Monkeypox virus is an orthopoxvirus sharing the common genus with variola and vaccinia virus. Most of the monkeypox (MPX) cases had been reported from the central and west African region (the main endemic areas) prior to 2022 but there was a sudden outbreak in May, 2022 disseminating the infections to thousands of people even in non-endemic countries, posing a global public health emergency. MPX was considered a rae and neglected disease, however the 2022 MPX outbreaks in multiple countries attracted attention of worldwide researchers to pace up for carrying out researches on various aspects of MPXV including attempts to design and develop diagnostics, vaccines, drugs and therapeutics counteract MPX. Apart from being a zoonotic disease, the current outbreaks highlighted rapid human-to-human transmission of MPXV, besides the reverse zoonosis has also been documented with recent first report of human-to-dog transmission, urging a call for the importance of one health approach. Atypical and unusual disease manifestations as well asymptomatic MPXV infections have also been observed during 2022 MPX outbreak. The affected patients typically develop a rash resulting in a mild disease followed by recovery with some supportive care and use of antivirals such as tecovirimat, cidofovir and brincidofovir in severe disease cases. Modified vaccinia Ankara (MVA) vaccine with an excellent safety profile has been recommended to patients with higher risk exposure and immunocompromised individuals. Moreover, another vaccine the replication-competent vaccine (ACAM2000) could be a suitable alternative to MVA’s non-availability to some selective immunocompetent individuals. Current review highlights the salient aspects of management and treatment of monkeypox along with underlying promises in terms of therapeutics and a variety of challenges posed due to current global public health emergency situation to counteract MPX.
Monkeypox, Epidemiology, Disease, Therapeutics, Drugs, Vaccines, Clinical Management
The monkeypox virus (MPXV) is a double-stranded, deoxyribonucleic acid (DNA) orthopox virus of the Poxviridae family, that causes Monkeypox (MPX), and has been known to have a close association with smallpox. MPXV firstly was identified among crab-eating macaques, during a laboratory outbreak of vesiculopustular skin eruptions in Denmark in 1958.1 Further MPXV disease outbreak occurred in the Rotterdam Zoo in the year 1964, followed by in the year 1970 some more MPVX cases were identified in Central and Western Africa, among the children with mild fever, vesiculopustular rashes and lymphadenopathy symptoms. Subsequently, the cases of the virus constantly increased from hundreds to thousands across Congo Basin. The isolation of virus from animals was done firstly in the year 1985 from African squirrel in the Equatorial region of the Democratic Republic of the Congo followed by another case where MPXV was isolated in year 2012 from rainforests of the Ivory Coast; from dead baby primate of a monkey species which is known as “mangabey”.2,3
Later in the year 2003, the cases of MPXV have been reported in Midwest US, where the disease outbreak was linked to pet prairie dogs. These dogs were imported from African rodents’ resultant of cross-infection.4 Prior to year 2022, the MPXV disease was typically identified only to a few of the areas (Central/Western Africa) around the globe, however, in May 2022, the United Kingdom (UK) reported several MPXV disease infections to humans even without any clear history of travel or any animal links to these already reported areas.5 In the US alone, more than 5,000 cases were reported in June 2022 itself.6 Additional cases of the disease were reported thereafter in over multiple non-endemic countries in subsequent months due to rapid human-to-human transmission across many counties in the world. Presently as of December 16, 2022, around 82,809 confirmed disease cases (human infections) had been reported from 110 countries and locations, with 65 deaths.7,8
In West and Central African countries, MPX has replaced smallpox as the leading cause of human Orthopoxvirus infection since the 1980s. As such, it is crucial to have a firm grasp of the primary risk factors that contribute to the propagation of current outbreaks.9 Risk factors for the spread of the virus between humans include close physical contact, such as sleeping in the same bed or room, or sharing eating and drinking utensils. The risk of contracting zoonotic MPX increases when you spend time in the woods, whether you camp there or call it home. Interesting to note is that aiding a patient with hygiene and laundry has not been associated to an increased risk of spreading MPX.10,11 According to the World Health Organization (WHO), coming into physical contact with an infected individual is the most significant risk factor for contracting MPX. The danger of infection for those caring for the sick and their loved ones consequently increases. The World Health Organization (WHO) has acknowledged that this virus can be spread by unprotected contact with the flesh, blood, or other organs of ill or deceased animals. Risk factors for infection with MPXV include a lack of smallpox vaccination.12,13 It is possible that males are more at risk of catching the disease than females because they engage with wild animals more frequently in regions where the disease is endemic. MPXV is related to smallpox, although not in the same way. The smallpox vaccine has been proven to provide cross-protection against the MXPV at a rate of approximately 85%.14 According to recent statistics, MPX has become more common since the smallpox vaccination program was discontinued. The decline in immunity and the growing deforestation of endemic areas are two other possible explanations for the MPX spike. The potential resurgence of MPX zoonosis has been linked to the evolution of the virus. 15
The transmission of virus occurs from individual to individual by close contact with lesions, body fluids, respiratory droplets etc. Oral and nasopharyngeal fluid exchanges are also the cause of spread of virus which rapidly replicates at the inoculation site with spreads to the lymph nodes. Endemic MPX virus infection is usually self-limited, with the clade-dependent case fatality rates ranging from 1-10%.16 In general, the start of the disease takes place with fever, headache, body aches (muscle, backache) followed by fatigue.17,18 Phylogenetically, MPXV has two clades; one is the West African clade and the other one is the Congo Basin clade, which is also known as the central African clade.19 In Phylogenetic analyses, it has been reported that MPXV has circulated to some other areas where the virus has remained endemic.20,21
The present article presents an overview on monkeypox virus (MPXV) and the clinical disease (MPX), with a special focus on advances in vaccines and vaccination, drugs and therapeutics being made for management of patients for counteracting MPX outbreak.
