ISSN: 0973-7510
E-ISSN: 2581-690X
Solenostemma argel (Asclepiadaceae) is a beneficial folk medicine used to treat many diseases, including the kidney, liver, and stomach diseases. The aim of this study was to assess the anti-Staphylococcus aureus activity of an aqueous ethanolic extract of Solenostemma argel (AEESA) and identify its phenolic and volatile compounds using high-performance liquid chromatography (HPLC) and gas chromatography-mass spectroscopy (GC-MS). As revealed using the disc diffusion test and DNA cleavage analysis, S. argel extract was active against Staphylococcus aureus. The minimum inhibitory concentration (MIC) was 31.6 g/mL. Sixteen phenolic compounds of AEESA were analyzed using HPLC, wherein the phenolic compounds: pyrocatechol (9519.95 µg/g), ferulic acid (3221.41 µg/g), chlorogenic acid (3221.41 µg/g), and gallic acid (2730.85 µg/g) were the most abundant. Additionally, naringenin (2262.80µg/g) and quercetin (1750.25µg/g) were the major flavonoids. GC-MS analysis of AEESA revealed 30 volatile compounds, the major percentages of 9,12-octadecadienoic acid (z,z)-, methyl ester (33.44%), ascorbic acid 2,6-dihexadecanoate (7.22%), and butylated hydroxytoluene(5.46%), followed by 2-Pentadecanone, 6,10,14-trimethyl (4.77%), 2-Pentadecanone, 6,10,14-trimethyl (3.35%), Stigmasta-5,22-dien-3-ol, and acetate (3.Beta; 3.35%). The application of S. argel extract in reducing the growth of biofilms would subsequently diminish the development of antibiotic resistance and promote the future uses of plant extract components.
Solenostemma argel, Bioactive Compounds, GC-MS, HPLC, Staphylococcus aureus
Solenostemma argel Del. Hayenne (Family Asclepiadaceae) is a desert plant indigenous to Africa that is used in traditional medicine worldwide but is prominent in Saudi Arabia, Sudan, Chad, Egypt, Algeria, Libya, and Palestine.1,2 Extensive research has shown that S. argel contains several chemicals and offers a range of bioactivities without causing poisoning.2 Numerous researchers have examined the phytochemicals in S. argel.3-6 It contains multiple components, and crystalline compounds have been examined in several parts of S. argel (leaves, stems, and flowers).7 Solenostemma argel methanolic extract used as a source of bioactive substances with subsequent health advantages, such as antioxidant, antiinflammatory, anticancer, and antibacterial activities.3 Staphylococcus aureus is a well-known bacterial pathogen that causes millions of serious infections (such as pneumonia, cardiovascular disease, and nosocomial bacteremia) worldwide.8 Staphylococcus aureus is resistant to most antibiotics, even when used in conjunction with other medications. Excessive use of antibiotics to treat microbiological infections, such as Staphylococcus aureus may result in antibiotic resistance. To minimize the possible repercussions of antibiotic resistance, focusing on the extracts of halophytic wild plants as an alternative method to combat pathogenic microorganisms has become critical. Furthermore, information regarding the antibacterial properties of S. argel against the potentially harmful S. aureus is lacking. The current study used high-performance liquid chromatography(HPLC) and gas chromatography-mass spectroscopy (GC-MS) to analyze the aqueous ethanolic extract of S. argel (AEESA) against S. aureus and to identify the polyphenols and volatile components present.
Plant material
Solenostemma argel was collected from Al-Ahsa, Saudi Arabia in March 2021. In order to prepare the fresh plant aerial parts for use in the experiments, they were cleaned, dried in an oven at 50°C until constant weight, and then ground to a fine powder.
Preparation of the Solenostemma argel extract
Using a Soxhlet apparatus at 80°C, 800 g of the dried aerial parts of S. argel has been extracted with 70% aqueous ethanol. The extraction process was continued until the solution became completely clear. The alcoholic extract was filtered, dried using reduced-pressure evaporation, and weighed (12.6 g).9 For future studies, the dried extracts were stored at room temperature(25°C-30°C).
