M.E Shilpa and G.P. Brahmaprakash
Department of Agricultural Microbiology, UAS, GKVK, Bengaluru, Karnataka, India.
ABSTRACT
The present investigation was conducted to find out the effect of vermicompost and urban compost as amendment with talc in maintaining the shelf life of microbial inoculants such as Bradyrhizobium japonicum, Bacillus megaterium and Pseudomonas fluorescens as single, dual and triple inoculants. Talc amended with organic materials maintained better shelf life of microbial inoculants than talc. Maximum viable cells and per cent survival of microbial inoculants were recorded in triple inoculants in all the carrier based formulations followed by dual and single inoculant formulations.
Keywords: Vermicompost, Urban compost, Talc and Microbial consortium.
INTRODUCTION
Population of introduced microbial inoculants starts to decline gradually (Roy et al., 2010), it is due to unpredictable and heterogeneous environment of soil. Survival of introduced microbial inoculants in soil depends on various factors like temperature, moisture and carrier material. Carrier material acts as a medium to transfer live microorganisms from laboratory to plant rhizosphere. It provides an environment to microbial inoculants which is conducive to their growth and development. Ideal properties of carrier materials are, should contain high organic matter content, high water holding capacity, it should have neutral pH, amenable to nutrient supplement, available in powder or granular form in abundant quantities at a reasonable price, manageable in mixing, curing and packaging operation, non toxic to the microbial inoculants and to the plant itself, supports growth and survival of microbial inoculants (Smith, 1992).
Various organic materials such as FYM, vermicompost and compost have been used as carrier materials for formulation of beneficial microbial inoculants (Mohammadi and Sohrabi, 2012). The amendment of carrier with organic materials during the preparation of microbial inoculants will improve inoculants quality such as better adherence to seed, stabilizing the product, and enhances the survivability of microorganisms during storage, exhibit better field performance and tolerance to extreme environmental conditions. Many kinds of additives have been used for inoculant production because of their good rheological properties and high water holding capacity (Mugnier and Jung, 1985).
Many investigations have suggested compost as carrier material for biofertilizers, but the role of compost in maintaining microbial population has not been studied much. In the present study, vermicompost and urbancompost are used as amendment with talc to develop carrier based formulation of microbial consortium and their effect on survival of microbial inoculants.
MATERIALS AND METHODS
The experiment was carried out in the Department of Agricultural Microbiology, University of Agricultural Sciences, Bengaluru. Bradyrhizobium japonicum as nitrogen fixer, Pseudomonas fluorescens as PGPR and Bacillus megaterium as phosphorus solubilizer were used to develop single, dual and triple inoculant formulation in three carrier materials.
Preparation of carrier material
Vermicompost and urban compost were collected sieved through 2 mm sieve and shade dried for 4-5 days, to bring the moisture level up to 5 per cent, powdered and sieved through 90 ìm sieve. The processed samples were mixed separately with talc in the ratio of 1:10, autoclaved and used to develop single, dual and triple inoculant formulation.
Preparation of microbial consortium
Three single inoculant formulations of Bradyrhizobium japonicum, Pseudomonas fluorescens and Bacillus megaterium were prepared. These microorganisms were grown in yeast extract mannitol broth, King’s B broth and Pikovaskaya’s broth respectively and incubated on shaker for 3-4 days. After incubation period, 25 ml of each single microorganism broth was mixed separately with 0.5 per cent carboxy methyl cellulose (CMC) + sterilized talc powder and stored in polythene covers for further use. Same procedure was followed to develop single inoculant formulations for sterilized talc + 10 per cent vermicompost and sterilized talc + 10 per cent urban compost.
Three dual inoculants formulations were prepared with combinations of Bradyrhizobium japonicum + Pseudomonas fluorescens, Bradyrhizobium japonicum + Bacillus megaterium and Pseudomonas fluorescens + Bacillus megaterium. These inoculants broth were mixed in 1:1 ratio. 25 ml of broth containing Bradyrhizobium japonicum and Pseudomonas fluorescens were added to sterilized talc powder and 0.5 per cent CMC, mixed throughly and stored for further study. In the similar way dual inoculants formulations were prepared for talc + 10 per cent vermicompost and talc + 10 per cent urban compost formulations. Similarly dual inoculants of Bradyrhizobium japonicum + Bacillus megaterium and Pseudomonas fluorescens + Bacillus megaterium were prepared.
