A new strain of the old pandemic, Monkeypox (MPX), has emerged with a more complicated clinical appearance. It is a source of relief that the fatality rate in the new monkeypox is lower, but communicability is higher. This infection’s diagnosis and therapy are still challenging and unknown. Researchers are reporting increased human-to-human transmission in the modified version of MPX. There have been several reports of the updated version of monkeypox in the European and American areas. Brazil, Colombia, France, Spain, Germany, Peru, the United Kingdom, and the United States of America have recorded over three thousand new cases of monkeypox through October 2022. Few antiviral medicines and vaccines are available on the market, making treatment of this condition difficult. MPX was previously declared an epidemic disease, but ignorance about it can bring devastation in the shape of the next pandemic-like COVID-19. This review aims to assess the virology, transmission, diagnosis, and therapy of MPX.
Monkeypox, International, New, Complication, COVID-19
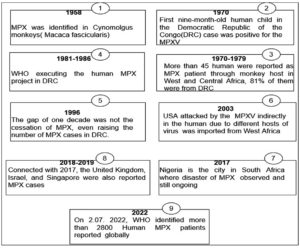
Since 1970, human monkeypox (MPX) has been recognized as an endemic illness in West Africa (WA) and Central Africa (CA).1 By the second of June 2022, the World Health Organization (WHO) has discovered over 2800 confirmed cases all around the world, which is cause for alarm.2 It was the most widespread illness in North America and Oceania. Meanwhile, the region had zero mortality rates at the time, although death happened on a regular basis in the epidemic region. Following the heinous tragedy of Corona Virus Disease (COVID-19), it is July 23rd, 2022, when Dr. Tedros Adhanom Ghebreyesus, Director-General of WHO, designates MPX as a worldwide public health emergency of international significance.3
MPX, COVID-19, Salmonellosis, Smallpox, Leptospirosis, Tuberculosis, Melioidosis, Cowpox, Rabies, Ebola, Lassa fever, Marburg hemorrhagic fever, and Giardiasis are examples of developing zoonoses (the ability of animals to transmit disease to humans).4-6 Monkeypox virus (MPXV) is one of the causal agents of the zoonosis uncommon illness MPX, commonly known as “monkey smallpox,” and was discovered in smallpox-free locations for the first time in 1958.7-9 It is a complex double-stranded DNA virus of the genus Orthopoxvirus (OPXV), the Oxviridae family, and the Chordopoxvirinae subfamily. There is no link between the disease’s name and the agent that causes it. It has been known as monkeypox since 1958 when it was isolated in monkeys in a Danish laboratory for the first time.10,11
Clinical signs such as the onset, length, frequency, and intensity of skin rashes, as well as their distribution, mirror smallpox; however, it is often less severe in terms of fatality and scarification or fissure of skin than smallpox.12 The United Nations Sustainable Development Goal 3 (indicator 3.3.5) calls for the cessation of outbreaks of Neglected Tropical Diseases and the provision of interventions to sufferers by 2030.13
Still, the exact natural host of this virus is unknown; nonetheless, a variety of rodents and non-human primates have been recorded. It was isolated from a Ropy squirrel in the Democratic Republic of the Congo (DRC), a sooty mangabey in Cote d’Ivoire, Gambian pouched rats, and dormice. The MPXV was discovered in cynomolgus macaques (old-world monkeys) imported from Singapore in 1958 by Staten’s Serum Institute in Copenhagen, Denmark. MPXV is found in a variety of genetic alterations.14-16
- Congo Basin is known as Clade I. t is caused through both human and non-human reservoirs, as well as worldwide spread. In humans and monkeys, it is more dangerous and contagious. The mortality rate is claimed to be more than 10%.17,18
- Nowadays, WA is known as Clade IIa & Clade IIb. Clade I and Clade IIa were reported during the MPX calamity of WA and CA. Clade IIb was found in the MPX wave occurring from 2017-2019 from the United Kingdom (UK), Israel, Nigeria, the United State of America (USA), and Singapore through the human reservoir. Clade IIa exhibited a mild and very less death rate associated with less communicability. Clinical manifestation and mortality rate of Clade IIb are still topics of investigation in the present scenario, although it is less communicable, unlike Clade I MPX.19-21
- Clade III is a new clinical epidemiologic type of MPX caused by the WA variation.22,23
MPX Reappearance