Disc diffusion assay
The S. aureus strain was obtained from King Faisal University College of Medicine. Staphylococcus aureus was cultivated in nutritional broth ( Cat. no. 7014), and the disc diffusion experiment was performed according to the method of Rad.10 The samples were grown at 37°C at 200 rpm until their turbidity at 600 nm reached 0.3. To inoculate 10 mL of nutritional broth, 100 µL of overnight bacterial culture were used. Five milliliters of each bacterial strain was then placed in separate nutrient agar plates, and each plate was gently mixed to ensure that the culture was dispersed evenly on the agar. Before applying the test solution discs to the agar, all the inoculation plates were kept uncovered in a biosafety cabinet to allow the residual liquid to sink into the agar. Four 6 mm diameter discs containing different test solutions were placed on the agar surface of each inoculation plate. Three of the discs contained AEESA test solutions extracted from aerial parts, whereas the positive control disc containing 10 µg of Meropenem was acquired from Condalab in Madrid, Spain. After each plate had been incubated at 37°C overnight, the diameters of the incubation zone were measured.
Calculating the minimum inhibitory concentration (MIC)
The minimum inhibitory concentration (MIC) of the extract against S. aureus was determined using the agar microdilution technique against S. aureus.11 The bacterial strain was cultured in Mueller-Hinton broth for 24 h at 30°C. (MHB, Oxoid, UK). The bacterial suspension was adjusted with a sterile saline solution until it reached 1×107 CFU/mL. After that, a 96-well microtiter plate was loaded with 50 µL MHB and 50 µL inoculum. A 5 mg/mL stock solution of plant extract in DMSO was prepared, and the wells were mixed with two-fold dilutions of the stock plant extract in MHB. Each well’s capacity was increased up to 150 µL by adding MHB. The microplates were incubated for 24 h at 30°C (160 rpm) in a rotating agitator. MHB with the inoculum was used as a positive control, whereas MHB with the plant extract served as a negative control for maximal growth. p-Iodonitrotetrazolium (MP Biomedicals, Salon) was used to determine the inhibitory concentration. After incubation, each microtiter well received 10 L (0.1 mg/mL)of p-iodonitrotetrazolium violet, then the bacterial growth was assessed by reincubating at 30°C for 1 h. The color shift from violet to orange-red indicated that the cells were alive. The inhibitory concentration was comparable to that required for metabolically active cell oxidation. A Bio-Rad ELISA reader was used to measure the absorbance of the 96-well microtiter plates at 630 nm. Testing was performed in triplicates. The concentration of the first well that exhibited no turbidity was used to calculate the MIC.12
Anti-biofilm activity
To determine the anti-biofilm activity of the plant extract, bacterial strains were inoculated into trypticase soy broth (TSB) supplemented with 0.1% glucose (w/v). After 18 h of incubation at 30°C, cells were collected via centrifugation at 10,000 rpm for 10 min. PBS was used to wash the cell pellets. Specifically, 100µL of bacterial suspensions in TSB (OD600 = 0.1) were added to 96-well plates and incubated at 37°C for 24 h under static conditions. After 1 h of incubation with the plant extracts at the specified concentrations, 0.1% crystal violet was added to each well to stain the biofilms for 10 min. After adding 30% (v/v) acetic acid to each well to dissolve the crystal violet, the optical density of each sample was measured at 540 nm using a microplate reader (Rayto, Germany).13,14 The tests were performed in triplicates.
Assay for DNA fragmentation
Staphylococcus aureus cells were injected into a 12-well plate. Following overnight incubation, the medium was replenished and IC50 doses of the test sample extracts were added. After 24 h of incubation, cells were centrifuged, washed with PBS, and counted. The cells (5 × 107) were then treated for 2 h at 37°C with lysis buffer (10 mM Tris (pH 8), 10 mM EDTA, and 0.5% Triton X-100). RNase A (20µg /mL) was applied to genomic DNA for 1 h at 37°C, followed by proteinase K (50µg/mL) for 2 h at 50°C. DNA was extracted using phenol, chloroform, and isoamyl alcohol (25:24:1), washed with 70% ethanol, air-dried, soaked in distilled water, and separated on a 1.5% agarose gel with 10 mg/mL EtBr. The DNA fragmentation pattern was observed using a UV transilluminator and photographed using gel documentation equipment.