Triple inoculant formulation was prepared by mixing the broth containing Bradyrhizobium japonicum, Pseudomonas fluorescens and Bacillus megaterium in 1:1:1 ratio. This broth culture was used for the development of talc, talc + 10 per cent vermicompost and talc + 10 per cent urban compost formulations.
Storage and survival study
Inoculant formulations of talc, talc + 10 per cent vermicompost and talc + 10 per cent urban compost formulations were packed in sterilized polythene covers under aseptic conditions and stored at ambient temperature. Survival of developed inoculant formulations was monitored upto 360 days at intervals of 0, 10, 20, 30, 60, 90, 120, 150, 180 and 360 days. Viable counts were enumerated by standard plate count method and number of colony forming units (cfu) were recorded at 10 6 dilutions and converted to log10 cfu per gram.
Statistical analyses of population data was analyzed by using complete randomized design and means were compared by the Duncan’s Multiple Range Test (DMRT) (Little and Hills, 1978).
RESULTS AND DISCUSSION
Survival of single, dual and triple inoculants in talc + 10 % vermicompost formulation
Among single, dual and triple inoculants of talc + 10 % vermicompost formulation, survival of microbial inoculants was found better in triple inoculants compared to single and dual inoculants. Initial 90 days of storage, population of viable cells increased in single, dual and triple inoculants. At the end of 360 days, population of Bradyrhizobium japonicum (log10 6.98 cells/ g), Pseudomonas fluorescens (log10 6.97 cells/ g) and Bacillus megaterium (log10 6.98 cells/ g) were recorded in single inoculant formulation. Dual inoculants containing Bradyrhizobium japonicum + Pseudomonas fluorescens recorded (log10 6.96 and 7.04 cells/ g), Bradyrhizobium japonicum + Bacillus megaterium (log10 6.78 and 6.69 cells/ g) and Pseudomonas fluorescens + Bacillus megaterium (log10 6.84 and 6.98 cells/ g) respectively. Population of Bradyrhizobium japonicum, Pseudomonas fluorescens and Bacillus megaterium was log10 6.30, 6.45 and 6.31 cells/ g respectively in triple inoculants at the end of 360 days (Fig 1). The per cent reduction of cells on log values was found to be 80.60, 82.56 and 80.10 per cent in triple inoculants of B. japonicum, P. fluorescens and B. megaterium respectively (Table 1).
Table 1: Per cent survival of microbial inoculants in talc + 10 % vermicompost based formulations
Note: Means with same superscript are statistically on par at P £ 0.01 by DMRT
Bj: Bradyrhizobium japonicum, Pf: Pseudomonas fluorescens, Bm: Bacillus megaterium
Table 2: Per cent survival of microbial inoculants in talc based formulation
Note: Means with same superscript are statistically on par at P £ 0.01 by DMRT
