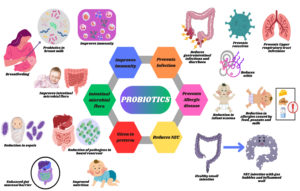
The breast milk microbiome has emerged as an essential determinant of infant health, influencing gut microbiota composition, immune system development, and overall regulation of physiological maturation. This review focuses on a comprehensive analysis of breast milk microbiota and its contributions in shaping infant health. Additionally, the potential of probiotics in establishing a microbial equilibrium to improve gut microbiota and immunity in both preterm and full-term infants is discussed in detail. The first encounter with probiotics in the human body occurs during infancy through breast milk. Complete breastfeeding stimulates the growth and development of beneficial probiotics such as Saccharomyces boulardii, Lactobacillus, Streptococcus, Enterococcus, and Bifidobacterium which play crucial roles in preventing conditions such as allergies, microbial infections, gastric and intestinal infections, urinary tract infections, necrotizing enterocolitis, and dermal infections. Most of these probiotics act as bacterial inhibitors by reducing pH levels, whereas some have specific cells that trigger immune cells to reduce infections. Thus, probiotics offer promising therapeutic effects for regulating overall infant health. This review emphasizes the importance of probiotic-based interventions in optimizing infant health outcomes.
Breast Milk Microbiome, Infant Health, Probiotics, Gut Microbiota, Immune Development
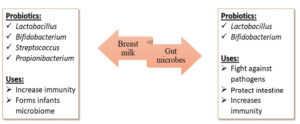
Consumption of probiotics has been practiced since ancient times, involving a wide variety of bacteria, including lactic acid bacteria and bifidobacteria.1 Lactic acid bacteria are present in human milk and are recognized for their probiotic potential.2 When supplemented in sufficient proportions, probiotic microorganisms benefit the health of the host by establishing their niche primarily in the gut.3 Immunoprotection against pathogens, infections, and metabolic disorders, achieved by boosting immunity, is the prime reason for their reception in the field of immunobiology.4 An increase in gut microbiota can enhance host immunity by reducing pathogen growth and stimulating the production of inhibitory compounds such as fatty acids, organic acids, aldehydes, acetyl groups, macromolecules, and bacteriocins.4 These compounds aid in pathogen limitation through competitive inhibition. Infants and newborns benefit from nutrient-rich breast milk, which comprises highly concentrated probiotics that shield the body from various intestinal and bacterial infections, allergies, and eczema.5 As colostrum contains fats, proteins, carbohydrates, vitamins, minerals, probiotics, macromolecules, and immunity-boosting compounds such as IgA, leukocytes, and lactoferrin, it promotes infant immunity and overall health; therefore, breastfeeding is advisable for six months.6,7 It also modulates the risk of weight gain, obesity, and diabetes by reducing insulin levels.5 Proteins in milk, along with the optimal intake of early proteins in colostrum, also prevent childhood obesity.8 Infants with low birth weight and weakened immunity are more prone to infections caused by bacteria, fungi, and parasites.9 According to the World Health Organization (WHO), these infections affect the human gastrointestinal and respiratory tracts causing pneumonia and sepsis. Hence, the consumption of breast milk increases immunity owing to the presence of antibodies (Figure 1). Notable probiotics found in breast milk include Bifidobacterium, Propionibacterium, Saccharomyces, Sphingomonas, and Lactobacillus, which aid in boosting immunity and fighting infections.10,11
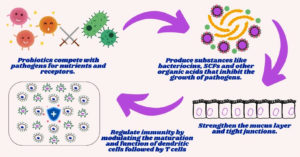
Bifidobacterium and Lactobacillus are the dominant genera found in breast milk that produce microbial metabolites such as lactate and short-chain fatty acids. Lactic acid and bacteriocins secreted by probiotic bacteria function as bacterial inhibitors by reducing pH levels and inhibiting pathogen growth.12 Probiotics also induce Toll-like Receptors (TLRs), which in turn stimulate IL-6 and IgA-like mediators. Some probiotics also have anti-inflammatory properties that inhibit TLR4 activity and reduce intestinal permeability.12,13 Probiotics stimulates the intestinal barrier immune response by modulating Th1 and Th2 activity, promoting tolerogenic dendritic cells and regulatory T cells (Treg), while suppressing Th17 cells. Lactobacillus GG signals gut-associated lymphoid tissue (GALT) and Tregs, which act as intestinal barriers and reduce allergic reactions.13
Probiotics in breast milk