HPLC analysis of phenolic compounds in AEESA
High-performance liquid chromatography (HPLC) was performed using the Agilent 1260 series. Separation was carried out using a 4.6× 250 mm i.d., 5 m, Eclipse C18 column. The mobile phase had a flow rate of 0.9 mL/min and was composed of water (A) and 0.05% trifluoroacetic acid in acetonitrile (B). The linear gradient was sequentially programmed into the mobile phase as follows: 12–15 min (82% A), 15–16 min (82% A), and 16–20 min (82% A), all of which were within acceptable ranges. A multi-wavelength detector was operated at 280 nm. For every one of the sample solutions, 5 L of injection volume was used. The column was maintained at a constant temperature of 40°C.
GC/MS analysis of volatile compounds in AEESA
The volatile compounds in the AEESA were analyzed, and the dried aqueous ethanolic extract (5 g) was dissolved in 30 mL distilled water (with warming) and further fractionated via extraction with 60 mL diethyl ether. The diethyl ether layer was concentrated on a water bath at 60°C to obtain diethyl ether fraction of aqueous ethanolic extract. The diethyl ether fraction was then analyzed using GCMS-QP2010 SE equipment (SHIMADZU, Japan) and a direct capillary column Rtx-5MS (30 m × 0.25 mm × 0.25 m film thickness). The column oven temperature was originally maintained at 60°C for 2 min before being raised by 10°C/min to 300°C for 4 min. The injector temperature was maintained at 300°C. Helium was used as a carrier gas at a constant flow rate of 2 mL/min. The extract sample(1 µl) was automatically injected using an autosampler AOC-20S attached to the GC in split mode. Full scan mode m/z 20–500 EI mass spectra were collected at 70 eV ionization voltage. Temperatures of 200 and 300°C were selected as the ion source and transfer lines, respectively. The components were identified by comparing their retention durations and mass spectra with those in the NIST 05 mass spectral database.15
The AEESA inhibits the growth of S. aureus
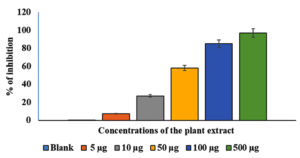
The effects of various AEESA concentrations on S. aureus proliferation are presented in Figure 1. The percentage of surviving microbes decreased as the concentration of the plant extract increased. At 5 µg/mL of the plant extract, robust growth was observed in comparison to the untreated control, whereas at 500 µg/mL, a substantial suppression (78%) was observed. With an MIC of 31.6µg/mL, plant extract exhibited significant antibacterial activity against S. aureus. (Figure 1).
Figure 1. Effects of various AEESA concentrations (µg/mL) on S. aureus growth. The AEESA concentrations used were 5 µg/mL, 10 µg/mL, 50 µg/mL, 100 µg/mL, and 500µg/mL
The inhibitory effects of the AEESA against S. aureus
The effects of various AEESA concentrations on the growth of S. aureus are shown in Figure 1. The survival percentage of the bacterial strains decreased as the plant extract concentration increased. In comparison to the untreated control, good growth was detected at 5 µg/mL of the plant extract, whereas a substantial suppression (78%) was observed at 500 µg/mL. Plant extract exhibited substantial antibacterial activity against S. aureus, with the MIC being 31.6 µg/mL (Figure 1).
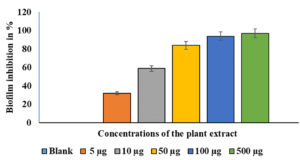
A crystal violet test was used to measure biofilm production.14 The effects of different EESB concentrations (5 µg/mL, 10 µg/mL, 50 µg/mL, 100 µg/mL, and 500 µg/mL) were evaluated, and the results are presented in Figure 2. As depicted in Figure 2, the treated cells exhibited substantially less biofilm formation than the control cells. At higher concentrations of AEESA, the proportion of biofilm formed was proportionally lower in the treated cells compared with the control cells. The amount of biofilm formation following treatment with AEESA at 10 µg/mL, 50 µg/mL, 100 µg/mL, and 500 µg/mL was significantly reduced by 50%, 80%, 92%, and 94%, respectively, when compared to the control. AEESA exhibited a comparable antibacterial effect against the existing S. aureus-generated biofilms (24 h old). Biofilm formation of 100% in the control corresponds to an OD530 of 0.48 0.05 in the absence of AEESA (0 µg/mL) and uninhibited biofilm formation by the bacterial strain. Significant biofilm inactivation was observed at 31.6 µg/mL, which was generally consistent with the MIC value.
