Strongyloidiasis is a neglected parasitic disease caused by the intestinal parasite, Strongyloides stercoralis. Most patients with strongyloidiasis are asymptomatic, but few present with varied clinical manifestations such as cutaneous, gastrointestinal, pulmonary, and disseminated disease. It creates a diagnostic dilemma and undue delay in the diagnosis of patients. We report the case of a 79-year-old male who presented with fever and abdominal pain due to strongyloidiasis with no history of immunosuppression. The infection resolved entirely on treatment with ivermectin.
Strongyloides Stercoralis, Immunocompetent Individual, Ivermectin, Harada Mori Technique, Rhabditiform Larvae, Stool Examination
Strongyloides stercoralis is an intestinal parasite, endemic in tropical and subtropical countries. The two known reported pathogenic species in humans are S. stercoralis and S. fuelleborni.1 Strongyloides spp frequently cause asymptomatic infections in humans and hence remains unnoticed. The presence of larvae in stool and eosinophilia are the only indications of this disease.2 The parasite is capable to cause autoinfection in the host. Complications of this infestation such as hyperinfection and disseminated disease, are seen in patients with co-morbidities such as diabetes mellitus, chronic obstructive pulmonary disease, and malnutrition. Walking with bare feet, contact with human waste and sewage, and occupation that increases the exposure to contaminated soil like farming and coal mining are common risk factors to acquire Strongyloides stercoralis infection. Previous studies have found an association between strongyloidiasis and Human T cell lymphotropic Virus-1(HTLV-1) infection. Patients with Human Immunodeficiency Virus(HIV) co-infection are documented to have poor clinical outcome.3
The life cycle of Strongyloides spp is complex. It exists in three forms: adult, larvae (4 stages), and egg. It involves only one host, humans. L3 (filariform larvae) is the infective form. It is transmitted to humans through penetration of the skin by L3 larvae. It travels to subcutaneous tissue and is carried to the lungs through venous circulation. They reach the alveolar space of the lungs and migrate to the pharynx and enter the gastrointestinal tract. In the intestine, female worms lay eggs without fertilization. This process is called parthenogenesis. These eggs hatch out immediately, releasing the rhabditiform L1 larva. This L1 larva is the diagnostic form.4 The clinical manifestations range from asymptomatic to mild symptoms to life-threatening infections. The acute infection is manifested by gastrointestinal symptoms (abdominal pain, vomiting, diarrhea) and pulmonary symptoms (productive cough due to migrating larva, dyspnoea). The chronic infection is presented by larva currens, an intensely pruritic urticarial rash. The hyperinfection is due to the disseminated strongyloidiasis seen in patients with co-morbidities.5
The laboratory diagnosis involves the examination of the stool specimen for the rhabditiform larvae. It is demonstrated by the saline mount and iodine mount of a stool sample (WHO). Serological diagnosis is available like radioallergosorbent assay for specific immunoglobulin E is usually elevated6 and enzyme-linked immunosorbent assay (ELISA for IgG antibodies.7 Strongyloidiasis is challenging to treat. Specific therapy is effective when an intestinal infection was diagnosed by stool examination. The recommendation given by CDC, for first-line treatment is oral administration of ivermectin 200 μg/kg for 1-2 days and the alternate being oral administration of twice a day albendazole 400mg for one week.8 After treatment, follow-up stool examination is done at 6 and 12 months to prove the absence of larva.9 For hyper infection, oral administration of ivermectin 200 μg/kg was given for two weeks and until the negative findings in stool & sputum specimens.10
Case report
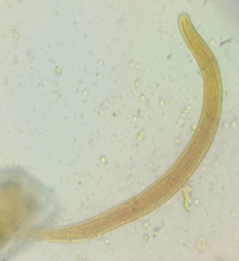
A 74-year-old male presented with a history of fever for three days and abdominal pain for three days. The patient was a known case of chronic obstructive pulmonary disease. He was evaluated for severe acute respiratory syndrome due to Coronavirus 2(SARS CoV2) infection by real-time PCR and was found to be negative. His routine laboratory investigations, including complete blood count, were normal, including eosinophil count. He was advised to send a stool sample for examination of ova and cysts. Stool examination of the saline and iodine mount showed multiple live larvae. The larva was identified as rhabditiform L1 larva of Strongyloides spp based on the short buccal cavity, bulbed esophagus, and measuring approximately 250 µm long (Figure). The patient was advised to provide additional stool samples on consecutive days which also demonstrated the rhabditiform L1 larva of Strongyloides spp.
The result was confirmed using the Harada Mori technique. Harada Mori filter paper technique was performed and rhabditiform L1 larva was observed by the seventh day of culture. The patient was treated with ivermectin, 200 mg/kg/day for seven days, inspite of the CDC recommended two days of treatment. Many systemic reviews as well as metaanalysis showed inconclusive results of using ivermectin in treatment of COVID-19. Manu et al., stated from the metaanalysis of eighteen randomised control trials that ivermectin can be used as a prophylaxis and treatment for COVID-19, which further reduces the morbidity and mortality among COVID-19 patients.11 Ivermectin has both antiviral and anti-parasitic properties, since patient was admitted as suspected COVID-19 he was treated for seven days.12 Follow-up stool examination after six months of therapy was negative for Strongyloides stercoralis.
