ISSN: 0973-7510
E-ISSN: 2581-690X
White root rot disease caused by Rigidoporus microporus was a detrimental disease of rubber plant. In controlling the disease, synthetic chemicals were applied. This control may cause environmental pollution and health problem. In this study, an alternative to control WWRD using biological control agent of local Trichoderma spp. isolates was examined. Trichoderma spp. were isolated from soil of healthy rubber, sugarcane and tobacco plantation, and of Sibolangit Forest Park. R. microporus was isolated from infected rubber tree. Sixteen Trichoderma spp. were isolated and subjected to antagonisms assay againt R. microporus. Trichoderma spp. isolates showed to have different ability in inhibiting R. microporus growth. Four isolates i.e. KA03 of rubber, TB03 of sugarcane, TM01 of tobacco plantations, and HU01 of Sibolangit Forest Parks showed relatively higher percentage of inhibition to R. microporus by 67.2, 66.3, 70.2, and 71%, respectively. Examination of inhibition of white root rot disease in rubber stump in polyethylene plastic bag showed that the four isolates were able to reduce disease intensity and severity of R. microporus after 60 days of control, but no improvement of stump performance was observed.
Biological control, Rigidoporus microporus, Trichoderma.
Indonesia is the world’s second largest rubber producer after Thailand. Indonesian rubber exports showed significant progress annually and contribute in raising revenue for the country (Ministry of Agriculture, 2014). However, rubber production faces a significan problem due to rubber plant diseases which causes economic losses not only because of the production loss, but also the high cost required in control the disease.
White root rot disease (WRRD), caused by Ridigoporus microporus is one of detrimental disease of rubber tree in Indonesia, India, Malaysia, Sri Lanka, Thailand, West and Central Africa (Kaewchai & Soytong, 2010). WRRD may lead to death of rubber plants. Plants of two to six years are particularly susceptible to this disease. After initial infection this disease may kill three-year old plants within six months and six-year old plant within 12 months, respectively, depends on number of the pathogen in soil.WRRD often causes damage to where there are many root stump or residual wood, and of sandy or loose soil (Situmorang & Budiman, 2003).
Early symptom of WRRD showed as decaying roots of rubber plant attacked, following by pale yellowing leave, folded leaf edges and ends, and sometime with early flowers and fruit appearance. White thread of rhizomorph is seen in the root, sometime with yellowish/orange fruiting bodie, mainly at the collar of the dead infected tree. Secondary symptoms such as increasing the number of branches bearing fruit earlier are frequently observed. Leaves of affected plants turn yellow and fall which is followed by the death of the plant twigs (Anwar, 2006).
To control WRRD, an integration of cultural and chemical methods such as removal and burning of the infected root, applying the chemical fungicides has been applied, but sometimes it is too late to control disease (Kaewchai et al., 2009). The cultural control may be loborous, while the chemical control may cause environmental pollution and health problems. Biological control is an alternative in controlling the disease, which is expected to reduce the reliance on synthetic chemical compounds. Biological control agent using facultative parasitic fungi such as Trichoderma spp. may be used to control the disease. Trichoderma spp. the very common and antagonistic fungi found in the soil are well-known as biological control agent of plant fungal disease (Benítez et al., 2004; Ikediugwu & Monday 2012; Jeyaseelan et al., 2012). This fungus with the help of their enzymes and toxic compounds damages its host and absorb food from the host cells by entering hyphae to the host (Benítez et al., 2004; Vinale et al., 2008).