Since 1970, during smallpox zoonosis monitoring, the first baby human kid in the DRC instance tested positive for MPXV and was identified as a human pathogen in WA and CA. Benin, Cameroon, the Central African Republic, the Democratic Republic of the Congo, Gabon, Cote d’Ivoire, Liberia, Nigeria, the Republic of the Congo, Sierra Leone, and South Sudan were also recognized as MXPV sufferers. From 1970 to 1978, just three MPX cases were discovered in Nigeria (WA).24,25
Since 2003, the United States has seen 47 positive cases of MPX from outside of Africa, all from pet prairie dogs. These canines were infected with MPXV at the pet distribution center by Gambian pouched rats (Cricetomys gambianus) from Ghana (WA). Aleksandar Radoni et al. discovered a dead wild sooty mangabey (Cercocebus atys) monkey in Cote d’Ivoire’s Tai National Park in 2012 and isolated the MPXV that resembled WA.26-28
After a 39-year absence, MPX resurfaced in South Africa in September 2017. (Nigeria). There have been 500 suspected cases and over 200 confirmed cases, with a case-fatality ratio of about 3%. Cases have been recorded up to the present day. From September 2018 to November 2021, numerous MPX patients were recorded in the United Kingdom, Israel, and Singapore, all of which are non-African nations, with most of them relating to Nigeria owing to trade links. It is still invading today. Figure 1 depicts the ongoing changes in the MPX outbreak around the world.29-31
Furthermore, the topic of what sort of settings are required to survive MPXV in nature remains a mystery, complicating the search for specific precautions and treatments for MPX to new hosts outside of endemic locations. Previous research indicates that the primary host of MPXV is mostly a few rodents, with primates such as monkeys becoming the secondary host via the primary host. Both types of hosts infected humans in distinct ways. It has only been isolated from wild animals twice before because they died because of severe pox-like lesions.33,34
Due to previous occurrences of undefined OPXV species, a field assessment of anti-OPXV seropositivity among wild animals was conducted. Rope squirrels (Funisciurus), dormice (Graphiurus), African giant pouched rats (Cricetomys), and sun squirrels (Heliosciurus) were discovered as highly seropositive for OPXV in Ghana and DRC; however other animals such as rufous-nosed rat, striped mouse, gerbil, and elepha shrew had a lower incidence. Anti-OPXV antibodies were detected in numerous non-human primates (NHP) in West and Central Africa.35-37
Ground squirrels in North American grasslands were especially vulnerable to MPXV, and the number of MPX patients was growing because of human repercussions for animals. All the evidence assumed that the MPXV virus is monitored by multiple host reservoirs. The mutation is another danger associated with the MPX vulnerability. MPXV is a DNA virus that is reproduced by viral DNA polymerase. The MPXV mutation rate ranged from 2×10-6 to 1×10-5 nucleotide replacement/site/year, implying that at least two nucleotides change each year. It has a modest mutation rate when compared to other RNA viruses. RNA virus substitution rates ranged from 10-2 to 10-5 nucleotide substitutions/site per year.38-41
MPXV is resistant to many chemicals and heat. It is very stable in diethyl ether, but it is inactivated in methane, sodium dodecyl sulfate, trichloromethane, methanol, and carbolic acid.42 MPX outbreaks on a global scale in 2022.
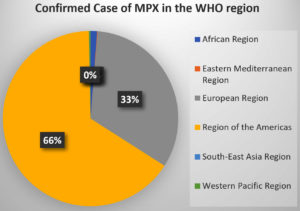
WHO designated the six MPX areas. During the third to fourth week of October 2022, MPX patients in Europe and America continued to decline after increasing dramatically in August 2022. Intervention for the MPXV is most needed in Africa, Southeast Asia, and the Eastern Mediterranean. Table 1 shows the total number of MPX cases from January to October 2022. The area of America accounts for 66% of confirmed cases, with Europe accounting for the second greatest share. The remainder of the WHO area has an extremely low percentage of MPX patients. Figure 2 depicts the percentage contribution of MPX cases from the WHO area.43-44
Table (1):
Data on MPX cases reported to WHO from January to October 2022.
WHO region |
Confirmed Case |
Death |
|---|---|---|
African Region |
934 |
14 |
Eastern Mediterranean Region |
72 |
1 |
European Region |
25303 |
4 |
Region of the Americas |
50716 |
16 |
South-East Asia Region |
30 |
1 |
Western Pacific Region |
209 |
0 |
The top eleven nations exhibiting the highest proportion of MPX cases till October 2022 are the United States, Brazil, Canada, Chile, Colombia, Mexico, and Peru in America, and the United Kingdom, Spain, France, and Germany in Europe (Table 2).45
Table (2):
Depicts the number of confirmed cases and deaths in the top thirty countries45.