Strongyloidiasis is caused by the intestinal threadworm Strongyloides stercoralis. It is an opportunistic infection seen in men, dogs, and cats. It can be transmitted from the animals to man and vice versa. The mortality rate of strongyloidiasis is 60-85% in patients with poor immune responses. Almost 100 million people are affected by strongyloidiasis worldwide. 16.7% worldwide require hospitalization due to strongyloidiasis. The disease is frequently seen in tropical and subtropical countries such as South East Asia, Central, and South America, and Sub-Saharan Africa. People living in low socio-economic groups, rural and institutional settings, farmers and coal miners are more exposed to this parasite. Poor personal hygiene and deplorable sanitary measures further add to the risk of the acquisition of the infection. The infection occurs in all seasons of the year. Previous studies indicate an association between Strongyloides stercoralis infection and diabetes. We report here a case of intestinal strongyloidiasis in an elderly male with no history of immunocompromised status.13
The lifecycle of strongyloidiasis alternates between the free-living cycle and parasitic cycle. The parasite enters the human body by penetration of the skin. The larvae of the parasite are seen in the stool of patients infected with the disease within one month of infection. The organs majorly affected by the parasite are the skin, intestine, and lungs. The clinical manifestation of the disease has varied presentations from asymptomatic individuals to those with multiorgan failure. Hyperinfection with Strongyloides stercoralis is seen in immunocompromised individuals. In a rare occurrence, larvae of Strongyloides stercoralis have been identified in the urine of a patient.14 Our patient presented with intestinal strongyloidiasis. Larvae of Strongyloides stercoralis were isolated multiple times from the patient’s stool. The larva was identified as rhabditiform L1 larva of Strongyloides spp. The parasite measured 250 µm in length and had a short buccal cavity and bulbed esophagus.
Eosinophilia is frequently seen in patients infected by Strongyloides stercoralis and can be used for screening for chronic strongyloidiasis. Serum IgE is elevated in patients with this disease. Feces of patients with this disease contains larvae of the parasite rather than ova. The larvae are released in the stool intermittently, and hence multiple stool examination is required to rule out the disease. A previous study indicates seven consecutive stool examinations for maximum detection of the parasite. Stimulation of secretion of the parasite can be done by the administering of oral albendazole. Fecal concentration techniques such as the formalin ether sedimentation technique enhance the detection of the parasite from feces. Strongyloides stercoralis larvae were isolated from multiple stool examinations in our patient.15
The Baermann culture method increases the detection rate of Strongyloides stercoralis by over four times when compared to direct fecal microscopy and fecal concentration techniques. The disadvantage of this method is the requirement of freshly passed stool samples. The Baermann culture method has better detection rates compared to the agar plate culture method or the Harada Mori filter paper technique.16 The water emergence method, where a stool sample is added to an agar plate with a central depression containing water and incubated at 37°C for one hour, also provides a good yield of larvae (sensitivity of 85%) for detection of the parasite. The above techniques use the principle of water tropism of larvae for the detection of the parasite. The charcoal culture method is beneficial for detection of parasites in remote areas. The diagnosis of Strongyloides stercoralis infection was confirmed in our patient using the Harada Mori technique. The larvae were identified in the water after the seventh day of inoculation onto the filter paper.
Serological tests such as enzyme-linked immunosorbent assay(ELISA) and indirect immunofluorescence microscopy(IFAT) can be used to diagnose the disease. Previous studies indicate ELISA has better sensitivity(93.5%) compared to IFAT(85%). The disadvantage of serological tests is their cross-reactivity with other parasitic infections such as filaria. The latest advancement in this field is the development of the luciferase immunoprecipitation system(LIPS) to identify Strongyloides sterocoralis antibodies. The test uses recombinant Strongyloides spp antigen which has a sensitivity of 97% and specificity of 100% when compared to ELISA. The test has a rapid turnaround time of less than 2.5 hours. This test is not currently available in the majority of laboratories. ELISA can also be used for coproantigen detection in fecal samples. Molecular detection for Strongyloides sterocoralis can be done with real-time polymerase chain reaction(RT PCR) using 18S rRNA, 28S RNA, cytochrome c oxidase subunit I as targets. These tests have 100% specificity and 100% sensitivity when compared to the agar culture method. The amount of parasite-specific DNA in the stool sample is in direct correlation with the immune response of the host and the intensity of the infection.17
Treatment must be provided for all clinical manifestations of strongyloidiasis to prevent hyperinfection and disseminated disease. Ivermectin and albendazole are used in chemotherapy. Pregnancy and lactation are contraindications for therapy. Our patient was treated with ivermectin for 7 days. Follow-up stool sample taken after therapy was negative for larvae of Strongyloides stercoralis.