Biological control of many plant fungal diseases using Trichoderma spp. has been widely reported. Some studies indicate that Trichoderma suppressed the growth of pathogens in both the leaf and root. For examples, Jeyaseelan et al. (2012) reported the ability of Trichoderma spp. to suppress the growth of Pythiuma phanidermatum soil borne diseases on tomato plants. Suppression Collelotrichum capsici agen of anthracnose disease of chilli using Trichoderma has also been reported (Ajith & Lakshmidevi, 2010). So far, almost no study on the isolation and utilization of local Trichoderma spp. in North Sumatra especially in controlling WRRD of rubber plants caused by R. microporus was reported. In this study, isolation of Trichoderma spp. of soil of healthy rubber, sugarcane and tobacco plantation, and from Sibolangit Forest Park was conducted. The isolates were then examined in vitro against R. microporus, followed by an in vivo examination on rubber stump infected with R. microporus in polyethylene plastic bag.
Isolation and characterization of Rigidoporus microporus
Suspected fungal infected root as well as fungal mycelia of the infected root was taken. The root was sliced to about 0.5 cm long. Infected roots and mycelia were put on PDA. Cultures were incubated at ambient temperature for 48 hours. Growing colonies was separated to get single colony. Morphological and microscope observation and characterization were conducted to the fungal colony.
Isolation and characterization of Trichoderma spp.
Soil samples were taken from healthy rubber, sugarcane, and tobacco plantations, and from Sibolangit Forest Park. The soil samples were cleaned from large particles such as roots and leave debris. Isolation of Trichoderma spp. were conducted using dilution method on a common fungal medium, Potato Dextrose Agar (PDA). Soil was diluted with sterile distilled water. One ml of soil dilution was spreaded on PDA added with 50 µg of chloramphenicol. Selected fungal colony was grown in PDA. All cultures were incubated at ambient temperature for 48 hours. Morphological and microscope observation, and characterization of the fungal colony were conducted.
Assay of antagonism of Trichoderma spp. against Rigidoporus microporus in vitro
Dual culture method was utilized to examine the ability of Trichoderma spp. to inhibit R. microporus growth. Actively growing hyphae of Trichoderma spp. and R. microporus on agar were taken with a 5 mm cork borer. Both fungal hyphae were grown side by side with a distance of 3 cm on PDA. Culture was made by growing single fungus on PDA. Cultures were incubated at ambient temperature. Hyphal diameter of both colonies was measured for 7 days. Percentage of inhibition was measeured as:
PIRG= [ R1 – R2 / R2 ] x 100%
PIRG = Percentage of inhibition of radial growth
R1 = hyphal diameter of R. microporus growing as a control
R2 = hyphal diameter of R. microporus in dual culture with Trichoderma spp.
To observed antagonism effect of Trichoderma spp. on R. microporus, slide culture was utilized for 7 days. Contacted hyphae were observed microscopically.
Assay on control of WWRD on rubber stump
Selected Trichoderma spp. isolates were subjected to growth in autoclaved rice media. Rubber stump with disease intensity of 25-50% of 1 month old were grown in polyethylene plastic bag of 30 x 40 cm. Trichoderma application of 50 g/stump was conducted after 1 week of the growth of rubber stump by pouring Trichoderma rice culture on digged soil around rubber stump. Poured Trichoderma culture was re-covered with soil. Observation was conducted on disease intensity by digging soil around stump 5-10 cm depth. Disease intensity was measured as 0% = no disease, 1-25% = mild, 25-50% = moderate, 50-75% = severe, and 75-100% = highly severe intensity, while disease severity was measured on a scales of 0 = no disease, 1 = light, 2 = moderate, and 3 = severe infection.
Isolation and characterization of Rigidoporus microporus
Rigidoporus microporus isolated from infected rubber root showed to have white flattened cottony-like colony, smoothy mycelium with no ring of growth observed (Figure 1.). In 4 days of incubation, the colony overgrew of 9 cm petridish on PDA. Hyphae was septate with hyaline basidiospore with diameter of 3.28 ìm. These characters were similar to that of Kaewchai et al. (2009) observation on R. microporus isolated from South part of Thailand.