Figure 2. Effects of various AEESA concentrations (µg/mL) on the biofilm formation (percent) of S. aureus. The following AEESA concentrations were utilised: 5 µg/mL, 10 µg/mL, 50 µg/mL, 100 µg/mL, and 500 µg/mL
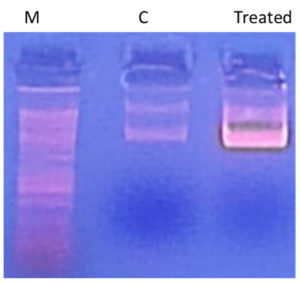
Using the standard “DNA ladder” technique, DNA is extracted from apoptotic cells and separated on an agarose gel to study DNA fragmentation. As shown in Figure 3, treatment of S. aureus genomic DNA with AEESA at the MIC (31.6 µg/mL) resulted in the fragmentation of chromosomal DNA into small internucleosomal fragments compared to untreated DNA.
Phenolic compounds in AEESA using HPLC
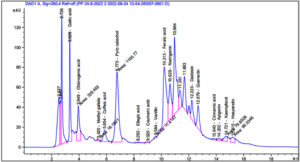
According to the findings in Figure 4 and Table 1, the phenolic components in the 70% aqueous ethanol extract of S. argel had notable concentrations of phenolic compounds, with pyrocatechol (9519.95 µg/g), ferulic acid (3221.41 µg/g), chlorogenic acid (3221.41 µg/g), and gallic acid (2730.85 µg/g), being the most abundant. Naringenin (2262.80µg/g) and quercetin (1750.25µg/g) were the major flavonoids observed.
Table (1):
Phenolic compounds in AEESA using HPLC
Retention time (min.) |
Compounds |
Conc. (µg/g) |
Compounds nature |
|---|---|---|---|
3.326 |
Gallic acid |
2730.85 |
Phenolic acid |
3.949 |
Chlorogenic acid |
2934.62 |
|
4.522 |
Catechin |
0.00 |
Flavonol |
5.420 |
Methyl gallate |
70.03 |
Phenolic acid |
5.854 |
Coffeic acid |
803.38 |
|
6.479 |
Syringic acid |
0.00 |
|
6.773 |
Pyrocatechol |
9519.95 |
Phenolic compound |
7.795 |
Rutin |
0.00 |
Flavonoid glycoside |
8.292 |
Ellagic acid |
208.52 |
Phenolic acid |
9.020 |
Coumaric acid |
7.10 |
phenolic derivative of cinnamic acid |
9.584 |
Vanillin |
23.25 |
phenolic aldehyde |
10.213 |
Ferulic acid |
3221.41 |
Phenolic acid |
10.629 |
Naringenin |
2262.80 |
Flavanone |
12.223 |
Daidzein |
654.07 |
Hydroxyisoflavone |
12.679 |
Quercetin |
1750.25 |
Flavonol |
13.843 |
Cinnamic acid |
25.01 |
Phenolic acid |
14.202 |
Apigenin |
139.33 |
Flavone |
14.721 |
Kaempferol |
399.74 |
Flavonol |
15.215 |
Hesperetin |
305.38 |
Flavanone |
Identification of volatile compounds in AEESA using the GC-MS analysis
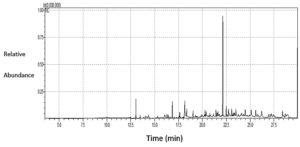
The results of the GC-MS analysis of S. argel extract are presented in Figure 5 and Table 2. The data revealed a high percentage of 9,12-octadecadienoic acid (z,z)- methyl ester (33.44%), ascorbic acid 2,6-dihexadecanoate (7.22%), and butylated hydroxytoluene (5.46%), followed by 2-Pentadecanone, 6,10,14-trimethyl (4.77%), 2-pentadecanone, 6,10,14-trimethyl (3.35%),stigmasta-5,22-dien-3-ol, and acetate, (3.Beta; 3.35%).