S.No. |
Country |
Confirmed cases of MPX |
Confirmed death caused by MPX |
|---|---|---|---|
1. |
Argentina |
675 |
0 |
2. |
Australia |
140 |
0 |
3. |
Austria |
323 |
0 |
4. |
Belgium |
785 |
1 |
5. |
Bolivia |
241 |
0 |
6. |
Brazil |
9162 |
8 |
7. |
Canada |
1437 |
0 |
8. |
Chile |
1149 |
0 |
9. |
Colombia |
3298 |
0 |
10. |
DRC |
206 |
0 |
11. |
Denmark |
191 |
0 |
12. |
Ecuador |
243 |
0 |
13. |
France |
4094 |
0 |
14. |
Germany |
3662 |
0 |
15. |
Ghana |
105 |
4 |
16. |
Ireland |
206 |
0 |
17. |
Israel |
262 |
0 |
18. |
Italy |
894 |
0 |
19. |
Mexico |
2654 |
0 |
20. |
Netherlands |
1235 |
0 |
21. |
Nigeria |
583 |
7 |
22. |
Peru |
3048 |
0 |
23. |
Poland |
206 |
0 |
24. |
Portugal |
944 |
0 |
25. |
Puerto Rico |
199 |
0 |
26. |
Spain |
7317 |
2 |
27. |
Sweden |
212 |
0 |
28. |
Switzerland |
546 |
0 |
29. |
UK |
3698 |
0 |
30. |
USA |
28379 |
6 |
MPXV Virus Biology
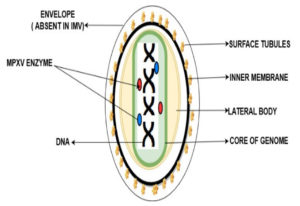
MPXV virions are brick-shaped particles with an average diameter of 280 nm 220 nm. It is composed of around 200 kilobase pairs with approximately. MPXV is an enveloped, double-stranded Virus that belongs to the genus Orthopoxviral of the Poxviridae family.46
The MPXV genome encompasses ~197,000 bp and includes hairpin termini as well as >190 non-overlapping open reading frames responsible for the replication or invade the infection, whereas the rest of the genes are called morons genes or non-open reading frames which are responsible for the virus-host interaction, immunomodulation, and pathogenesis, still there a lot of ORFs unaware regarding their role. The central coding area is enclosed by various layers. There are present dumbbell shape nucleoproteins in double-strand DNA genome mature poxvirus particles. MPXV has two mature forms of invasion. Extracellular enveloped virus (EEV) is assumed responsible for the early invasion, and the intracellular mature virus (IMV) ruptures during cell extrusion. EEV has an extra outermost membrane and more condensed viral protein in comparison to IMV. Figure 3 is the depiction of MPXV as reported earlier.47,48
Transmissibility and Pathogenesis of MPXV Across the Public Domain
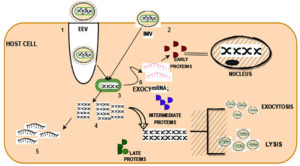
Even though it is a DNA virus, it grows in cells. Micropinocytosis and fusion processes allow viruses to enter the host cell. Then, both EEV and IMV genomes uncoated and released DNA material, which replicated and translated into early mRNA and late DNA. The release of early protein, intermediate protein, and late protein causes the cell nucleus to expand. Exocytosis and lysis mechanisms are used to release after maturation. Figure 4 depicts the invasion of MPXV among humans.49,50
Figure 4. Human transmission of MPXV 1) Fusion 2) Micropinocytosis 3) Unpacking of envelope in EEV 4) Late transcription 5) Late translation 6) Early transcription 7) Morphogenesis
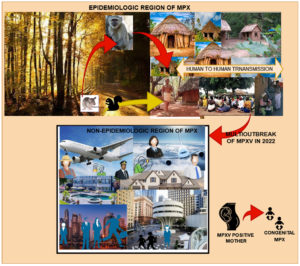
Although the actual natural host of the MPXV remains unknown, African rats have been blamed for the spread of Monkeypox. The virus enters the host through the surface layer of the infected animal and bodily excretion, as well as direct contact by scratch, bite, and food. Congenital monkeypox occurs when a fetus is infected through the placenta during pregnancy. Yet, no cases of sexual transmission have been documented. Figure 5. depicts the transmission of MPXV across humans and nonhumans.51-53
Clinical Symptoms of Former Epidemiologic MPX(EMPX) and New Epidemiologic MPX(NMPX)
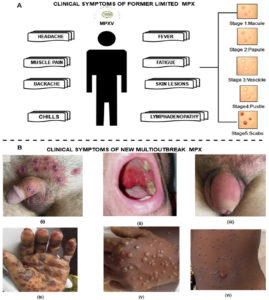