Epidemiology of monkeypox and underlying etiological factors
MPXV infection is one of the most significant orthopoxvirus infections reported ever since the smallpox infection was eliminated about four-decades back. First report of the virus isolation was reported way back in the year 1958 in monkeys having pox-like disease in them while in humans the monkeypox disease was discovered in the year 1970.11 The story dates back to the year 1968 in the Democratic Republic of Congo (DRC) which became completely free of smallpox, even then a boy around 9 years of age was diagnosed with the monkeypox disease. Since then, the cases of human monkeypox spread to more than 11 African nations showing an upward trend especially in the central and western regions of Africa along with rain forest areas of the Congo Basin in DRC.22 United States of America was the first country outside Africa to report the first MPX outbreak with over 70 cases reported in the year 2003 which supposedly came through contacts with pet prairie dogs suffering from the disease as these pets were kept with dormice and pouched rats that were imported from Gambia. The MPX disease recently has been reported in travelers from African nations to Israel and the UK (September 2018; December 2019; May 2021; May 2022), Singapore (May 2019), and the United States of America (July 2021 and November 2021) (World Health Organization, 2022.23
MPX was considered as a neglected disease prior to the current year, however its larger outbreaks have been reported most recently in May 2022. The first incidence of the current outbreak was discovered in a patient from London on May 6, 2022, who had recently travelled to Nigeria (where the disease is endemic). The UK Health Security Agency (UKHSA) later identified four new cases on May 16 that had no connection to travel to a nation where MPC is an endemic disease. Cases began to be recorded from an expanding number of nations and areas starting on May 18, primarily from Europe, then in North and South America, Asia, Africa, and Oceania.24 Brazil announced the first death outside of endemic Africa on July 29, 2022, involving a 41-year-old man with underlying comorbidities (F, 2022). On the same day, a 44-year-old man in Alicante became the first person to pass away in Spain. His cause of death was encephalitis brought on by infection with MPX. On July 30, 2022, Spain reported the death of a 31-year-old male in Córdoba from MPX-related infection. This man also had encephalitis.25 A 22-year-old man who passed away from MPX in Thrissur, Kerala, on July 30 was India’s first verified case on August 1, 2022.26 The crucial aspect of the current MPX 2022 epidemic is that multiple countries are still reporting cases of MPX while cases have been recorded significantly from nations where the illness was not endemic and thus has expanded significantly outside endemic regions of Central and West Africa. For the first time, several MPX cases and clusters have been reported simultaneously in endemic and non-endemic nations across extremely dissimilar geographic regions. Tedros Adhanom Ghebreyesus, the director-general of the World Health Organization (WHO), recognized the urgency of the situation and declared the outbreak, a public health emergency of international concern on July 23, 2022. (PHEIC).
The major signs include a rash that looks like a pimple or blister and flu-like symptoms like fever, tiredness, and enlarged lymph nodes. People can exhibit only a rash and no flu-like symptoms, or the opposite may occur. In the current outbreak, cases frequently appear with genital or anus lesions, occasionally with penile enlargement or rectal pain, or proctitis.27 Lesions can also develop on hands, feet, cheeks, and other body regions. Within three weeks of infection, symptoms typically start to show. Eventually, the lesions scab up and peel off; this typically takes from two to four weeks. Multi-origins and complex virus transmission routes along with atypical and unusual disease manifestation as well as asymptomatic MPXV infections have also been reported during the current 2022 MPX outbreaks, hence greater measures for checking its transmission and adequate prevention and control strategies are suggested.28,29
MPX is a zoonotic pathogen,30 and more recently the first report of human-to-dog transmission of MPXV31 has highlighted risk of reverse zoonosis of the virus, which calls for increasing the screening of different animal species including pets, adopting advanced global surveillance system, and strengthening of one health approach for effectively countering MPX transmission and circulation.32-35 Various wild animals, including monkeys and rodents (such as squirrels, rats, and dormice), can spread the virus to people by bites or scratches; through activities like hunting, trapping, skinning, cooking, or ingesting infected animals; or through contact with infected bodily fluids36 Human-to-human transmission, which mostly occurs through direct skin-to-skin contact with infectious sores, is less common than animal-to-human transmission but has occasionally resulted in small-scale, confined human outbreaks.12 Direct contact with infected skin lesions, mucocutaneous lesions, and respiratory droplets is required for human-to-human transmission (and possibly short-range aerosols requiring prolonged close contact). Transmission through fomite can also happen (for example, through infected clothing or linens).37
Although the virus can spread during sexual activity, MPX is not regarded as a sexually transmitted infection because it can also be contracted without having sex.38 It is also unknown if the MPXV may spread through semen, vaginal fluids, urine, or faeces, despite the fact that the virus has been found in semen.39 When a woman is pregnant, transmission to the fetus may happen via the placenta.40 It is uncertain if people without skin lesions can spread the virus or how frequently the virus is disseminated by respiratory secretions. Casual contact does not spread the infection.41