It is essential to evaluate the patient after chemotherapy for Strongyloides stercoralis infection to confirm the eradication of the disease. This is due to the risk of autoinfection in strongyloidiasis. Baermann technique or Agar plate culture technique should preferentially be used for follow-up due to their high sensitivity compared to the other tests. Follow-up for strongyloidiasis must be done using multiple stool sample examination over one year. Serological tests are not useful for follow-up as they continue to remain positive for an extended duration after the eradication of the parasite. However, antibody ratio <0.6 (antibody level after treatment divided by initial antibody level before therapy) is considered as a cure for the disease.18
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Institutional Ethics Committee, SRM Medical College Hospital & Research Centre, India with reference number 2895/IEC/2021.
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- Arsic-Arsenijevic V, Dzamic A, Dzamic Z, Milobratovic D, Tomic D. Fatal Strongyloides stercoralis infection in a young woman with lupus glomerulonephritis. J Nephrol. 2005;18(6):787-790. PMID: 16358242
- Dalela G, Sabharwa ER, Mehta P. Strongyloides Stercoralis Infection in an Immunocompetent Patient Presenting with Shock. National Journal of Laboratory Medicine. 2012;1(1):38-40.
- Krolewiecki AJ, Lammie P, Jacobson J, et al. A public health response against Strongyloides stercoralis: time to look at soil-transmitted helminthiasis in full. PLoS Negl Trop Dis. 2013;7(5):e2165.
Crossref - Olsen A, van Lieshout L, Marti H, et al. Strongyloidiasis–the most neglected of the neglected tropical diseases? Trans R Soc Trop Med Hyg. 2009;103(10):967-972.
Crossref - Toledo R, Munoz-Antoli C, Esteban JG. Strongyloidiasis with emphasis on human infections and its different clinical forms. Adv Parasitol. 2015;88:165-241.
Crossref - Peeling RW, Smith PG, Bossuyt PMM. A guide for diagnostic evaluations. Nat Rev Microbiol. 2006;4(9 Suppl):S2-S6.
Crossref - Buonfrate D, Formenti F, Perandin F, Bisoffi Z. Novel approaches to the diagnosis of Strongyloides stercoralis infection. Clin Microbiol Infect. 2015;21(6):543-552.
Crossref - Henriquez-Camacho C, Gotuzzo E, Echevarria J, et al. Ivermectin versus albendazole or thiabendazole for Strongyloides stercoralis infection. Cochrane Database Syst Rev. 2016;2016(1):CD007745.
Crossref - Biggs BA, Caruana S, Mihrshahi S, et al. Management of chronic strongyloidiasis in immigrants and refugees: is serologic testing useful? Am J Trop Med Hyg. 2009;80(5):788-791. PMID: 19407125
- CDC. Parasites – Strongyloides: Resources for Health Professionals. Centers for Disease Control and Prevention. 2018. https://www.cdc.gov/parasites/strongyloides/health_professionals/index.html#tx. Accessed: February 16, 2022
- Chaccour C, Casellas A, Blanco-Di Matteo A, et al. The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: A pilot, double-blind, placebo-controlled, randomized clinical trial. EClinical Medicine. 2021;32:100720.
Crossref - Manu P. Expression of Concern for Kory P, Meduri GU, Varon J, Iglesias J, Marik PE. Review of the Emerging Evidence Demonstrating the Efficacy of Ivermectin in the Prophylaxis and Treatment of COVID-19. Am J Ther. 2021;28(3):e299-e318. Am J Ther. 2022;29(2):e231.
Crossref - Requena-Mendez A, Chiodini P, Bisoffi Z, Buonfrate D, Gotuzzo E, Munoz J. The laboratory diagnosis and follow up of strongyloidiasis: a systematic review. PLoS Negl Trop Dis. 2013;7(1):e2002.
Crossref - Tachamo N, Nazir S, Lohani S, Karmacharya P. Strongyloidiasis in the immunocompetent: an overlooked infection. J Community Hosp Intern Med Perspect. 2016;6(4):32038.
Crossref - Greaves D, Coggle S, Pollard C, Aliyu SH, Moore EM. Strongyloides stercoralis infection. BMJ. 2013;347:f4610.
Crossref - Khanna V, Gopinathan A, Manjunatha HH, Vishwanath S, Mukhopadhyay C. Strongyloides stercoralis Hyperinfection Presenting as Subacute Intestinal Obstruction. Int J Sci Res. 213;2(9):352-53.
Crossref - Balachandra D, Ahmad H, Arifin N, Noordin R. Direct detection of Strongyloides infection via molecular and antigen detection methods. Eur J Clin Microbiol Infect Dis. 2021;40(1):27-37.
Crossref - Nutman TB. Human infection with Strongyloides stercoralis and other related Strongyloides species. Parasitology. 2017;144(3):263-273.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.