Clinical symptoms are resembling with smallpox except for lymphadenopathy or swollen lymph nodes. Initial signs are fever, fever, chills, headache, muscle aches(myalgia), backache, and fatigue leading the extreme exhaustion for EMPX. 1-2 weeks up to 3 weeks is the possible incubation period. After getting high-temperature, skin lesions appear in the whole body, which becomes severe overtime a period. Generally, skin lesions start from the oropharynx. The severity of this disease leads to deep abscesses or ulcers associated with secondary infections. The skin lesion is a severe symptom that evolved in distinct stages. Initially, red flat spots appeared which leads the hard swollen bumps in stage 2. Furthermore, these bumps are enclosed with transparent liquid which changes into pus in stage 4. In the end, the bumps dried & fall off as scabs. It is not compulsory to show all the symptoms in all patients. Briefly, rashes are hard and well distributed all over the body part followed by maculopapular, vesicular, and pustular, and progressively change into the next stage.54,55 Complications associated with MPX are reported secondary skin infections, pneumonia, confusion, bone pain, vomiting, diarrhea, sepsis, and eye problems. Complications one of them is proctitis means, painful sores and swelling inside the rectum. Figure 6 shows the clinical symptoms of EMPX and NPMX. EMPX exists from 1970 to till date in the CA and WA while NMPX exists in those regions where MPX is considered as nonepidemic till 2022 i.e. Europe, North, and South America, the Middle East, Australia NMPX areas.
- EMPX mostly attacks children and young adult, while NMPX mostly affects male who is 31-40 years old.
- EMPX is transmitted through infected animal reservoirs which are followed by human transmission, while NMPX is spread mainly by human-to-human transmission.
- Propagation of EPMX was limited, but unsafe sex and multiple sexual relationships caused more propagation of NMPX.
- Skin lesions were a common clinical sign in EMPX, but genital lesions, penial rashes, ulcerative lesions, proctitis, and pharyngitis is seen as common sign in NMPX. The mortality rate was 1 in 15 people for EMPX, but it is 1 in 1000 for the NMPX.56,57
Figure 6. A) Clinical symptoms of former EMPX; B) Clinical symptoms associated with NMPX i) Genital lesion in males ii) Pharyngitis in males iii) Erythema and swelling of penis iv) Hyperpigmentation, crusts, and desquamation on the hand v) Uneven distribution of papules and rashes on hand vi) Different lesion and papules58
Identification Test
Sample collection from lesions, live viral culture, and biopsy is the methods by which MPX can find.
Real-time Polymerase Chain Reaction
RT-PCR is the identification test for MPX-specific DNA signatures which can find the presence of MPX either current or earlier infection. Well-skilled specialists and laboratories are needed for the testing which needs huge capital.
Anti- Orthopox Virus Immunoglobulins (IgM/IgG)
This is the peculiar test for all kinds of Orthopox virus antibodies either recent or earlier exposure to the virus. This is specific for smallpox identification but not monkeypox.
Viral Cultures
A live MPXV is cultured; a specific gene can be found the live virus is grown. It is a time-consuming test and needs well-skilled specialists, and laboratories are needed for the testing like Real-time polymerase chain reaction.
Immunohistochemistry
It is specific Orthopoxvirus antigens test. The biopsy sample is used for the MPXV, unlike another test. It needs well-skilled specialists and laboratories needed for testing.59,60
The Intervention of MPX from 1958 to 2022
Vaccines
Vaccination is an effective method to prevent MPX in public. As smallpox completely eradicates after proper vaccination, MPX can be eradicated at 85% reported previously whether there is limited facility toward the prevention and cure of MPX. Still, mass vaccination is not allowed by the WHO.
II generation ACAM 2000 (Emergent BioSolutions)
It is a second-generation live, attenuated vaccine approved by Food and drug administration in 2003. It was examined to reduce the lethality of MPX but serious adverse effects in those who were victims of cardiac complication, atopic dermatitis or atopic eczema, and immunocompromised persons. It can be used under an Expanded Access Investigational New Drug (EA-IND) protocol.