MPXV is a double-stranded DNA virus, though in the past it was thought to be RNA-based given to the presence of MPXV RNA showing significant amounts of human beta-2-microglobulin (β2M) RNA. In some of studies, it has been reported that the presence/detection of β2M from either wastewater or from excretions of certain organisms showed the presence of nucleic acids within human cells.42 Upon the exposure of MPXV-infected human cells into wastewater, revealed the presence of MPXV RNA. In a study, the extraction was performed using a specialized type of polymerases [Volcano 2nd Generation (V2G)] that is capable of amplifying both DNA and RNA templates.43,44 In another study, the MPXV RNA detection was carried out from wastewater treatment plants. The obtained samples were amplified using qPCR, and the sequencing studies were performed. The sequences thus obtained were used in performing alignment studies, which revealed that the query sequence obtained had similarity to MPXV genome.45,46 The natural reservoir of the monkeypox virus is still unknown, though some of the rodents have been acknowledged to be naturally susceptible to the disease (MPXV).33
Symptomatic trial and clinical management
The incubation period of the virus is usually 6 to 13 days long but in some of the cases it has been observed from 5 to 21 days. The disease is contagious from 1-2 days prior to the rash until when all of the scabs have fallen off or become pus-filled. The virus transmission in humans occurs primarily through direct contacts such as respiratory droplets, body secretions or lesions or through contaminated clothing or linens of an infected person.11 In animals, the transmission occurs by bite or scratch of infected animals. 47
Initially, the cases observed for the disease investigation were as follows: a single person of any age group with a travel history to the affected countries within the last 21 days presenting with unexplained acute rashes and one or more of the symptoms including mild fever with headache, body ache with weakness, and swollen lymph nodes, etc.48,49 Any individual who meets the case description for a suspected case, suffering from a clinically compatible condition, and has an epidemiological relationship.50,51 The confirmation of the disease among humans have been verified by the detection of viral DNA sequence either by sequencing or by running polymerase chain reaction (PCR).52-54
The disease symptoms of MPXV lasts for about 2 to 4 weeks, the children are more likely to suffer from severe instances. The case fatality rates (CFR) of the MPXV disease have been known to fluctuate traditionally (from 0% to 11%) in the general population, with small children are likely to be more vulnerable. Recently, the CFR has been at 3-6%. General symptoms (fever, body ache, lymphadenopathy, sore throat & coughing etc.) last for about 5 days. In other series of symptoms, skin rashes start at day one and lasts for about 2-4 weeks.55,56 The skin rashes occur in different stages; initially in the stage-1, tongue and mouth lesions appear followed by centrifugal distribution within 24 hours.57 Specificity of rashes in the body includes as; 98% facial, 95% on palms and soles, 70% on oral mucous membranes, while 28% on genitalia and 20% on conjunctiva.50,58 By the end of 3rdday the lesions get grown/raised and fluid filled and last for about 1 to 2 days, which then turn into the pustule on 6th or 7th day of the infection. The lesions dry up by the end of the second week, the scabs last a week before dropping off.59,60
Supportive treatment regimens for clinical management of MPX cases require rehydration with orally or intravenously administered fluids, hemodynamic balance, oxygen support, symptomatic case management and to tackle superinfections of bacterial skin lesions and eye complications. 11
Anti-viral Drugs and Therapeutic interventions
Presently there is no as such approved treatment for the infections associated with MPXV. The therapeutic interventions in the present scenario from the Strategic National Stockpile (SNS) as treatment option for MPX disease includes some of the approved products; tecovirimat, brincidofovir, and Cidofovir which are available commercially for the treatment of the disease (Table 1).61-64
Table (1):
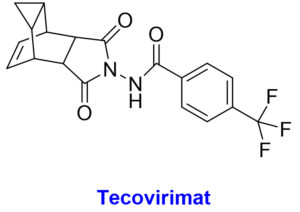
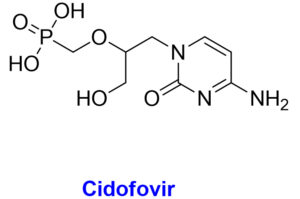
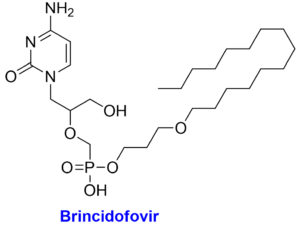
The chemical structures with structural features and possible mechanism of action of antiviral drugs (Tecovirimat, Brincidofovir, Cidofovir) against the monkeypox virus.
Chemical Structure |
Structural features |
Mechanism of Action |
|---|---|---|
|
|
|
Acyclic monophosphate nucleotide analog of deoxycytidine
|
|
|
Acyclic nucleotide analogue and lipid conjugate pro-drug of the cidofovir
|
|
The Food and Drug Administration (FDA) approved tecovirimat (TPOXX/ ST-246), a 4-trifluoromethylphenol derivative, against orthopoxviruses, has been recommended to be useful in treating MPX cases.11,65 Animal studies have indicated that this medication is effective against multiple orthopoxviruses, including MPX. Compassionate use of tecovirimat for vaccinia and cowpox was being conducted, and no concerning adverse effects were being observed.66 Tecovirimat inhibits the growth of the MPX virus envelope by inhibiting the production of the viral protein p37, which is highly conserved throughout orthopoxviruses. Those interested can have it in the form of capsules to be taken orally twice daily for a total of 14 days. On May 19, 2022, the US Food and Drug Administration greenlighted a solution for intravenous use.67,68 An extended access program for tecovirimat is currently being created for the Central African Republic, which is experiencing widespread MPX epidemics.