Table (2):
GC-MS spectral analysis of AEESA
Peak No |
RT |
Area % |
Compounds |
|---|---|---|---|
1. |
13.037 |
5.46 |
Butylated Hydroxytoluene |
2. |
13.483 |
1.01 |
2(4H)-Benzofuranone, 5,6,7,7a-tetrahydro-4,4,7a-trimethyl- |
3. |
14.344 |
0.73 |
18-Methyl-nonadecanol, trimethylsilyl ether |
4. |
16.277 |
1.81 |
5-Caranol, trans,trans-(+)- |
5. |
16.849 |
4.77 |
2-Pentadecanone, 6,10,14-trimethyl- |
6. |
17.632 |
2.11 |
Cyclodecasiloxane, eicosamethyl- |
7. |
18.158 |
7.22 |
Ascorbic acid 2,6-dihexadecanoate |
8. |
18.361 |
2.02 |
Docosanoic acid, ethyl ester |
9. |
19.019 |
2.16 |
1,3-dioxolane,4-methyl-2-pentadecyl |
10. |
19.257 |
0.67 |
9,9-Dimethoxybicyclo[3.3.1]nona-2,4-dione |
11. |
20.264 |
2.18 |
Cyclononasiloxane, octadecamethyl- |
12. |
20.387 |
1.75 |
N-(2-Methylbutyl)undeca-(2E,4E)-diene-8,10-diynamide |
13. |
20.777 |
1.04 |
2-Heptanone, 6-methyl-, O-methyloxime |
14. |
21.41 |
2.2 |
Phenol, 2,2′-methylenebis[6-(1,1-dimethylethyl)-4-methyl- |
15. |
21.519 |
1.28 |
E-11-Hexadecenal |
16. |
21.596 |
0.81 |
2H-Pyran-2-one, tetrahydro-6-tridecyl- |
17. |
21.631 |
1.48 |
Eicosanoic acid, 2,3-bis(acetyloxy)propyl ester |
18. |
22.134 |
33.44 |
9,12-octadecadienoic acid (z,z)-, methyl ester |
19. |
22.49 |
3.58 |
linalool |
20. |
22.733 |
2.76 |
Hexanoic acid, octadecyl ester |
21. |
22.788 |
1.01 |
Estra-1,3,5(10)-trien-17.beta.-ol |
22. |
23.057 |
2.1 |
di(2- ethylhexyl) phthalate |
23. |
23.233 |
1.04 |
1,4-Piperazinediethanol, .alpha.,.alpha.’-bis(phenoxymethyl)- |
24. |
23.33 |
0.72 |
cis-13-Eicosenoic acid |
25. |
23.503 |
2.08 |
Hexadecanoic acid, methyl ester |
26. |
24.479 |
2.64 |
Nonanoic acid, 9-oxo-, methyl ester |
27. |
25.1 |
3.35 |
14-Methyl-pentadecane-1,2-diol, isopropylidene derivative |
28. |
25.585 |
3.33 |
10-octadecenoic acid, methyl ester |
29. |
26.189 |
1.9 |
Lupeol |
30. |
28.384 |
3.35 |
Stigmasta-5,22-dien-3-ol, acetate, (3.beta.)- |
In this study, multiple investigations indicated that the aerial parts of S. argel ethanolic extract are rich in bioactive compounds, and the major phenolic compounds(pyrocatechol, ferulic acid, chlorogenic acid, and gallic acid) and flavonoids (naringenin and quercetin) were identified. These results were in line with the findings of Soliman et al.,3 which revealed that gallic acid was the most abundant phenolic acid, followed by syringic, p-coumaric, caffeic, and ferulic acids.3 Catechin, quercetin, luteolin, and rutin were detected in the methanolic extract. Staphylococci are common constituents of the human skin microbiome and are among the most common bacteria responsible for serious infections worldwide.8 Overuse of antibiotics may result in the spread of antibiotic resistance. Alternatively, natural compounds derived from wild heterotrophic plants may be used to fight pathogenic bacteria without negatively affecting health or the environment. AEESA was tested against S. aureus using disc diffusion, MIC determination, biofilm development, and DNA fragmentation tests. HPLC and GC-MS were used to identify the main components of AEESA. The diameter of the zone of inhibition and MIC are inversely related. When bacteria become increasingly sensitive to antimicrobial agents, their MIC decreases and the zone of inhibition increases. In contrast, as the bacteria become more resistant to antibiotics, the MIC increases, and the zone of inhibition decreases.