II generation IMVAMUNE
Both agencies, the Food and Drug Administration and the European Medicine Agency certified the IMVAMUNE vaccine for the prevention of MPX in the population more than eighteen years old. It is a modified form of the vaccinia Ankara vaccine. It does not produce adverse effects like ACAM 2000. Unfortunately, both vaccines are still not approved for mass vaccination. So, it is a challenge staying in front of scientists that the vaccine which is effective in eradicating smallpox is equally effective in the prevention of MPX.61-65
Symptomatic Treatment (Antiviral Drug)
Until now, there are unavailable any certain medications for the cure of MPX, and most of the availability of cures and prevention are symptomatic and supportive therapy. Drugs effective in Smallpox have been approved for the treatment of MPX.
Tecovirimat (ST-246) (TPOXX)
It is certified in 2022 in Europe. It inhibits the function of major envelope proteins p37 which manage the production of extracellular viruses.
Cidofovir-diphosphate (CDV-PP or Vistide) and Brincidofovir Derivative (CMX001)
It is certified in 2022 in the United State. Both are acyclic nucleoside phosphate derivatives’ that can prevent MPXV replication in vitro and in vivo. It inhibits the synthesis of Viral DNA Polymerase and causes DNA genome synthesis.
Nioch-14
It is a nucleoside inhibitor strong anti-MPXV and is produced easily.
Ribavirin and Tiazofurin
Both are inosine monophosphate dehydrogenase (IMP) inhibitors and can minimize the replication of poxviruses; meanwhile, MPXV is more susceptible to this drug.
C-CA3-ADO and C3-NPC A
The first one is S-adenosylhomocysteine hydrolase inhibitors while the latter one is DNA polymerase inhibitors HPMA and adenosine N1 oxide. Both derivatives showed opposing action MPXV potentially.66-70
Public Health Implication
According to the survey, males with an average age of 33 to 36 years old are the most vulnerable to new monkeypox; however, females, pregnant women, children, and the elderly should not be overlooked. Since October 2022, there have been 77,264 confirmed MPX cases and 36 fatalities. The widespread MPX epidemic in 2022 is a hint of the coronavirus pandemic’s impact on healthcare. It has been observed that smallpox immunization creates partial immunity against MPX for approximately twenty-five years, although the risk of MPX increases when smallpox is no longer present. There are few resources available for MPX diagnosis, surveillance, and treatment. The COVID-19 pandemic raises difficulties such as global relocation, mutation, violence, desertification, climate change, and overcrowding, which invites new developing illnesses such as MPX. In terms of diagnostic equipment and laboratories, there is an economic difference around the world. The Global North nations provide a more effective involvement in the MPX. If universal health policy is adopted globally, it will provide a better platform for the study and innovation of developing zoonotic diseases, as well as aid in MPX prevention.71-73
While MPX is a zoonotic virus, it may be transmitted from human to human, resulting in greater MPXV displacement in the nonepidemic zone from 2022. It looks to be civic unrest and the loosening of international travel restrictions. More study is needed to better understand the many aspects connected to disease transmission and dissemination in the most recent outbreak of the human diseases MPX and COVID-19. MPX, like smallpox and polio, should be examined on a regular basis over the world. Raising awareness and educating the public about the virus, creating rapid, accurate diagnoses, and testing the efficacy of therapy and vaccine are all important steps toward the abolition of MPX.
ACKNOWLEDGMENTS
The authors would like to thank Mr. Jitender Joshi, Chancellor, and Prof. Dharam Buddhi, Vice Chancellor of Uttaranchal University for encouraging us to write this communication.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
KD and PG conceived the literature, wrote the original draft, and critically reviewed and edited the draft. VJ and ANMA have approved the final article for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies on human participants or animals performed by any of the authors.
- Antinori A, Mazzotta V, Vita S, et al. Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy. Euro Surveill. 2022;27(22):2200421.
Crossref - Antunes F, Cordeiro R, Virgolino A. Monkeypox: From A Neglected Tropical Disease to a Public Health Threat. Infect Dis Rep. 2022;14(5):772-783.
Crossref - Ren SY, Li J, Gao RD. 2022 Monkeypox outbreak: Why is it a public health emergency of international concern? What can we do to control it?. World J Clin Cases. 2022;10(30):10873-10881.
Crossref - Rahman MT, Sobur MA, Islam MS, et al. Zoonotic Diseases: Etiology, Impact, and Control. Microorganisms. 2020;8(9):1405.