Brincidofovir is an antiviral medication for treating poxviruses that has been licensed by the European Medicines Agency and the FDA.69 Prairie dogs with chronic MPX infection exhibited a moderate survival benefit from this medication. In 2018, the first individuals in the United Kingdom were diagnosed with MPX, however neither brincidofovir nor tecovirimat were approved for use in treating the disease.70 In 2018, however, Brincidofovir was selected for the patients since it was readily accessible through an approved, urgent repurposing of an existing supply from a local clinical trial. A rise in hepatic transaminases, diarrhoea, nausea, vomiting, and stomach discomfort have all been recorded as adverse reactions to this medication.8 Pregnant women are not encouraged to take brincidofovir due to the risk of harm to the developing embryo or fetus. While cidofovir is also active in vitro for MPX, it is not a viable first-line treatment due to its high nephrotoxicity profile and electrolytic abnormalities. Another study, however, claims that cidofovir is more effective than the smallpox vaccine in treating fatal MPXV infections.71 For the treatment of cytomegalovirus, the Food and Drug Administration has just approved cidofovir. Cidofovir and brincidofovir both work by inhibiting viral DNA polymerase. To counter this, tecovirimat blocks a specific viral product to prevent intracellular viruses from escaping the cell.39,66 Vaccinia immune globulin intravenous (VIGIV) has also been found useful in treating severe cases of MPX as well as prospective passive antibody-based therapies such as hyperimmune globulin (HIG), monoclonal antibodies (mAbs), and convalescent plasma (CP) have also been suggested to play useful role for treating MPX, particularly beneficial against severe cases.72
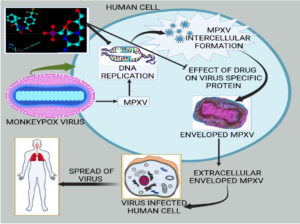
The replicative cycle of MPOX virus in humans and the therapeutic interventions by the anti-MPX viral drugs have been elucidated in Figure. Tecovirimat, which is an orthopoxvirus-specific protein (VP37) inhibitor harbors the property of inhibiting the systemic spread of the virus to other cells in humans. Tecovirimat and cidofovir in combination has been shown as DNA replication inhibitors which could show synergistic effects as well.
Figure. The replicative cycle of MPXV followed by the MPOX virus and putative mechanism of action of various drugs applicable at different levels. Whereas the drug Tecovirimat is an orthopoxvirus-specific protein (VP37) inhibitor that has a property of inhibiting the virus’s systemic spread to other cells in the humans. Tecovirimat and cidofovir in combination work as DNA replication inhibitors which may provide synergistic effects
Vaccines and Vaccination
JYNNEOS and ACAM2000 vaccines, the smallpox vaccines, are considered to be effective to treat MPX cases, with booster doses being more effective, which are presently being used as pre-exposure prophylaxis.73 Vaccinia-virus-based vaccines including current MVA-BN and ACAM2000 vaccines can elicit highly cross-reactive immunity against newly arrived MPXV.74 The use of Vaccinia-based vaccines and anti-vaccinia immunoglobulin for post-exposure prophylaxis and management against MPX has also been highlighted.75 Modified Vaccinia Ankara-Bavarian Nordic (MVA-BN) is a live non-replicating third-generation vaccine, and is approved for both smallpox and MPX. The same vaccine in USA is marketed as JYNNEOSTM. This vaccination reveals about 85% effectiveness in protecting people suffering with MPX. However, in its overall effectiveness and safety against pregnant women is still in doubt. For the use of vaccination, the Disease Control and Prevention Center recommends vaccination for the MPX virus within four days of exposure. It also has been observed that if anybody getting vaccine administered between 4 and 14 days of exposure, the effect of the dose may lessen to symptoms of the disease but will not prevent the disease.76-78 The smallpox vaccines are used for the treatment of MPXV, as these have been shown to be protective against the disease. These are genetically recombinant engineered products, in which some agents are encoded to produce antigens to the pathogens. These are made up of a live viral or bacterial vector. Even engineered vaccines are there in order to express a variety of exogenous antigens in the target cell cytoplasm. Few of the important vaccines, antiviral drugs and therapeutic agents used against monkeypox virus are presented in Table 2.
Table (2):
Various vaccines and antiviral therapeutic agents used against the monkeypox virus.