Bj: Bradyrhizobium japonicum, Pf: Pseudomonas fluorescens, Bm: Bacillus megaterium
Table 3: Per cent survival of microbial inoculants in talc + 10 % urbancompost based formulation
Note: Means with same superscript are statistically on par at P £ 0.01 by DMRT
Bj: Bradyrhizobium japonicum, Pf: Pseudomonas fluorescens, Bm: Bacillus megaterium.
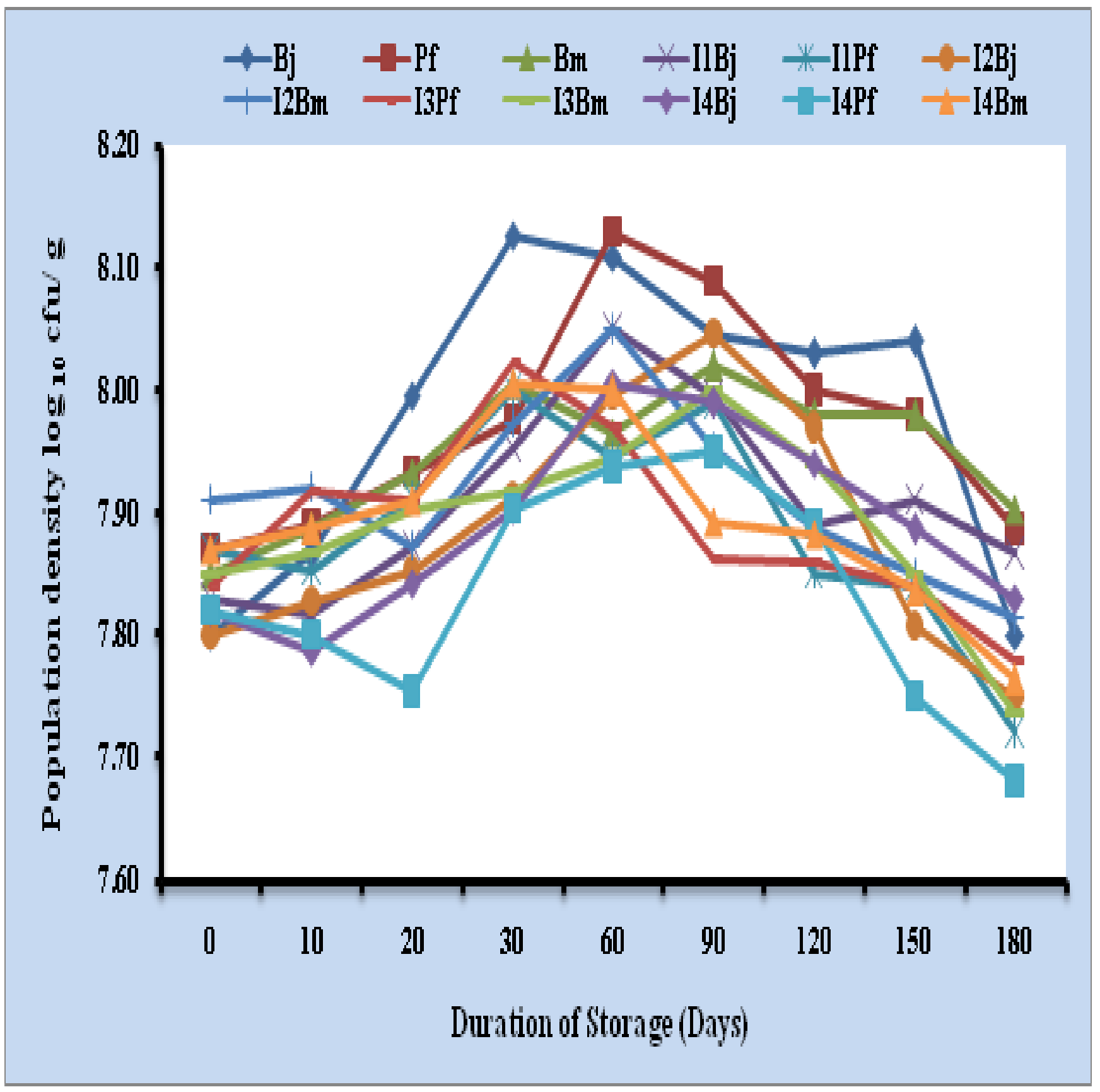
Fig. 1: Survival of microbial inoculants in talc + 10 per cent vermicompost based formulation upto 180 days
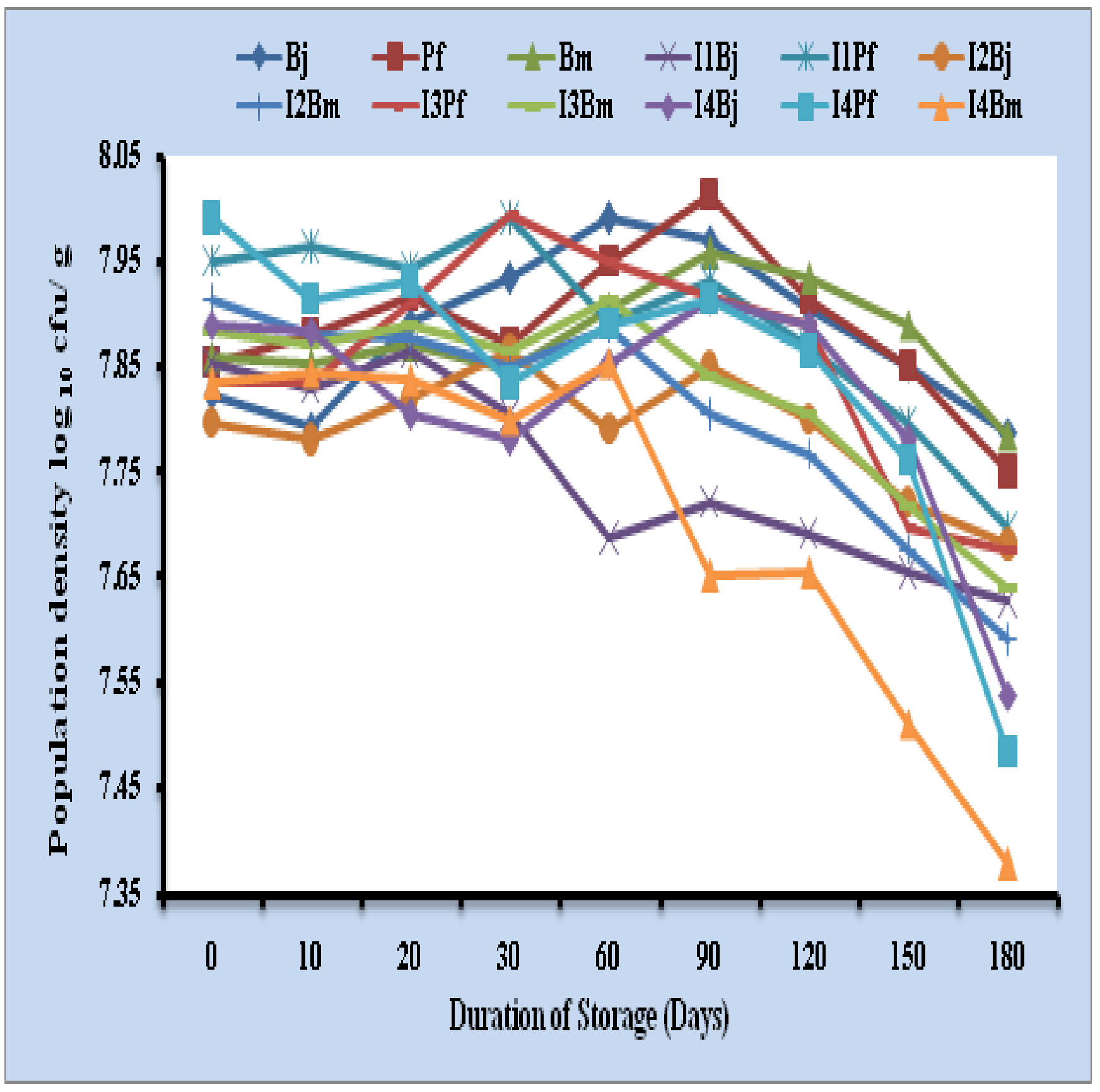
Fig. 2: Survival of microbial inoculants in talc based formulation upto 180 days
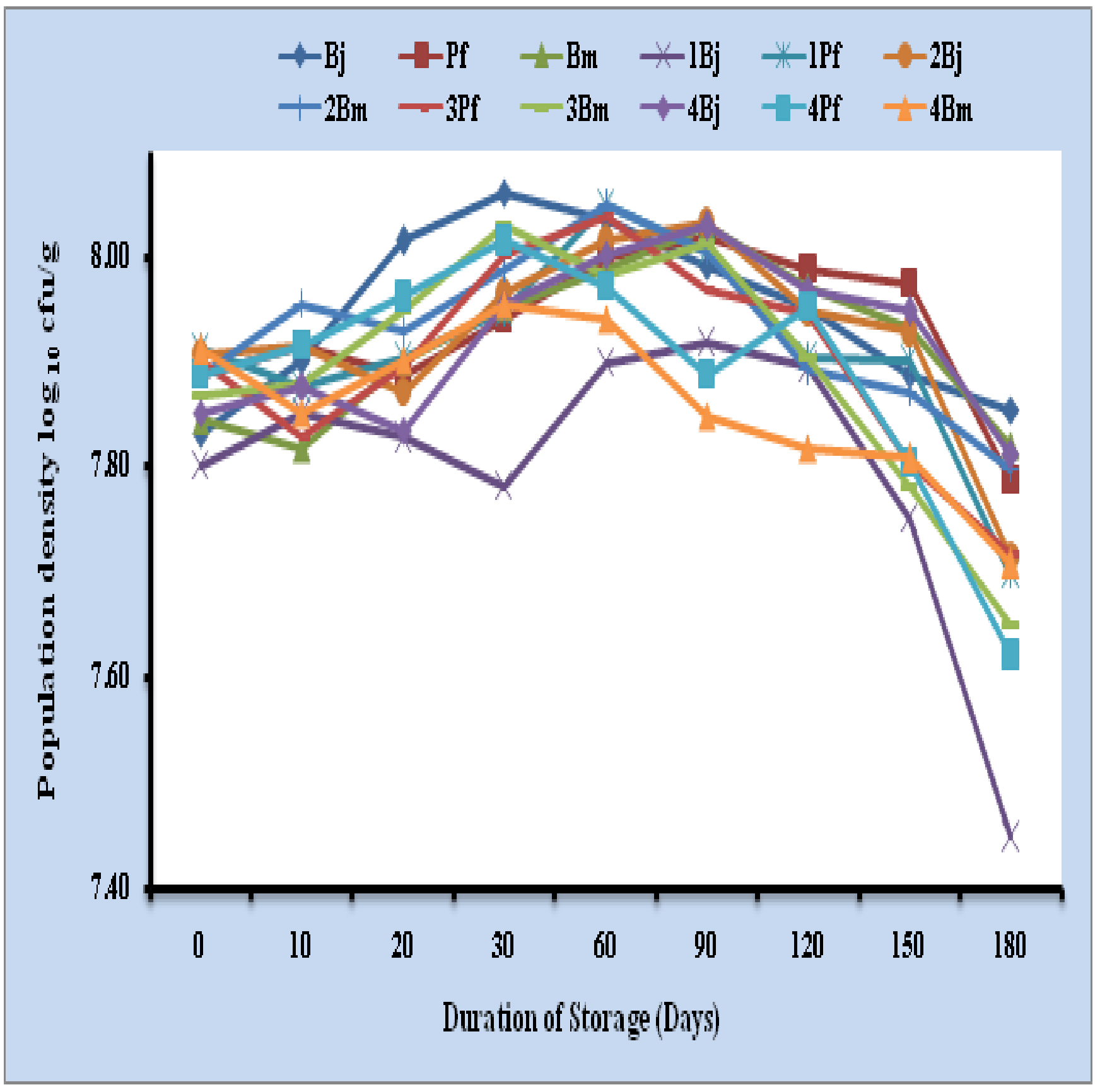
Fig. 3: Survival of microbial inoculants in talc + 10 per cent urban compost based formulation upto 180 days
Bj: Bradyrhizobium japonicum, Pf: Pseudomonas fluorescens, Bm: Bacillus megaterium, I: Inoculant
Survival of single, dual and triple inoculants in talc formulation
Talc formulation maintained population density of single inoculants of Bradyrhizobium japonicum, Pseudomonas fluorescens and Bacillus megaterium (log10 6.94, 6.82 and 6.64 cells/ g respectively) at the end of storage period. Consortium containing Bradyrhizobium japonicum + Pseudomonas fluorescens, Bradyrhizobium japonicum + Bacillus megaterium and Pseudomonas fluorescens + Bacillus megaterium maintained population of (log10 6.91 and 6.98 cells/ g), (log10 6.45 and 6.49 cells/ g) and (log10 