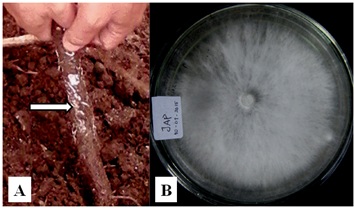
Fig. 1. A. Infected rot (arrowed) with hyphae of R. microporus, and B. Overgrown colony of R. microporus in petri dish.
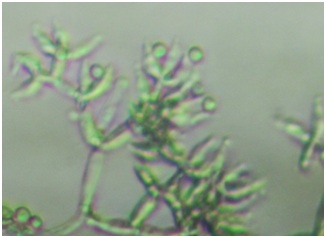
Fig. 2. Conidia of one isolate of Trichoderma
Isolation and characterization of Trichoderma spp.
Sixteen Trichoderma spp. were isolated from different soil samples, i.e. 5 isolates of rubber (KA01, KA02, KA03, KA04, KA05), 3 isolates of sugarcane (TB01, TB02, TB03), 5 isolates of tobacco plantations (TM01, TM02, TM03, TM04, TM05), and 3 isolates of Sibolangit Forest Park (HU01, HU02, HU03). Morphological observation showed that isolates KA01, KA03, TB03, HU01 and HU02 have smooth and cottony colony surface and isolates KA02, KA04, KA05, TM01, TM02, TM03, TM04, TM05, TB01, TB02, and HU03 were granulated. The isolates had a variety in colors such as white-green, light green, green, and green-yellowish, and spherical shape of colony. Seven colonies grew laterally with ring of growth, however, others did not show ring of growths. These features belong to Trichoderma spp. Extended growth showed to have dark green colony (Table 1.). Microscopic observation of the hyphae showed that conidiophore was with pyramide-like branch (Figure 2.) as specific to Trichoderma (Samson et al., 1995).
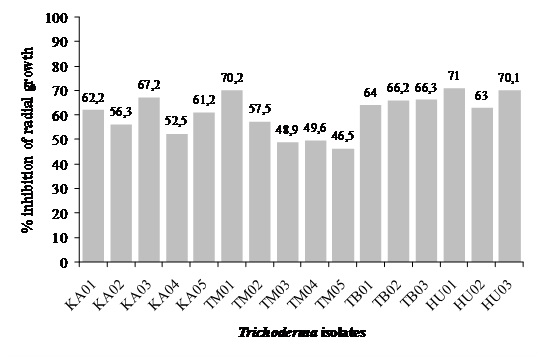
Fig. 3. Trichoderma spp. ability to inhibit R. microporus hyphal growth
Table (1):
Morphological dan microscopic observation of Trichoderma spp. characteristics
| Soil Samples of | Isolate Code | Morphological characteristics | ||||
|---|---|---|---|---|---|---|
| Structure | Color | Shape | Type of growth | Conidiofore | ||
| Rubber plantation | KA01 | Smooth-cottonlike | White-greenish | Circular | With growing circle | Branched, pyramide-like |
| KA02 | Granulated | Greenish | Circular | With growing circle | Branched, pyramide-like | |
| KA03 | Smooth-cottonlike | Green | Circular | With growing circle | Branched, pyramide-like | |
| KA04 | Granulated | White-greenish | Circular | With growing circle | Branched, pyramide-like | |
| KA05 | Granulated | White-greenish | Circular | Without growing circle | Branched, pyramide-like | |
| Tobacco plantation | TM01 | Granulated | Green | Circular | With growing circle | Branched, pyramide-like |
| TM02 | Granulated | White-greenish | Circular | Without growing circle | Branched, pyramide-like | |
| TM03 | Granulated | Greenish | Circular | With growing circle | Branched, pyramide-like | |
| TM04 | Granulated | Greenish | Circular | Without growing circle | Branched, pyramide-like | |
| TM05 | Granulated | White-greenish | Circular | With growing circle | Branched, pyramide-like | |
| Sugarcane plantation | TB01 | Granulated | White-greenish | Circular | Without growing circle | Branched, pyramide-like |
| TB02 | Granulated | Greenish | Circular | Without growing circle | Branched, pyramide-like | |
| TB03 | Smooth-cottonlike | Green-yellowish | Circular | Without growing circle | Branched, pyramide-like | |
| Sibolangit Forest Park | HU01 | Smooth-cottonlike | Greenish | Circular | Without growing circle | Branched, pyramide-like |