Breast milk supplemented for newborns comprises a unique combination of macromolecules and essential nutrients that ensure the average growth and development of the baby.14,15 It also contains various bioactive compounds responsible for numerous health benefits, such as immune system maturation and protection against infections. Isolation of probiotics with beneficial effects on the host provides scientific support for incorporating these bacteria into infant formula at levels of 104-106 per day.16 Breastfed newborns have better gut microbiota, as human milk contains lactic acid bacteria and other bifidogenic substances such as oligosaccharides.17,18 Under normal conditions, a newborn’s aseptic gut becomes initially colonized during delivery when exposed to the mother’s vaginal flora and the usual flora of the parents. As a result, various Bifidobacteria, Enterobacteria, Bacteroides, Clostridia, and Gram-positive cocci become inoculated.19,20 Following the initial colonization, the flora quickly changes and becomes nourished. Exclusively breastfed infants have Bifidobacteria-dominated gut microbiome within a few weeks, presumably because of the selection of agents (Bifidobacterial factors) found in human milk. The microbiome becomes more diverse and starts to resemble that of an adult after weaning. Therefore, breastfeeding and the probiotics present in breast milk are significant determinants of intestinal colonization in infants, among other factors.15 In contrast, infants fed with formula develop a more varied gut flora that includes Bacteroides, Enterobacteria, Enterococci, and Clostridia, in addition to Bifidobacteria.21,22 The growth of pathogenic microorganisms such as Staphylococcus aureus, Salmonella typhimurium, Yersinia enterocolitica, and Clostridium perfringens is known to be inhibited by Lactobacilli and Bifidobacteria (Figure 2). These bacteria aggressively colonize the bowel of a child and inhibit pathogen attachment. In addition to gastrointestinal benefits, microbiome modification with beneficial bacteria has been shown to modulate immune function and enhance defense against intestinal pathogens. Heikkila et al. has reported that the bacteria in human breast milk protects both the mother and the newborn from several infections caused by Staphylococcus aureus Cutibacterium, Enterococcus, and Veillonella.15,16 Probiotic bacteria such as L. gasseri CECT571423, L. salivarius CECT571324, and L. fermentum CECT571623, L. reuteri ATCC55730, L. rhamnosus LGG, L. gasseri CECT5714, L. salivarius CECT5713, and L. fermentum CECT5714 isolated from human breastmilk contains potential bacteria that are required for infant gut development.21
An imbalance in the composition of the gut microbiota and mammary glands is reported to cause many problems such as mastitis and atopic dermatitis in the mother, diarrhea, and colic in the newborn.14 Randomized controlled studies indicate that infant-specific lactobacilli supplementation may reduce the risk of nonspecific gastrointestinal infections or decrease colic and fussiness. However, adequate prospective randomized double-blind controlled studies should be conducted with structured clinical questions related to the study population, type, duration of intervention, and type of comparison. Two European research teams independently established the presence of lactic acid bacteria L. gasseri and E. faecium in human milk and explored their probiotic potential.18-21 Accordingly, Heikkila et al. reported that these human milk bacteria shield both mothers and newborns from Staphylococcus aureus infections.19,21 Martin et al. isolated lactic acid bacteria from human milk, specifically L. gasseri CECT5714, L. salivarius CECT5713, and L. fermentum CECT5716, along with other strains, and observed that breast milk provided immunity against infections. According to Bjorksten et al. and Lara-Villoslada et al., variations in the composition of the intestinal microbiota affect the frequency of pathologies, such as allergic and inflammatory conditions.19,22 The Hygiene hypothesis and the TH1/TH2 balance could be used to explain the anti-allergic effects of probiotics. Probiotics cause a TH1 response, which inhibits the synthesis of TH2 cytokines that cause allergic reactions. According to previous in vitro studies, the immunomodulatory effects of probiotics depend on the cell environment.19,22 In the absence of additional stimuli, the TH1 cytokines IL-2 and IL-12 and the inflammatory mediator tumor necrosis factor were produced more in the presence of the probiotics L. fermentum CECT5716 and L. salivarius CECT5713 which are present in breast milk. Probiotic strains such as L. fermentum CECT5716 and L. salivarius CECT5713 stimulate the production of the immunosuppressive cytokine IL-10, which is the basis of the regulatory mechanism. This immunostimulatory action may also play a role in the anti-infective function of animal models of Salmonella infection.22,23 Administration of L. gasseri CECT5714 and L. coryniformis CECT5711 diminished the frequency and intensity of allergic responses.19,24 In a recent study, L. fermentum CECT5716 reduced intestinal damage and inflammation in an animal model of inflammatory bowel disease.19,25 Infectious diseases such as gastroenterocolitis and respiratory infections are a significant cause of death in the pediatric age group in developing countries. Especially in newborns who had not been nursed for at least 13 weeks, a 17-fold increase in the risk of pneumonia was observed.19,26,27 Weaned infants have a 14.2-fold higher risk of dying from diarrhea.19,28 Administration of breast milk to children up to six months of age decreased acute urinary infections in infants.19,29