The MIC of AEESB against S. aureus was 31.6 µg/mL. This MIC value was less than those for S. villosa (6.25 mg/mL) and S. persia (1.56 mg/mL).16 Oueslati17 reported similar findings, revealing that an ethyl acetate extract from the roots of S. imbricata demonstrated possible antibacterial action against S. aureus, as shown by a relatively low MIC value (16 µg/mL).17 However, it has recently been reported that the ethanolic extract of S. argel displayed no inhibitory effects against several bacterial strains, including S. aureus.18 The differences in MIC values may be related to various variables, including the plant species used, plant portion extracted, extraction procedures, type of solvent used, and the bacterial strain. As a result, the chemical makeup, including the active components, changed, as did the possible mechanisms of action against microbial cells. Furthermore, flavonoids have been proposed to exhibit antibacterial activities that are mediated by the inhibition of nucleic acid synthesis, cytoplasmic membrane function, energy metabolism, attachment and biofilm formation, porin inhibition on the cell membrane, and membrane alteration.19,20
Some chemicals in S. argel extract, such as pyrocatechol, may have antibacterial activity against S. aureus. A possible explanation for this is the toxic effects of the reactive oxygen species (ROS) generated during catechol oxidation. ROS are highly reactive radicals that freely attack vital molecules within cells, including DNA and RNA, and cause undesired redox reactions.21 Similar results have been recently reported for Uncaria Gambir leaf extract and ester derivatives of catechol against peri-implantitis-related bacteria.22 In addition, 9,12-octadecadienoic acid (z,z)- methyl ester interacts with the cell membrane, destroying its integrity because of its lipophilic nature. Bacterial cell-selective permeability, efflux pumping systems, ATPase machinery activity, and RNA synthesis are compromised, resulting in bacterial mortality.21 All of these processes contribute to the high antibacterial activity of the chemicals. The methanolic extracts of aerial parts of S. argel have been found to be high in 2-(5-methyl-5 vinyl tetrahydro-2-furanyl)-2-propanol (11.63%), hexanoic acid methyl ester (10.93%), 3-dioxolane,4-methyl-2-pentadecyl (9.69%), and phenol, 2-(1,1-dimethylethyl) (8.50%).18 The variability in the GC-Mass profile could be attributed to multiple factors, including differences in the chemical properties of the solvent used, part of the plant, and the growing phase of the plant. It was recently reported that the ethyl acetate extract of S. argel possesses antifungal, antioxidant, and wound-healing properties.23
Bacterial biofilms are formed when cell aggregates attach to surfaces or interfaces and are embedded in a self-produced extracellular matrix composed of proteins and polysaccharides.24 Biofilm production is critical for bacterial survival, particularly in challenging environments containing antimicrobial substances.24 According to our findings, AEESA displayed an antibiofilm-forming activity comparable to its antibacterial activity. Antibiotic resistance in bacteria is associated with their propensity to form biofilms rather than their presence as free bacteria. Antimicrobial agents cannot reach bacterial cells in the presence of biofilms, because biofilms render them ineffective.24 Biofilm development is a well-documented multi-step process that includes adhesion, maturation/proliferation, and separation. Nonspecific antibiotic resistance is also involved in biofilm-associated S. aureus infection.8 The use of AEESA to reduce biofilm development may minimize antibiotic resistance.
