to acknowledge Al-Qasemi Research Foundation and the Arab American University-Palestine (AAUP) Research Foundation for providing the financial support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
KE conducted antioxidant assays and physicochemical characterization. MM and BA performed antibacterial activity studies. ST contributed to physicochemical characterization and conducted statistical analysis. BL and BS performed supervision, wrote the manuscript, and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
ETHICS STATEMENT
Not applicable.
- Saad B. Exploring natural products: Novel insights and therapeutic potential of plant-based compounds. Microbes & Immunity. 2024;1(2):1-2.
Crossref - Eteraf-Oskouei T, Najafi M. Uses of Natural Honey in Cancer: An Updated Review. Adv Pharm Bull. 2021;12(2):248-261.
Crossref - Navaei-Alipour N, Mastali M, Ferns GA, Saberi-Karimian M, Ghayour Mobarhan M. The effects of honey on pro and anti inflammatory cytokines: A narrative review. Phytother Res. 2021;35(7):3690-701.
Crossref - Bouddine T, Laaroussi H, Bakour M, et al. Organic Honey from the Middle Atlas of Morocco: Physicochemical Parameters, Antioxidant Properties, Pollen Spectra, and Sugar Profiles. Foods. 2022;11(21):3362.
Crossref - Saad, B. History, Present and Prospect of Greco-Arab and Islamic Herbal Medicine. History, Present and Prospect of World Traditional Medicine, 2024:235-300.
Crossref - Salam MA, Al-Amin MY, Salam MT, et al. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare. 2023;11(13):1946.
Crossref - Almasaudi S. “The antibacterial activities of honey. Saudi J Biol Sci. 2021;28(4):2188-2196.
Crossref - Abu-Farich B, Masalha M, Egbaria E, et al. Physicochemical Properties, Chemical Composition, Antioxidant Properties, and Antibacterial Effects of Four Palestinian Honey Varieties. J Pure Appl Microbiol. 2024;18(4):2315-2327.
Crossref - Becerril-Sanchez AL, Quintero-Salazar B, Dublan-Garcia O, Escalona-Buendia HB. Phenolic compounds in honey and their relationship with antioxidant activity, botanical origin, and color. Antioxidants. 2021;10(11):1700.
Crossref - Alaerjani WMA, Mohammed MEA. Impact of floral and geographical origins on honey quality parameters in Saudi Arabian regions. Sci Rep. 2024;14(1):8720.
Crossref - Albu A, Radu-Rusu CG, Pop IM, Frunza G, Nacu G. Quality assessment of raw honey issued from eastern Romania. Agriculture. 2021;11(3):247.
Crossref - Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999;269(2):337-341.
Crossref - Abu-Farich B, Hamarshi H, Masalha M, et al Polyphenol Contents, Antibacterial and Antioxidant Effects of Four Palestinian Honey Samples, and their Anticancer Effects on Human Breast Cancer Cells. J Pure Appl Microbiol. 2024;18(2):1372-1385
Crossref - Bazaid AS, Aldarhami A, Patel M, et al. The antimicrobial effects of Saudi sumra honey against drug resistant pathogens: phytochemical analysis, antibiofilm, anti-quorum sensing, and an-tioxidant activities. Pharmaceuticals. 2022;15(10):1212.
Crossref - Al-Kafaween MA, Alwahsh M, Hilmi ABM, Abulebdah DH. Physicochemical characteristics and bioactive compounds of different types of honey and their biological and therapeutic properties: a comprehensive review. Antibiotics. 2023;12(2):337.
Crossref - Rani GN, Budumuru R, Bandaru NR. Antimicrobial activity of honey with special reference to methicillin resistant Staphylococcus aureus (MRSA) and methicillin sensi-tive Staphylococcus aureus (MSSA). J Clin Diagn Res. 2017;11(8):DC05-DC08.
Crossref - Mohammed SEA, Kabbashi AS, Koko WS, Ansari MJ, Adgaba N, Al-Ghamdi A. In vitro activity of some natural honeys against Entamoeba histolytica and Giardia lamblia trophozoites. Saudi J Biol Sci. 2019;26(2):238-243.
Crossref - Al-kafaween MA, Hilmi ABM, Al-Jamal HAN. The Beneficial Effects of Stingless Bee Kelulut Honey against Pseudomonas aeruginosa and Streptococcus pyogenes Planktonic and Biofilm. Trop J Nat Prod Res. 2021;5(10):1788-1796.
Crossref - Ibarguren C, Raya RR, Apella MC, Audisio MC. Enterococcus faecium isolated from honey synthesized bacteriocin-like substances active against different Listeria monocytogenes strains. J Microbiol. 2010;48(1):44-52.
Crossref - Aween MM, Hassan Z, Muhialdin BJ, Noor HM, Eljamel YA. Evaluation on antibacterial activity of Lactobacillus acidophilus strains isolated from honey. Am J Appl Sci. 2012;9(6):807-817.
Crossref - Mathialagan M, Edward YSJT, David PMM, Senthilkumar M, Srinivasan MR, Mohan-kumar S. Isolation, characterization and identification of probiotic lactic acid bacteria (LAB) from honey bees. Int J Curr Microbiol Appl. Sci. 2018;7(4):849-906.
Crossref - Brudzynski K. A current perspective on hydrogen peroxide production in honey. A review. Food chem. 2020;332:127229.
Crossref - Majtan J, Bucekova M, Kafantaris I, Szweda P, Hammer K, Mossialos D. Honey anti-bacterial activity: A neglected aspect of honey quality assurance as functional food. Trends Food Sci Technol. 2021;118(Part B):870-886.
Crossref - Nweze AJ, Olovo CV, Nweze EI, John OO, Paul C. Therapeutic properties of honey. Honey Anal New Adv Chall. 2020;15;332:1-21.
Crossref - Hasam S, Qarizada D, Azizi M. A review: honey and its nutritional composition. Asian J Res Biochem. 2020;7(3):34-43.
Crossref - Lawag IL, Islam MK, Sostaric T, Lim LY, Hammer K, Locher C. Antioxidant activity and phenolic compound identification and quantification in western australian honeys. Antioxidants. 2023;12(1):189.
Crossref - Tananaki C, Rodopoulou MA, Dimou M, Kanelis D, Liolios V. The Total Phenolic Content and Antioxidant Activity of Nine Monofloral Honey Types. Appl Sci. 2024;14(10):4329.
Crossref - Imtara H, Elamine Y, Lyoussi B. Physicochemical characterization and antioxidant activity of Palestinian honey samples. Food Sci Nutr. 2018;6(8):2056-2065.
Crossref - Aazza S, Lyoussi B, Antunes D, Miguel MG. Physicochemical characterization and antioxidant activity of 17 commercial Moroccan honeys. Int J Food Sci Nutr. 2014;65(4):449-457.
Crossref - Khalil MI, Moniruzzaman M, Boukraa L, et al. Physicochemical and antioxidant properties of Algerian honey. Molecules. 2012;17(9):11199-11215.
Crossref - Abdellah F, Makhloufy C, Boukraa L, et al. Physico-chemical properties and antibacterial and antioxidant activity of two varieties of honey from Algerian steppe. Journal of Apitherapy and Nature. 2020;3(2):59-74.
Crossref - Zawawi N, Chong PJ, Tom NNM, et al. Establishing Relationship between Vitamins, Total Phenolic and Total Flavonoid Content and Antioxidant Activities in Various Honey Types. Molecules. 2021;26(15):4399.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.