| HU02 | Smooth-cottonlike | White-greenish | Circular | Without growing circle | Branched, pyramide-like | |
| HU03 | Granulated | Green-yellowish | Circular | Without growing circle | Branched, pyramide-like | |
Assay of antagonism of Trichoderma spp. to Rigidoporus microporus in vitro
In vitro antagonism assay was conducted using a dual method on PDA. Observation of growth inhibition on R. microporus was carried out in 2-8 days of incubation. Trichoderma spp. ability to inhibit R. micoporus growth varied, four potential isolates were to showed to inhibit more (Figure 3.). Antagonistic effect was observed on 2-days of incubation, but clearly seen on 4-days of incubation, in which R. micoporus was inhibited by Trichoderma spp. At the end of observation, Trichoderma spp. growth almost over entire dish, suppressing R. microporus growth (Figure 4.). Microscope observation of four potential isolates of Trichoderma spp. showed that their hyphae penetrated and wrapped R. microporus hyphae around (Figure 5.). Kubicek et al. (2001) reported that mycoparasitism is one of the most common mechanism showed by Trichoderma spp. After recognizing the host, Trichoderma hyphae attached to host hyphae by twisting and then penetrate host cell wall through secretion of cell wall degrading enzymes (Viterbo et al., 2002). Mycoparasite producing cell wall degrading enzymes is to perforate host hyphae to takes nutrients from host and uses it for growing. Chitinase and â -1,3-glucanase has been observed to get directly involved in mycoparasitic mechanism between Trichoderma spp. with the host (Kubicek et al., 2001), or antibiosis, antibiosis by producing an antibiotic 6-pentyl-a-pyrone (6PP), and peptaibol heptilidic acid (Vinale et al., 2008), competition for nutrients and space, and induce local and systemic resistance of the plant (Harman, 2006).
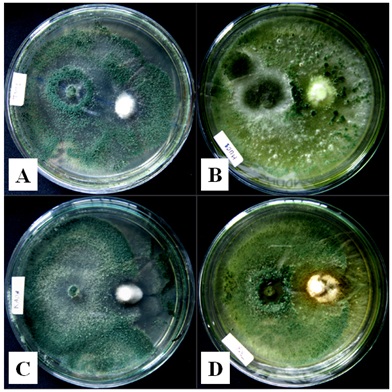
Fig.4. Trichoderma spp. hyphae overgrown on R. microporus
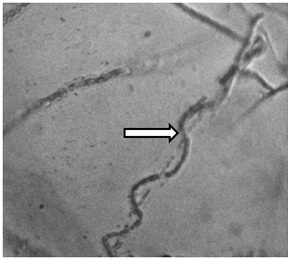
Fig.5. Trichoderma spp. hyphae twisted R. microporus hyphae around
Reduction of WRRD severity and intensity by Trichoderma spp. isolates
Trichoderma isolates with relatively higher inhibition zone from different locations i.e. KA03, TM01, TB03 and HU01 were used in examination to reduce WWRD intensity in rubber stump in vivo. Observations were carried out every 30 days of 90 days. In all observation, a decline of disease severity and intensity, and improving rubber stump healthy of all Trichoderma treatments was observed compared to that of (+) control (Table 2.). Number of rubber stump survival and its root were also observed. Number of rubber stump survival was seen by making small wound to rubber stump to see a greeny tissue, while number of root was observed by gently digging soil around growing stump. The result showed that Trichoderma spp. isolates was not able to enhance stump performance, such as root number, however, all Tricoderma isolates were able to keep the stump alive (Table 3.). It seemed that the effect of Trichoderma spp. in increasing plant performance might not occur immediately.