Name of Vaccine/ Antiviral agents used for MPXV |
General Features |
|---|---|
JYNNEOS Vaccine |
The vaccine has been approved for smallpox and monkeypox |
Dryvax |
It is a live vaccinia virus (VACV), which protects against smallpox and monkeypox |
4pox |
This is comprised of L1R, A27L, A33r and B5R genes of VACA providing protective efficacy in rhesus macaques upon lethal monkeypox virus challenge |
ACAM2000 |
By pricking of the skin surface this vaccine is administered into the skin as a live vaccinia virus preparation |
IMVAMUNE |
An attenuated strain of the vaccinia virus. |
Tecovirimat |
This drug was shown to inhibit the functions of a major protein of MPXV. |
Brincidofovir |
This drug was shown to act as an inhibitor of viral DNA polymerase. |
Cidofovir |
This compound has the capability to block the viral replication. The blockage is performed by the selective inhibition of viral DNA polymerases. |
JYNNEOS vaccine so far looks like a safer option with about 85% efficacy against MPX, still we are not very clear about the stability, efficacy and durability of the vaccine.79 Two-dose vaccination strategy and its ability to induce the immune response as well the long-lasting effects on immunity, still needs further evaluation in order to further decide the future course of action especially regarding the frequency of the booster doses. Although ACAM2000 has shown promise in trials but concrete data is required for JYNNEOS. Another challenge is about the efficacy and safety of the above vaccines for the lactating and pregnant women especially for the infants whether they are passively immunized.79,80 Immunization undoubtedly is going to hold the key as far as to minimize the MPX cases in humans and preferences to be given to healthcare professionals at high risk, immunodeficient individuals and young ones with impaired immune system. Of note, ring vaccination approach has been suggested to counter the ongoing MPX outbreak for limiting the virus transmission and spread, and to be useful during mass gathering events.28,30, 81-83
In the current scenario, the data on the effectiveness of newer MPX vaccines in the prevention of MPX is still under investigation and specific vaccines for MPX are being attempted to be developed such as novel and next-generation vaccine, multi-epitope vaccine using pan-genome and reverse vaccinology, multi-epitope based MPXV-specific vaccine exploring immunoinformatics, and nucleic acid-based universal MPX vaccine candidates. 84-89
The need to reinforce measures targeted at preparing for an outbreak is urgent in light of the current knowledge gaps, the shifting epidemiology and clinical presentation of MPX, and other challenges. Increase in public health and disease monitoring infrastructures are urgently needed in all countries to combat the growing threat posed by emerging and re-emerging infectious diseases like MPX. For public health to be prepared and for priority research to be conducted, community-led, locally coordinated, interdisciplinary initiatives focused on capacity building and education are required. Those who work in healthcare’s front lines and with the world’s most vulnerable populations must be safeguarded in order to stop the spread of the current MPX outbreak. To effectively manage new or reemerging viral threats, it will be essential to implement proactive, continuous, comprehensive surveillance, quick risk assessments, response measures, early detection, and contact tracing. Increased attention to national healthcare systems and the creation of international laws, regulations, and response mechanisms are urgently needed in the wake of the COVID-19 pandemic. Due to poor health care infrastructure and scarce resources, MPX may go unreported in impoverished countries. Concerns regarding the potential for human-to-human transmission of the disease are shared by members of the general public and healthcare professionals alike. Given the potential for a global pandemic, MPX illness is an issue of paramount public health importance that must not be dismissed. Understanding the dynamic epidemiology of this reemerging disease requires the detection and surveillance of MPX cases, which requires international cooperation.
To better treat and control this viral illness, more in-depth research into the disease and international collaboration are required. However, antiviral drugs, immunotherapeutics, and immunizations against MPX are not widely available at this time, thus there is a pressing need to get these treatments into the hands of countries reporting outbreaks. Although the prognosis for MPX varies depending on a number of factors such as immunization history, general health at the time of infection, and the presence of any preexisting medical disorders, in most cases, patients with MPXV suffer mild or self-limiting disease even without therapy.
In addition, there is a dearth of information from clinical trials on currently available MPX vaccines and antivirals. Current vaccinia vaccines, such as JYNNEOS, and antivirals, such as tecovirimat and brincidofovir, need more data from clinical studies to be fully effective. Though the emergency approval of the JYNNEOS for vaccination against MPXV is a major headway to contain MPX infection but still concrete research is desired as far as the overall efficacy of the vaccine is concerned along with assessing the side effects, risks and contraindications. Moreover, the duration of immunity following the 2-dose JYNNEOS vaccination series needs more investigation. It would also be helpful to get clinical data on the use of both the JYNNEOS and mRNA COVID-19 vaccines in tandem. Closer monitoring, vigilance of the situation along with enhanced surveillance, innovative treatment and control measures are the need of the hour so that effective and safer new generation anti-MPXV therapeutic agents could be developed. Specific reservoirs of the orthopox viruses need to be identified in order to be aware of the different properties of the virus and to know the high-risk behaviors for acquiring viral infections. It is vital to promote awareness among potentially impacted groups, healthcare professionals, and laboratory personnel in order to discover and stop future cases and successfully manage the present outbreak. Vaccines, medicines, and therapies that are both effective and specific against MPXV are urgently needed to aid in the control of the disease and in the clinical management of MPX patients. In brief, health systems need to raise public awareness, actively monitor for disease, diagnose patients quickly, and promptly report data in order to take any public intervention measures against MPXV’s potential to cause an epidemic.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript..
ETHICS STATEMENT
Not applicable.
- Parker S, Buller RM. A review of experimental and natural infections of animals with monkeypox virus between 1958 and 2012. Future Virology. 2013;8(2):129-157.
Crossref - Vaughan A, Aarons E, Astbury J, et al. Human-to-human transmission of monkeypox virus, United Kingdom, October 2018. Emerging Infectious Diseases. 2020;26(4):782-785.
Crossref - Altindis M, Puca E, Shapo L. Diagnosis of monkeypox virus – An overview. Travel Medicine and Infectious Disease.2022;50:102459.
Crossref - Pastula DM, Tyler KL. An overview of monkeypox virus and its neuroinvasive potential. Annals of Neurology. 2022;92(4):527-531.
Crossref - WHO. Monkeypox outbreak. July 29. https://www.who.int/emergencies/situations/monkeypox-oubreak-2022. Accessed July 30, 2022. 2022;
- Minhaj FS, Ogale YP, Whitehill F, et al. Monkeypox outbreak—Nine states, May 2022: American Journal of Transplantation. 10, 2022/71 (23); 764–769.
Crossref - Nuzzo JB, Borio LL, Gostin LO. The WHO declaration of monkeypox as a global public health emergency. JAMA. 2022;328(7):615-617.
Crossref - Desai AN, Thompson GR, Neumeister SM, Arutyunova AM, Trigg K, Cohen SH. Compassionate use of tecovirimat for the treatment of monkeypox infection. JAMA. 2022;328(13):1348-1350.