6.78 and 6.81 cells/ g) respectively and triple inoculants maintained log10 5.95, 6.13 and 6.03 cells/ g of Bradyrhizobium japonicum, Pseudomonas fluorescens and Bacillus megaterium respectively at the end of 360 days (Fig 2). Maximum per cent survival was observed to be 75.45, 76.73 and 76.94 in B. japonicum, P. fluorescens and B. megaterium in triple inoculants respectively at 360 days (Table 2).
Survival of single, dual and triple inoculants in talc + 10 % urban compost formulation
Viable cells of Bradyrhizobium japonicum (log10 6.15 cells/ g), Pseudomonas fluorescens (log10 6.25 cells/ g) and Bacillus megaterium (log10 6.17 cells/ g) were noticed in triple inoculants of talc + 10 % urban compost formulation where as single inoculants of Bradyrhizobium japonicum (log10 6.96 cells/ g), Pseudomonas fluorescens (log10 6.93 cells/ g) and Bacillus megaterium (log10 6.91 cells/ g) at the end of 360 days. Consortium of Bradyrhizobium japonicum + Pseudomonas fluorescens (log10 6.98 and 7.00 cells/ g), Bradyrhizobium japonicum + Bacillus megaterium (log10 6.68 and 6.56 cells/ g) and Pseudomonas fluorescens + Bacillus megaterium (log10 6.76 and 6.85 cells/ g) were maintained at the end of storage period (Fig 3). 78.39, 79.46 and 78.04 per cent cells were recorded at the end of 360 days in triple inoculants of B. japonicum, P. fluorescens and B. megaterium respectively (Table 3).