Crossref - Alcami A. Was smallpox a widespread mild disease? Science. 2020;369(6502):376-377.
Crossref - Bavinger JC, Shantha JG, Yeh S. Ebola, COVID-19, and emerging infectious disease: lessons learned and future preparedness. Curr Opin Ophthalmol. 2020;31(5):416-422.
Crossref - Durski KN, McCollum AM, Nakazawa Y, et al. Emergence of Monkeypox – West and Central Africa. MMWR Morb Mortal Wkly Rep. 2018;67(10):306-310.
Crossref - Muller J, Kretzschmar M. Forward thinking on backward tracing. Nat Phys. 2021;17:555-556.
Crossref - Kuthyar S, Anthony CL, Fashina T, Yeh S, Shantha JG. World Health Organization High Priority Pathogens: Ophthalmic Disease Findings and Vision Health Perspectives. Pathogens. 2021;10(4):442.
Crossref - McEntire CRS, Song KW, McInnis RP, et al. Neurologic Manifestations of the World Health Organization’s List of Pandemic and Epidemic Diseases. Front Neurol. 2021;12:634827.
Crossref - Petersen E, Kantele A, Koopmans M, et al. Human Monkeypox: Epidemiologic and Clinical Characteristics, Diagnosis, and Prevention. Infect Dis Clin North Am. 2019;33(4):1027-1043.
Crossref - Ladnyj ID, Ziegler P, Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ. 1972;46:593-597.
- Shafaati M, Zandi M. Monkeypox virus neurological manifestations in comparison to other orthopoxviruses. Travel Med Infect Dis. 2022;49:102414.
Crossref - Ramirez-Rubio O, Daher C, Fanjul G, et al. Urban health: an example of a “health in all policies” approach in the context of SDGs implementation. Global Health. 2019;15(1):87.
Crossref - Berthet N, Descorps-Declere S, Besombes C, et al. Genomic history of human monkey pox infections in the Central African Republic between 2001 and 2018. Sci Rep. 2021;11(1):13085.
Crossref - Bunge EM, Hoet B, Chen L, et al. The changing epidemiology of human monkeypox-A potential threat? A systematic review. PLoS Negl Trop Dis. 2022;16(2):e0010141.
Crossref - Bethineedi LD, Kutikuppala LVS, Kandi V. Monkeypox Epidemic: A Throwback From Smallpox Eradication. Cureus. 2022;14(7):e26577.
Crossref - Li Y, Zhao H, Wilkins K, Hughes C, Damon IK. Real-time PCR assays for the specific detection of monkeypox virus West African and Congo Basin strain DNA. J Virol Methods. 2010;169(1):223-227.
Crossref - Nagarajan P, Rajathy Port Louis L, Howlader A, Rangarajalu K. The re-emerging human monkeypox virus: An urgent global health alert. Health Sci Rep. 2022;5(6):e928.
Crossref - Beer EM, Rao VB. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl Trop Dis. 2019;13(10).
Crossref - Scarpa F, Sanna D, Azzena I, et al. Genetic Variability of the Monkeypox Virus Clade IIb B.1. J Clin Med. 2022;11(21):6388.
Crossref - Shete AM, Yadav PD, Kumar A, et al. Genome characterization of monkeypox cases detected in India: Identification of three sub-clusters among A.2 lineage. J Infect. 2022; S0163-4453(22)00554-0.
Crossref - Likos AM, Sammons SA, Olson VA, et al. A tale of two clades: monkeypox viruses. J Gen Virol. 2005;86(Pt 10):2661-2672.
Crossref - Thornhill JP, Barkati S, Walmsley S, et al. Monkeypox Virus Infection in Humans across 16 Countries.
N Engl J Med. 2022;387(8):679-691.
Crossref - Orviz E, Negredo A, Ayerdi O, et al. Monkeypox outbreak in Madrid (Spain): Clinical and virological aspects. J Infect. 2022;85(4):412-417.
Crossref - Lu T, Wu Z, Jiang S, Lu L, Liu H. The current emergence of monkeypox: The recurrence of another smallpox? Biosaf Health. 2022.
Crossref - Minhaj FS, Ogale YP, Whitehill F, et al. Monkeypox Outbreak – Nine States, May 2022. MMWR Morb Mortal Wkly Rep. 2022;71(23):764-769.
Crossref - Radonić A, Metzger S, Dabrowski PW, et al. Fatal monkeypox in wild-living sooty mangabey, Côte d’Ivoire, 2012. Emerg Infect Dis. 2014;20(6):1009-1011.