Crossref - Mohapatra RK, Tuli HS, Sarangi AK, et al. Unexpected sudden rise of human monkeypox cases in multiple non-endemic countries amid COVID-19 pandemic and salient counteracting strategies: Another potential global threat? International Journal of Surgery (London, England). 2022;103:106705.
Crossref - Mauldin MR, McCollum AM, Nakazawa YJ, et al. Exportation of monkeypox virus from the African continent. The Journal of Infectious Diseases. 2022;225(8):1367-1376.
Crossref - Chandran D, Dhama K, Chakraborty S, Mohapatra R, Yatoo M. Monkeypox: An update on current knowledge and research advances. J Exp Biol Agric Sci. 2022;10:679-688. https://jebas.org/ojs/index.php/jebas.
- Cheema AY, Ogedegbe OJ, Munir M, Alugba G, Ojo TK. Monkeypox: a review of clinical features, diagnosis, and treatment. Cureus. 2022;14(7).
Crossref - Cohen J. Monkeypox outbreak questions intensify as cases soar. Science (New York, NY). 2022;376(6596):902-903. https://www.science.org/doi/10.1126/science.add1583
- Shanmugaraj B, Khorattanakulchai N, Phoolcharoen W. Emergence of monkeypox: Another concern amidst COVID-19 crisis. Asian Pacific Journal of Tropical Medicine. 2022;15(5):193.
Crossref - Saied AA, Metwally AA, Choudhary OP. Monkeypox: an extra burden on global health. International Journal of Surgery (London, England). 2022;104:106745.
Crossref - Bunge EM, Hoet B, Chen L, et al. The changing epidemiology of human monkeypox—A potential threat? A systematic review. PLoS Neglected Tropical Diseases. 2022;16(2):e0010141.
Crossref - Kumar N, Acharya A, Gendelman HE, Byrareddy SN. The 2022 outbreak and the pathobiology of the monkeypox virus. Journal of Autoimmunity. 2022;131:102855.
Crossref - Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. The Journal of Infectious Diseases. 2017;216(7):824-828.
Crossref - Seang S, Burrel S, Todesco E, et al. Evidence of human-to-dog transmission of monkeypox virus. The Lancet. 2022;400(10353):658-659.
Crossref - Thornhill JP, Barkati S, Walmsley S, et al. Monkeypox virus infection in humans across 16 countries—April–June 2022. New England Journal of Medicine. 2022;387(8):679-691.
Crossref - Sharma A, Sharma A, Sharma V, et al. Ab-initio density functional and docking studies of α-Santalol molecule derived from Santalum album: endorsing its reactive and inhibitory potential against Monkeypoxgp158 protein. Journal of Xidian University. 2022;16:158-167.
Crossref - Beer EM, Rao VB. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Neglected Tropical Diseases. 2019;13(10):e0007791.
Crossref - WHO. Multi-country monkeypox outbreak in non-
endemic countries 2022. Accessed date 21 May 2022. Available from:
https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON385 - Monkeypox-United Kingdom of Great Britain and Northern Ireland. URL: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON383 2022.
- Fonseca P (2022) Brazil reports first monkeypox death outside Africa in current outbreak. https://www.reuters.com/world/americas/brazil-confirms-its-first-monkeypox-related-death-2022-07-29/.
- Sah R, Abdelaal A, Asija A, et al. Monkeypox virus containment: the application of ring vaccination and possible challenges. Journal of Travel Medicine. 2022;29(6):taac085.
Crossref - Bragazzi NL, Kong JD, Mahroum N, et al. Epidemiological trends and clinical features of the ongoing monkeypox epidemic: A preliminary pooled data analysis and literature review. Journal of Medical Virology. 2022;95(1): e27931.
Crossref - Farahat RA, Khan SH, Dergaa I, Rabaan AA, Dhama K, Memish ZA. Asymptomatic Transmission of Monkeypox: Implications for Mass Gatherings? New Microbes and New Infections. 2022, 49:101056.
Crossref - Reda A, El-Qushayri AE, Shah J. Asymptomatic monkeypox infection: a call for greater control of infection and transmission. The Lancet Microbe. 2022;
Crossref - Beig M, Mohammadi M, Monfared FN, Nasereslami S. Monkeypox: An emerging zoonotic pathogen. World Journal of Virology. 2022;11(6):426-434.
Crossref - Sykes JE. A call for more evidence documenting human-to-dog transmission of monkeypox virus. Lancet. 2022;400(10357):993.
Crossref - Sah R, Mohanty A, Siddiq A, et al. Monkeypox reported in India–South East Asia region: health and economic challenges. The Lancet Regional Health-Southeast Asia.2022;4;100063.
Crossref - Mohapatra RK, Mishra S, Kandi V, et al. Monkeypox plays a similar role like SARS-CoV-2; intensive animal screening is crucial after the first human-to-dog transmission report – Correspondence. International journal of surgery (London, England), 2022; 106: 106925.
Crossref - Afrooghe A, Damavandi AR, Ahmadi E. Reverse zoonosis and monkeypox: Time for a more advanced global surveillance system for emerging pathogens. New Microbes and New Infections. 2022;48:101013.
Crossref - Shepherd W, Beard PM, Brookes SM, et al. The risk of reverse zoonotic transmission to pet animals during the current global monkeypox outbreak, United Kingdom, June to mid-September 2022. Eurosurveillance. 2022;27(39):2200758.
Crossref - Song T-Z, Zheng Y-T. Monkeypox, wild animals, and potential big problem. Zoological Research. 2022;43(4):612-614.
Crossref - Guarner J, Del Rio C, Malani PN. Monkeypox in 2022—what clinicians need to know. JAMA. 2022;328(2):139-140.