The investigation aimed on two different aspects, one is carrier based formulation and another was consortium development. The survival of any microbial inoculants mainly depends on the carrier material. Carrier materials used in the study satisfies the essential criteria of ideal carrier such as high water holding capacity, rich organic matter content, neutral pH, easy availability and low cost (Arangarasan et al., 1998). The maximum viable cells of Bradyrhizobium japonicum, Pseudomonas fluorescens and Bacillus megaterium in single, dual and triple inoculants were maintained in talc amended with vemicompost and urbancompost formulation compared to talc formulation. This may be due to high nutrient content present in organic materials supported the survival of microbial inoculants during storage period. Similar results were obtained by Rajasekar and Karmegam (2010) reported more than 1 X 107 viable cells/ g (Azotobacter chroococcun, Bacillus megaterium and Rhizobium leguminosarum) observed in different combination of vermicast with lignite upto 10 months. Survival of Pseudomonas fluorescens, Bacillus subtilis, Azospirillum brasilense were reported in different organic carriers like vermicompost, spent mushroom, farm yard manure, pressmud, rice bran (Karunya and Reetha, 2014; Gade et al., 2014 and Singh et al., 2014).
Highest survival rate of microbial inoculants were found in triple inoculants in all the developed carrier based formulations. The significant difference in survival was mainly due to synergistic effect of the inoculants on each other. The factors that affect the longevity of the cells of microbial inoculants include temperature, moisture and carrier materials. The result of the study is on par with the findings of Sahu et al., 2013 and Lavanya et al., 2015, reported triple inoculant formulation maintained maximum population of Acinetobacter sp., Pseudomonas fluorescens, Bacillus megaterium and Azotobacter chroococcum in consortium.
Commonly used carrier for microbial inoculants are peat, lignite, talc etc., which support the growth of microbial inoculants. Use of organic materials like vermicompost, urban compost as carrier increases the survival rate of microbial inoculants in biofertilizer formulation by providing nutrients for growth and metabolic activities of microbial inoculants during storage. The study indicated that the use of organic materials as amendment with talc was found to be successful in maintaining the viable cells of microbial inoculants in consortium for longer period of time which could meet the Bureau of Indian Standards and also possible delivery of microbial consortium in a single carrier. Hence organic materials can be used as amendment with talc which can be an efficient carrier for development of microbial inoculant formulation.
REFERNCES
- Arangarasan, V., Palaniappan, S.P., Chelliah, S. Inoculation effects of diazotrophs and phosphobacteria on rice. Indian J. Microbiol., 1998; 38(2): 111-112.
- Gade, R.M., Chaithanya, B.H., Khurade, K.C. A comparative study of different carriers for shelf life of Pseudomonas fluorescens. The Bioscan, 2014; 9(1): 287-290.
- Karunya, S.K., Reetha, D. Survival of saline tolerant PGPR in different carriers and liquid formulations. Int. J. Adv. Res. Biol. Sci., 2014; 1: 179-183.
- Lavanya, G., Sahu, P.K., Manikanta, D.S., Brahmaprakash, G.P. Effect of fluid bed dried formulation in comparison with lignite formulation of microbial consortium on finger millet (Eleusine coracona Gaertn.). J. Pure Appl. Microbiol., 2015; 9(2): 193-199.
- Little, T.M., Hills, J.F. Agricultural experimentation. John Wiley and sons, New York, USA. 1978.
- Mohammadi, K., Sohrabi, Y. Bacterial biofertilizer for sustainable crop production, A review. J. Agril. Biol. Sci., 2012; 7: 2.
- Mugnier, J., Jung. G. Survival of bacteria and fungi in relation to water activity and the solvent properties of water in biopolymer. Appl. Environ. Microbiol., 1985; 50: 108-114.
- Rajasekar, K., Karmegam, N. Earthworm casts as an alternate carrier material for biofertilizers: Assessment of endurance and viability of Azotobacter chroococcum, Bacillus megaterium and Rhizobium leguminosarum. Scientia Horticulturae., 2010; 124: 286–289.
- Roy, B.D., Deb, B., Sharma, G.D. Evaluation of carrier based inoculants of Azotobacter Chroococcun strain SDSA-112/2 in improving growth and yield of summer rice. IR-36. Biofrent, 2010; 1: 36-40.
- Sahu, P.K., Lavanya, G., Brahmaprakash, G.P. Fluid bed dried microbial inoculants formulation with improved survival and reduced contamination level. J. Soil Biol. Ecol., 2013; 33(1-2): 81-94.
- Singh, S., Govind Gupta, Ekta Khare, Behal, K.K., Naveen, K.A. Effect of enrichment material on the shelf life and field efficiency of bioformulation of Rhizobium sp. and P-solubilizing Pseudomonas fluorescens. Sci. Res. Reporter, 2014; 4(1): 44-50.
- Smith, R.S. Legume inoculant formulation and application. Can. J. Microbiol., 1992; 38: 485-492.