Crossref - Taylor L. Monkeypox: WHO to rename disease to prevent stigma. BMJ. 2022;377:o1489.
Crossref - Catala A, Clavo-Escribano P, Riera-Monroig J, et al. Monkeypox outbreak in Spain: clinical and epidemiological findings in a prospective cross-sectional study of 185 cases. Br J Dermatol. 2022;187(5):765-772.
Crossref - Mileto D, Riva A, Cutrera M, et al. New challenges in human monkeypox outside Africa: A review and case report from Italy. Travel Med Infect Dis. 2022;49:102386.
Crossref - Hraib M, Jouni S, Albitar MM, Alaidi S, Alshehabi Z. The outbreak of monkeypox 2022: An overview. Ann Med Surg (Lond). 2022;79:104069.
Crossref - Isidro J, Borges V, Pinto M, et al. Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of monkeypox virus [published correction appears in Nat Med. 2022;28(10):2220-2221.
Crossref - Adler H, Gould S, Hine P, et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022;22(8):1153-1162.
Crossref - Yadav PD, Reghukumar A, Sahay RR, Sudeep K, Shete AM, Raman A, et al. First two cases of Monkeypox virus infection in travellers returned from UAE to India, July 2022. J Infect. 2022;S0163-4453(22):00471-00476.
Crossref - McCollum AM, Damon IK. Human monkeypox. Clin Infect Dis. 2014;58(2):260-267.
Crossref - Kozlov M. Monkeypox goes global: why scientists are on alert. Nature. 2022;606(7912):15-16.
Crossref - Morand A, Delaigue S, Morand JJ. Review of poxvirus: emergence of monkeypox. Panorama des poxvirus: emergence du monkeypox. Med Sante Trop. 2017;27(1):29-39.
Crossref - Adnan N, Haq ZU, Malik A, et al. Human monkeypox virus: An updated review. Medicine (Baltimore). 2022;101(35):e30406.
Crossref - Babkin IV, Babkina IN, Tikunova NV. An Update of Orthopoxvirus Molecular Evolution. Viruses. 2022;14(2):388.
Crossref - Kugelman JR, Johnston SC, Mulembakani PM, et al. Genomic variability of monkeypox virus among humans, Democratic Republic of the Congo. Emerg Infect Dis. 2014;20(2):232-239.
Crossref - Wurtzer S, Levert M, Dhenain E, et al. First Detection of Monkeypox Virus Genome in Sewersheds in France: The Potential of Wastewater-Based Epidemiology for Monitoring Emerging Disease. Environ Sci Technol Lett. 2022;9(11):991-996.
Crossref - Shchelkunov SN, Totmenin AV, Safronov PF, et al. Analysis of the monkeypox virus genome. Virology. 2002;297(2):172-194.
Crossref - Noe S, Zange S, Seilmaier M, et al. Clinical and virological features of first human monkeypox cases in Germany. Infection. 2022;1-6.
Crossref - Luna N, Ramirez AL, Munoz M, et al. Phylogenomic analysis of the monkeypox virus (MPXV) 2022 outbreak: Emergence of a novel viral lineage? Travel Med Infect Dis. 2022;49:102402.
Crossref - Xiang Y, White A. Monkeypox virus emerges from the shadow of its more infamous cousin: family biology matters. Emerg Microbes Infect. 2022;11(1):1768-1777.
Crossref - Alakunle E, Moens U, Nchinda G, Okeke MI. Monkeypox Virus in Nigeria: Infection Biology, Epidemiology, and Evolution. Viruses. 2020;12(11):1257.
Crossref - Kumar N, Acharya A, Gendelman HE, Byrareddy SN. The 2022 outbreak and the pathobiology of the monkeypox virus. J Autoimmun. 2022;131:102855.
Crossref - Minasov G, Inniss NL, Shuvalova L, Anderson WF, Satchell KJF. Structure of the Monkeypox virus profilin-like protein A42R reveals potential functional differences from cellular profilins. Acta Crystallogr F Struct Biol Commun. 2022;78(Pt 10):371-377.
Crossref - Kaler J, Hussain A, Flores G, Kheiri S, Desrosiers D. Monkeypox: A Comprehensive Review of Transmission, Pathogenesis, and Manifestation. Cureus. 2022;14(7):e26531.
Crossref - Peter OJ, Kumar S, Kumari N, Oguntolu FA, Oshinubi K, Musa R. Transmission dynamics of Monkeypox virus: a mathematical modelling approach. Model Earth Syst Environ. 2022;8(3):3423-3434.