Crossref - Kozlov M. Pig organs partially revived in dead animals — researchers are stunned. Nature. 2022;608: 247-248.
Crossref - Jaiswal V, Nain P, Mukherjee D, et al. Symptomatology, prognosis, and clinical findings of Monkeypox infected patients during COVID-19 era: A systematic-review. Immunity, Inflammation and Disease. 2022;10(11):e722.
Crossref - Arotolu TE, Afe AE, Wang H, et al. Spatial modeling and ecological suitability of monkeypox disease in Southern Nigeria. Plos One. 2022;17(9):e0274325.
Crossref - Lapa D, Carletti F, Mazzotta V, et al. Monkeypox virus isolation from a semen sample collected in the early phase of infection in a patient with prolonged seminal viral shedding. The Lancet Infectious Diseases. 2022;22(9):1267-1269.
Crossref - Faye O, Pratt CB, Faye M, et al. Genomic characterisation of human monkeypox virus in Nigeria. The Lancet Infectious Diseases. 2018;18(3):246.
Crossref - Sharkey ME, Kumar N, Mantero AMA, et al. Lessons learned from SARS-CoV-2 measurements in wastewater. The Science of the Total Environment. 2021;798:149177.
Crossref - Isidro J, Borges V, Pinto M, et al. Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of monkeypox virus. Nature Medicine. 2022;28(8):1569-1572.
Crossref - Sharkey ME, Babler KM, Amirali A, et al. First detection of the Monkeypox virus using wastewater-based surveillance in Miami-Dade County. 2022; https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4236277
- Sharma V, Sharma AK, Yadav M, et al. Prediction models based on miRNA-disease relationship: Diagnostic relevance to multiple diseases including COVID-19. Current Pharmaceutical Biotechnology. 2022.
Crossref - Ježek Z, Szczeniowski M, Paluku K, Mutombo M. Human monkeypox: clinical features of 282 patients. Journal of Infectious Diseases. 1987;156(2):293-298.
Crossref - Minhaj FS, Ogale YP, Whitehill F, et al. Monkeypox outbreak—nine states, May 2022. Morbidity and Mortality Weekly Report. 2022;71(23):764.
Crossref - Mali TR, Mairal P. Monkeypox: An Overview. International Journal of Research Publication and Reviews, 3,11, 2304-2310. https://ijrpr.com/uploads/V3ISSUE11/IJRPR8096.pdf.
- Luo Q, Han J. Preparedness for a monkeypox outbreak. Infectious Medicine. 2022; 1(2): 124-134.
Crossref - Chan C. What pharmacists need to know about monkeypox. Pharmacy Today. 2022;28(10):20-21.
Crossref - Kulesh DA, Loveless BM, Norwood D, et al. Monkeypox virus detection in rodents using real-time 3′-minor groove binder TaqMan® assays on the Roche LightCycler. Laboratory Investigation. 2004;84(9):1200-1208.
Crossref - Vandenbogaert M, Kwasiborski A, Gonofio E, et al. Nanopore sequencing of a monkeypox virus strain isolated from a pustular lesion in the Central African Republic. Scientific Reports. 2022;12(1):1-13.
Crossref - World Health Organization. (2022). Laboratory testing for the monkeypox virus: interim guidance. 2022. https://www.who.int/publications/i/item/WHO-MPX-laboratory-2022.1.
- Iqbal SP, Jaffri SA. Monkeypox: A Global Challenge. Liaquat National Journal of Primary Care. 2022; 4(2): 134-140.
Crossref - Bothra A, Maheswari A, Singh M, Pawar M, Jodhani K. Cutaneous manifestations of viral outbreaks. Australasian Journal of Dermatology. 2021;62(1):27-36.
Crossref - Patel A, Bilinska J, Tam JC, et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ. 2022;378:e072410.
Crossref - Singhal T, Kabra S, Lodha R. Monkeypox: a review. Springer; 2022. 89, 955–960.
Crossref - Lahariya C, Thakur A, Dudeja N. Monkeypox disease outbreak (2022): epidemiology, challenges, and the way forward. Indian Pediatrics.2022;59(8):636-642.
Crossref - Long B, Koyfman A, Gottlieb M, et al. Monkeypox: A focused narrative review for emergency medicine clinicians. The American Journal of Emergency Medicine. 2022;61: 34-43.
Crossref - Chakraborty S, Mohapatra RK, Chandran D, et al. Monkeypox vaccines and vaccination strategies: Current knowledge and advances. An update-Correspondence. International Journal of Surgery (London, England). 2022;105:106869-106869.
Crossref - Ortiz-Saavedra B, León-Figueroa DA, Montes-Madariaga ES, et al. Antiviral Treatment against Monkeypox: A Scoping Review. Tropical Medicine and Infectious Disease. 2022;7(11):369.
Crossref - Diaz JH. The disease ecology, epidemiology, clinical manifestations, management, prevention, and control of increasing human infections with animal orthopoxviruses. Wilderness & Environmental Medicine. 2021;32(4):528-536.
Crossref - Rodríguez-Cuadrado F, Pinto-Pulido E, Fernández-Parrado M. FR-Potenciales tratamientos en viruela símica (monkeypox). Actas Dermo-Sifiliográficas. 2022; AD-310.
Crossref - Merchlinsky M, Albright A, Olson V, et al. The development and approval of tecoviromat (TPOXX®), the first antiviral against smallpox. Antiviral Research. 2019;168:168-174.