DNA fragmentation is a fundamental mechanism of microbial mortality. The release of the intercellular component DNA suggests a ruptured membrane. After 72 h of combined treatment with bacterial cells, our findings showed distinct smear production in contrast to the untreated cells. Previous studies have shown that herbal components exert their antibacterial effects through DNA fragmentation. Treatment with Caesalpinia coriaria glycosides and flavonoids, for example, causes the apparent synthesis of damaged DNA in pathogenic bacteria such as S. aureus, Escherichia coli, and Klebsiella pneumoniae. The antibacterial activity of Syzygium cumini ethanolic leaf extract has also been demonstrated through an underlying method of action involving DNA breakage. DNA fragmentation activity was used to determine the antibacterial activity of S. cumini methanolic seed extract.25 Certain carbohydrates such as chitosan have been shown to be efficient against S. aureus through DNA fragmentation.26 Genomic DNA fragmentation is fatal and irreversible. Endonucleases cleave linker DNA selectively into mono- and oligonucleosomal DNA pieces. Our results indicate that AEESA contains major phenolic compounds (pyrocatechol, ferulic acid, chlorogenic acid, and gallic acid) and flavonoids (naringenin and quercetin), as reported by Elsanhoty et al.,18 who found that gallic acid, synergic acid, and p-coumaric acid are the primary phenolic acids, and catechin, quercetin, luteolin, and kaempferol are the common flavonoids present in the methanolic extract of S. argel. A GC-MS study also revealed that the high percentages of volatile components in AEESA were 9,12-octadecadienoic acid (z,z), methyl ester, ascorbic acid 2,6-dihexadecanoate (7.22%), and butylated hydroxytoluene, as reported by Elsanhoty et al.18
AEESA shows promising antibacterial activity against S. aureus in disc diffusion, biofilm formation, and DNA fragmentation tests. The MIC was 31.6 g/mL. The ability of AEESA to inhibit S. aureus. The polyphenolic and volatile compounds in AEESB act as antibacterial agents. The use of AEESA to inhibit biofilm formation would thereby reduce antibiotic resistance and pave the way for the future deployment of AEESA components for potentially useful applications. The use of AEESA to inhibit biofilm formation would prevent antibiotic resistance and promote the future applications of AEESA components in therapeutics.
ACKNOWLEDGMENTS
The author is thankful to the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia, for funding support [Grant No 4,608].
FUNDING
This work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia [Grant No 4,608].
DATA AVAILABILITY
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
ETHICS STATEMENT
Not applicable.
- Shayoub M, Haj E, Makawy A, Rasha R, Mona A. Adverse reaction of Solenostemmaargel leaves, extraction and alkaloids tablets administered to patients. Global J Trad Med Sys. 2013;2(1):14-18
- Teia FKF. A review of Solennostemmaargel: Phytochemical, pharmacological activities and agricultural applications. J Ayurvedic Herb Med. 2018;4(2):99-101.
Crossref - Soliman MS, Abdella A, Khidr YA, Osman HG, Aladadh MA, Elsanhoty RM. Pharmacological Activities and Characterization of Phenolic and Fla-vonoid Compounds in Solenostemma argel Extract. Research Square. 2022.
Crossref - Sulieman AME, Elzobair WM, Abdelrahim AM. Antimicrobial ac-tivity of the extract of Solenostemmaargel (harjal) plant. J Sci Technol. 2009;10(3):120-134.
- Roos SA, Medgalla SE, Dishay DW, Awad AH. Studies for determining anti-biotic substances in some Egyptian plants: Screening for antimicrobial activities. Fitoterapia. 1980;5:303-308.
- Kamel MS, Ohtani K, Hasanain HA, Mohamed MH, Kasai R, Yamasaki K. Monoterpene and pregnaneglucosides from Solenostemma argel. Phytochemistry. 2000;53(8):937-940.
Crossref - Idris TI, Ibrahim AM, Mahdi EM, Taha AK. Influence of argel (Solenostemma argel Del. Hayne) soil applications on flowering and yield of date palm (Phoenix dactylifera L.). Agric Biol J North Am. 2011;2(3):538-542.
Crossref - Kranjec C, Angeles DM, Marli TM, et al. Staphylococcal Biofilms: Challenges and Novel Therapeutic Perspec-tives. Antibiotics. 2021;10(2):131.
Crossref - Lala PK. Lab manuals of Pharmacognosy. CSI Publishers and Distributors, Calcutta, 1993;5:38-48.