Crossref - Soheili M, Nasseri S, Afraie M, et al. Monkeypox: Virology, Pathophysiology, Clinical Characteristics, Epidemiology, Vaccines, Diagnosis, and Treatments. J Pharm Pharm Sci. 2022;25:297-322.
Crossref - Weaver JR, Isaacs SN. Monkeypox virus and insights into its immunomodulatory proteins. Immunol Rev. 2008;225:96-113.
Crossref - Kmiec D, Kirchhoff F. Monkeypox: A New Threat? Int J Mol Sci. 2022;23(14):7866.
Crossref - Patel A, Bilinska J, Tam JCH, et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ. 2022;378:e072410.
Crossref - Shukri AMA, Wang SM, Chia SL, Nawi SFAM. The SARS-CoV-2 Variants and their Impacts. J Pure Appl Microbiol. 2022;16(3):1409-1424.
Crossref - Ferre VM, Bachelard A, Zaidi M, et al. Detection of monkeypox virus in anorec- tal swabs from asymptomatic men who have sex with men in a sexually transmit- ted infection screening program in Paris, France. Ann Intern Med. 2022;175(10):1491-1492.
Crossref - Gessain A, Nakoune E, Yazdanpanah Y. Monkeypox. N Engl J Med. 2022;387(19):1783-1793.
Crossref - Cheema AY, Ogedegbe OJ, Munir M, Alugba G, Ojo TK. Monkeypox: A Review of Clinical Features, Diagnosis, and Treatment. Cureus. 2022;14(7):e26756. Published 2022 Jul 11.
Crossref - Kim SB, Jung J, Peck KR. Monkeypox: the resurgence of forgotten things. Epidemiol Health. 2022;e2022082.
Crossref - Erez N, Achdout H, Milrot E, et al. Diagnosis of Imported Monkeypox, Israel, 2018. Emerg Infect Dis. 2019;25(5):980-983.
Crossref - Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for Preexposure Vaccination of Persons at Risk for Occupational Exposure to Orthopoxviruses: Recommendations of the Advisory Committee on Immunization Practices – United States, 2022 [published correction appears in MMWR Morb Mortal Wkly Rep. 2022 Jul 08;71(27):886]. MMWR Morb Mortal Wkly Rep. 2022;71(22):734-742. Published 2022 Jun 3.
Crossref - Monkeypox Vaccine. In: Drugs and Lactation Database (LactMed). Bethesda (MD): National Institute of Child Health and Human Development; July 18, 2022
- Davies DH, Molina DM, Wrammert J, et al. Proteome-wide analysis of the serological response to vaccinia and smallpox. Proteomics. 2007;7(10):1678-1686.
Crossref - Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the advisory committee on immunization practices – United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71 (22):734-742.
Crossref - Decker MD, Garman PM, Hughes H, et al. Enhanced safety surveillance study of ACAM2000 smallpox vaccine among US military service members. Vaccine. 2021;39(39):5541-5547.
Crossref - Martín-Delgado MC, Martín Sánchez FJ, Martínez-Sellés M, et al. Monkeypox in humans: a new outbreak. Rev Esp Quimioter. 2022;35(6):509-518.
Crossref - Hung YP, Lee CC, Lee JC, Chiu CW, Hsueh PR, Ko WC. A brief on new waves of monkeypox and vaccines and antiviral drugs for monkeypox. J Microbiol Immunol Infect. 2022;55(5):795-802.
Crossref - Baker RO, Bray M, Huggins JW. Potential antiviral therapeutics for smallpox, monkeypox and other orthopoxvirus infections. Antiviral Res. 2003;57(1-2):13-23.
Crossref - Bajrai LH, Alharbi AS, El-Day MM, Bafaraj AG, Dwivedi VD, Azhar EI. Identification of Antiviral Compounds against Monkeypox Virus Profilin-like Protein A42R from Plantago lanceolata. Molecules. 2022; 27(22):7718.
Crossref - Al-Tawfiq JA, Barry M, Memish ZA. International outbreaks of Monkeypox virus infection with no established travel: A public health concern with significant knowledge gap. Travel Med Infect Dis. 2022;49:102364.
Crossref - Khodakevich L, Jezek Z, Messinger D. Monkeypox virus: ecology and public health significance. Bull World Health Organ. 1988;66(6):747-752.
- Yang X, Tang G, Shi L, Xu F. Transmission, and detection of monkeypox virus in saliva: Implications for dental practice and public health. J Dent Sci. 2022.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.