Table (2):
Reduction of WRRD severity and intensity by Trichoderma spp. isolates
| Treatments | Disease severity after | Disease intensity after | Severity/recovery level | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 30 days | 60 days | 90 days | 30 days | 60 days | 90 days | 30 days | 60 days | 90 days | |
| (-) control | 0 | 0 | 0 | 0 | 0 | 0 | No infection observed | No infection observed | No infection observed |
| (+) control | 2 | 3 | 3 | 50 | 100 | 100 | Severely rotted | Heavily rotted | Heavily rotted |
| KA03 | 1 | 0 | 0 | 15 | 0 | 0 | Moderate recovery | Moderate recovery | Moderate recovery |
| TM01 | 1 | 0 | 0 | 20 | 0 | 0 | Moderate recovery | Well-recovered | Well-recovered |
| TB03 | 1 | 0 | 0 | 17.5 | 0 | 0 | Moderate recovery | Moderate recovery | Moderate recovery |
| HU01 | 1 | 0 | 0 | 17.5 | 0 | 0 | Moderate recovery | Well-recovered | Well-recovered |
Table (3):
Stump condition and root number of rubber stump after treated with Trichoderma spp.
Treatments |
Stump Survival |
Root Number |
|---|---|---|
(-) control |
Alive |
5 |
(+) control |
Death |
0 |
KA03 |
Alive |
1 |
TM01 |
Alive |
0 |
TB03 |
Alive |
1 |
HU01 |
Alive |
1 |
WRRD caused by R. microporus is an important disease in rubber. The disease attacks all stage of rubber plants, especially to newly rubber plantation, and may cause high economic losses compared to other diseases. Situmorang (2004) reported that dry latex production declines at least 2.7 kilograms /tree /year. This disease attacks the roots by forming an overgrown flattened mycelia strands called rhizomorf of white or white-yellowish threadlike resembling root hairs, and later on develops to form its fruiting bodies. Infected trees show a general foliage discoloration, proceed sometimes by premature flowering and fruiting (Omorusi, 2012). Root eventually rots and the rubber plant crashes. Rotting of the roots is due to destruction of chemical structure of wood as a result of fungal enzyme activity. In addition, mechanically penetration through colonized natural openings or wounds were also contribute to plant damage (Omorusi 2012).
In vitro examination of Trichoderma spp. isolates showed to inhibit R. microporus hyphae in vitro. Trichoderma is known as effective biological control agents for many plant fungal diseases, and also were known for promoting plant growth (Ikediugwu & Monday 2012; Benítez et al., 2004). Biological control activity of Trichoderma occurs directly and indirecty through several mechanisms competing for nutrients and space, modifying the environmental conditions, or promoting plant growth and plant defensive mechanisms and antibiosis, and by mechanisms such as mycoparasitism (Howell, 2003; Diby et al., 2005; Benítez et al., 2004; Harman, 2006; Ikediugwu & Monday 2012).
Kubicek et al. (2001) reported that mycoparasitism is one of the most common mechanism showed by Trichoderma spp. After recognizing the host, Trichoderma hyphae attached to host hyphae by twisting and then penetrate host cell wall through secretion of cell wall degrading enzymes (Viterbo et al., 2002). Mycoparasite producing cell wall degrading enzymes is to perforate host hyphae to takes nutrients from host and uses it for growing. Chitinase and â -1,3-glucanase has been observed to get directly involved in mycoparasitic mechanism between Trichoderma spp. with the host (Kubicek et al., 2001).
Penetration of some Trichoderma hyphae into infected plant tissue might inhibit the pathogen growth, and might recover to plant growth (Harman et al., 2004). In this study, even thought Trichoderma spp. application decreased disease severity and intensity of WRRD as well, but it seemed that the isolates could not completely recover the stump that was previously infected with R. microporus with moderate disease intensity of 25-50%. Application of biological control agents prior fungal pathogen infected the plant possibly shows a different result.