Crossref - Sherwat A, Brooks JT, Birnkrant D, Kim P. Tecovirimat and the treatment of monkeypox—past, present, and future considerations. New England Journal of Medicine. 2022;387(7):579-581.
Crossref - Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for Preexposure Vaccination of Persons at Risk for Occupational Exposure to Orthopoxviruses: Recommendations of the Advisory Committee on Immunization Practices—United States, 2022. Morbidity and Mortality Weekly Report. 2022;71(22):734.
Crossref - Huang Y, Mu L, Wang W. Monkeypox: epidemiology, pathogenesis, treatment and prevention. Signal Transduction and Targeted Therapy. 2022;7(1):1-22.
Crossref - Islam M, Sangkham S, Tiwari A, et al. Association between Global Monkeypox Cases and Meteorological Factors. International Journal of Environmental Research and Public Health. 2022;19(23):15638.
Crossref - Saxena SK, Ansari S, Maurya VK, et al. Re-emerging human monkeypox: a major public-health debacle. Journal of Medical Virology. 95(1); e27902.
Crossref - Gong Q, Wang C, Chuai X, Chiu S. Monkeypox virus: a re-emergent threat to humans. Virologica Sinica. 2022;37(4): 477-482.
Crossref - Wei D-W, Wong N-K, Song Y, et al. IS 26 Veers Genomic Plasticity and Genetic Rearrangement toward Carbapenem Hyperresistance under Sublethal Antibiotics. Antimicrobial Chemotherapy. 2022;13(1):e03340-21.
Crossref - Sah R, Humayun M, Baig E, et al. FDA’s authorized “JYNNEOS” vaccine for counteracting monkeypox global public health emergency; an update–Correspondence. International Journal of Surgery (London, England). 2022;107:106971.
Crossref - Ahmed SF, Sohail MS, Quadeer AA, McKay MR. Vaccinia-Virus-Based Vaccines Are Expected to Elicit Highly Cross-Reactive Immunity to the 2022 Monkeypox Virus. Viruses. 2022;14(9):1960.
Crossref - Webb E, Rigby I, Michelen M, et al. Availability, scope and quality of monkeypox clinical management guidelines globally: a systematic review. BMJ Glob Health. 2022;7(8):e009838.
Crossref - Carvalho LB, Casadio LVB, Polly M, et al. Monkeypox Virus Transmission to Healthcare Worker through Needlestick Injury, Brazil. Emerg Infect Dis. 2022;28(11):2334-2336.
Crossref - Rizk JG, Lippi G, Henry BM, Forthal DN, Rizk Y. Prevention and treatment of monkeypox. Drugs. 2022:1-7.
Crossref - Hammarlund E, Lewis MW, Carter SV, et al. Multiple diagnostic techniques identify previously vaccinated individuals with protective immunity against monkeypox. Nature Medicine. 2005;11(9):1005-1011.
Crossref - Reda A, Sah R, Rodríguez-Morales AJ. More Evidence about Monkeypox Sexual Transmission in the Current 2022 Multi-Country Outbreak. Reply to Vera et al. Comment on “Sah et al. Monkeypox and Its Possible Sexual Transmission: Where Are We Now with Its Evidence? Pathogens 2022, 11, 924”. Pathogens. 2022;11(12):1418.
Crossref - Ajmera KM, Goyal L, Pandit T, Pandit R. Monkeypox–An emerging pandemic. IDCases. 2022;29:e01587.
Crossref - Farahat RA, Essar MY, Memish ZA. Monkeypox and ring vaccination during the FIFA world cup 2022 in Qatar: a call for action. Journal of Travel Medicine. 2022; taac103.
Crossref - Yuan P, Tan Y, Yang L, et al. Modelling vaccination and control strategies of outbreaks of monkeypox at gatherings. Frontiers in Public Health. 2022:4485.
Crossref - Sah R, Hada V, Mohanty A, et al. Recent first report of human-to-dog transmission of Monkeypox virus emphasizes an urgent need of enhancing surveillance and strengthen further explorative research to reveal its real magnitude of reverse zoonosis from other animals including pets as like that happened with SARS-CoV-2/COVID-19 pandemic–Correspondence. International Journal of Surgery (London, England). 2022; 106, 106949.
Crossref - Bhattacharya M, Dhama K, Chakraborty C. A call for a novel and next-generation vaccine against monkeypox disease. Annals of Medicine and Surgery (2012). 2022;84:104968-104968.
Crossref - Papukashvili D, Rcheulishvili N, Liu C, Wang X, He Y, Wang PG. Strategy of developing nucleic acid-based universal monkeypox vaccine candidates. Frontiers in Immunology. 2022;13.
Crossref - Hayat C, Shahab M, Khan SA, et al. Design of a novel multiple epitope-based vaccine: an immunoinformatics approach to combat monkeypox. Journal of Biomolecular Structure and Dynamics. 2022:1-12.
Crossref - Swetha RG, Basu S, Ramaiah S, Anbarasu A. Multi-Epitope Vaccine for Monkeypox Using Pan-Genome and Reverse Vaccinology Approaches. Viruses. 2022;14(11):2504.
Crossref - Ullah A, Shahid FA, Haq MU, et al. An integrative reverse vaccinology, immunoinformatic, docking and simulation approaches towards designing of multi-epitopes based vaccine against monkeypox virus. Journal of Biomolecular Structure and Dynamics. 2022:1-14.
Crossref - Zaib S, Rana N, Khan I, et al. Designing multi-epitope monkeypox virus-specific vaccine using immunoinformatics approach. Journal of infection and Public Health. 2022; 16(1): 107-116.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.