- Rad ZM, Nourafcan, H, Mohebalipour, N, Assadi, A, Jamshidi, S. Effect Of Salicyllc Acid Foliar Application On Phyto-chemical Composition, Antioxidant And Antimicrobial Activity Of Silybum Marianum. Iraqi J Agric Sci. 2021;52(1):63-69.
Crossref - Tsukatani T, Suenaga H, Shiga M, et al. Comparison of the WST-8 colorimetric method and the CLSI broth microdilution method for susceptibility testing against drug-resistant bacteria. J Microbiol Methods. 2012;90(3):160-166.
Crossref - Mousavi SM, Wilson G, Raftos D, Mirzargar SS, Omidbaigi R. Antibacterial activities of a new combination of essential oils against marine bacteria. Aquac Int. 2011;19(1):205-214.
Crossref - Stepanovic S, Vukovic D, Hola V, et al. Quantification of biofilm in micro-titer plates: Overview of testing condi-tions and practical recommendations for assessment of biofilm production by staph-ylococci. APMIS. 2007;115(8):891-899.
Crossref - Shukla SK, Rao TS. An improved crystal violet assay for biofilm quantification in 96-well microtitre plate. Biorxiv. 2017;10:100214.
Crossref - Suleiman RK, Iali W, El Ali B, Umoren SA. New Constituents from the Leaves of Date Palm (Phoenix dactylifera L.) of Saudi Origin. Molecules. 26(14):4192.
Crossref - Amin E, Abdel-Bakky MS, Mohammed HA, Chigrupati S, Qureshi KA, Hassan MH. Phytochemical Analysis and Evaluation of the Antioxidant and Antimicrobial Activities of Five Halophytes from Qassim Flora. Pol J Environ Stud. 2022;31(4):3005-3012.
Crossref - Oueslati MH, Bouajila J, Jannet HB. Two New Bioactive Biphenylpropanoids from the Roots of Salsolaimbricata (Chenopodiaceae) Growing in Saudi Arabia. Orient J Chem. 2017;33(4):1871-1878.
Crossref - Elsanhoty RM, Soliman MS, Khidr YA, et al. Pharmacological Activities and Characterization of Phenolic and Flavonoid Compounds in Solenostemma argel Extract. Molecules. 2022;27(23):8118.
Crossref - Gupta D, Guliani E. Flavonoids: Molecular mechanism behind natural chemoprotective behavior-a mini review. Biointerface Res Appl Chem. 2022;12(5):5983-5995.
Crossref - Thebti A, Meddeb A, Ben Salem I, et al. Antimicrobial Activities and Mode of Flavonoid Actions. Antibiotics. 2023;12(2):225.
Crossref - Razaviamri S, Wang K, Liu B, Lee BP. Catechol-based antimicrobial polymers. Molecules. 2021;26(3):559.
Crossref - Chinnasamy V, Gupta R, Sharma PK. Antibacterial Response of UncariaGambir Leaves Extract and Ester Derivative of Catechol Against Periimplantitis Re-lated Bacteria. J Sur Fish Sci. 2023;10(1S):4280-4298.
Crossref - Abdel-Motaal FF, Maher ZM, Ibrahim SF, El-Mleeh A, Behery M, Metwally AA. Comparative studies on the antioxidant, antifungal, and wound healing activities of Solenostemma arghel ethyl acetate and methanolic extracts. Appl Sci. 2022;12(9):4121.
Crossref - Yin W, Wang Y, Liu L, He J. Biofilms: The microbial “protective clothing” in ex-treme environments. Int J Mol Sci. 2019;20(14):3423.
Crossref - Yadav AK, Saraswat S, Sirohi P, et al. Antimicrobial action of methanolic seed extracts of Syzygium cumini Linn. on Bacillus subtilis. AMB Expr. 2017;7(1):196.
Crossref - Yadav AK, Sirohi P, Saraswat S, et al. Inhibitory Mechanism on Combination of Phytic Acid with Methanolic Seed Extract of Syzygium cumini and Sodium Chloride over Bacillus subtilis. Curr Microbiol. 2018;75(7):849-856.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.