ACKNOWLEDGMENTS
This research was supported in part by Directorate of Research and Community Service, Ministry of Research, Technology, and Higher Education of Republic of Indonesia.
- Ajith PS, Lakshmidevi N. Effect of volatile and non-volatile compounds from Trichoderma spp. against Colletotrichum capsici incitant of Anthracnose on bell peppers. Nature and Science, 2010; 8(9): 1-5
- Anwar C. 2006. Majemen dan teknologi budidaya karet (Management and technology of rubber plant culture). Makalah Pelatihan. Pusat Penelitian Karet, Medan.
- Benítez T, Rincón AM, Limón MC, Codón AC. Biocontrol mechanisms of Trichoderma strains. Int. Microbiol. 2004; 7:249-260.
- Diby P, Sju KA, Jisha PJ, Sarma YR, Kumar A, Anandaraj M. Mycolytic enzyme produced by Pseudomonas fluorescens and Trichoderma spp. against Phytophthora capsici, the foot rot pathogen of black pepper (Piper nigrum, L.). Annals Microbiol., 2005; 55(2): 129-133.
- Harman GE. Overview of mechanisms and uses of Trichoderma spp. Phytopathol. 2006; 96: 190-194.
- Howell CR. Mechanisms employed by Trichoderma species in the biological control of plant diseases: The history and evolution of current concepts. Plant Dis. 2003; 87: 4–10.
- Jeyaseelan EC, Tharmila S, Niranjan K. Antagonistic activity of Trichoderma spp. and Bacillus spp. against Pythium aphanidermatum isolated from tomato damping off. Arch Appl. Scie. Res. 2012; 4(4): 1623-1627.
- Ikediugwu FEO, Monday U. Root zone microflora is responsible for suppressiveness of the white root rot disease in Akwete Rubber Plantations. J. Plant Pathol. Microb., 2012; 3-7. Open Acces. http://dx.doi.org/10.4172/2157-7471.1000151.
- Kaewchai S, Soytong K, Hyde KD. Mycofungicides and fungal biofertilizers. Fungal Diversity, 2009; 38: 25-50.
- Kaewchai S, Soytong K. Application of biofungisida against Rigidoporus microporus causing white root disease of rubber trees. J. Agric. Technol. 2010; 6(2): 349-363.
- Kubicek CP, Mach RL, Peterbauer CK, Lorito M. Trichoderma: From genes to biocontrol. J Plant Pathol. 2001; 83: 11–24.
- Omorusi VI. 2012. Effects of white root rot disease on Hevea brasiliensis (Muell. Arg.) – Challenges and control approach. Intech. Pp. 139-152. http://dx.doi.org/10.5772/54024.
- Samson RA, Hoekstra ES, Frisvad JC, Filtenborg O. Introduction to food borne fungi. 4th edition. Posen and Looyen, Netherland 1995.
- Situmorang A. 2004. Status dan manajemen pengendalian jamur akar putih di perkebunan karet (Status and management of white root rot control in rubber plantation). Prosiding Pertemuan Teknis. Pusat Penelitian Karet, Balai Penelitian Sembawa. hlm 66-86.
- Situmorang A, Budiman A. 2003. Penyakit tanaman karet dan pengendaliannya (Rubber plant disease and its control). Palembang: Pusat Penelitian Karet, Balai Penelitian Sembawa.
- Vinale F, Sivasithamparam K, Ghisalberti EL, Marra R, Woo SL, Lorito M. Trichoderma–plant–pathogen interactions. Review article. Soil Biol. Biochem., 2008; 40: 1–10.
- Viterbo A, Ramot O, Chemin L, Chet I. Significance of lytic enzymes from Trichoderma spp in the biocontrol of fungal plant pathogens. Antonie van Leeuwenhoek. 2002; 81: 549-556.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