The preservation of immune system homeostasis is also significantly influenced by commensal bacterial colonization of the intestinal tract.19,30 These bacteria promote TH1 responses and counteract the tendency of the newborn immune system towards TH2.19,31 Lactobacillus rhamnosus LGG supplementation in babies lower the risk of atopic symptoms and inflammatory conditions that requires TH2 response, such as necrotizing enterocolitis. Dietary intake is one of the most important factors influencing the gut microbiome of a baby.16,32 The Enterobacteriaceae family, which includes the genera Staphylococcus, Streptococcus, Bifidobacterium, Bacteroides, Clostridium, Eubacterium, and Enterococcus, is among the first facultative anaerobes to colonize the baby gut.30,33 Before weaning, the newborn gut was dominated by Bifidobacterium species such as B. longum, B. breve, and B. bifidum, in contrast to the mature gut microbiota.30,34-36 The milk microbiome may contain novel species that influence the composition of the gut microbiota. Bifidobacterium sp., a predominant taxon in maternal milk, persists in infant feces.30,34,37-39
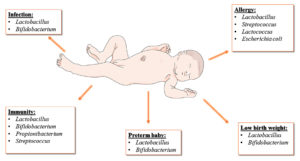
Probiotics given to infants to improve immunity and intestinal microbial flora
The innate and acquired immune systems function together to protect the host. The adaptive immune system remains untrained and undeveloped at birth. Therefore, intermediate-term neonates rely heavily on maternal IgG levels. Pregnancy-related Th1-polarizing cytokines are linked to an increased risk of abortion.40 Consequently, the method used to prevent adverse effects in the fetus involves the expression of Th2 cytokines. However, premature birth increases infants’ vulnerability to illnesses.39,41 Neutrophil transmigration is an essential step in immune cell recruitment to inflamed tissues. Lactoferrin, nucleotides, lymphocytes, maternal antibodies, and glycolytic enzymes contribute to the development of infant immunity. Probiotics such as Lactobacillus, Bifidobacterium, Propionibacterium and Streptococcus are the most preferred probiotics for infants that provide immunity against infections in the gut.42,43 Bifidobacterium are one of the primary species of human milk oligosaccharides (HMOs) that improve infant immunity.39,43 It is used to protect infants against microbial infection by cell signaling and cell-cell recognition, which enriches the microbial gut of infants by enhancing the adhesion of microbes.
Probiotics used to prevent infection in newborns
Newborns with immature immunity are prone to infection because their immunity is not fully developed. These conditions further lead to sepsis, which causes inflammation and affects blood circulation, leading to lower respiratory tract infections, culture-positive sepsis, and culture-negative sepsis.3 Bioactive molecules, including immune and non-immune cells, in breast milk contain substances such as cytokines, chemokines, and hormones that can assist in improving gut barriers and even promote immune defense against infections. Antibiotic treatment and the oral administration of live probiotics can prevent bacterial sepsis. Lactobacillus plantarum protects the gut barrier by enhancing epithelial defense and helps minimize the symptoms of diarrhea, umbilical stump infection, and pneumonia, thereby improving gut health, the immune system, and lung diseases.
According to Weizman et al, supplementation with probiotics, such as Lactobacillus reuteri and Bifidobacteria lactis, reduces the symptoms of diarrhea, fever, respiratory infection, pneumonia, and otitis by inducing the colonization and recruitment of immune cells. Improved humoral and cellular responses resolve intestinal problems such as constipation and diarrhea and gastrointestinal problems such as gastric infections. Induction of CD4+ T helper cells aides in improving gastric ailments.44-47 The daily intake of microbial probiotics (1.2 × 109 CFU/day) and oral administration of Lactobacilli (108-1010 CFU/day) were used to eliminate infections by increasing the expression of IgA-specific antibody-secreting cells.48 Lactobacillus GG and Lactobacillus reuteri reduced the duration of diarrhea; however, Lactobacillus GG showed better results in limiting gastrointestinal and upper respiratory tract infections.
Bifidobacteria supplementation prevents diarrhea caused by Salmonella sp., Enterobacter cloacae, E. coli, Klebsiella pneumoniae and viruses such as rotavirus.49 Administration of bifidobacterio species, such as Bifidobacterio animalis subsp. and Bifidobacterio longum along with Lactobacillus lactis and reuteri improved infant health by stimulating immunity.44,47,49 According to a study conducted by Di Gioia et al. A 28.3% to 11.9% reduction in the incidence of diarrhea was observed. Lactobacillus reuteri (ATCC 55730) prevents gas colic symptoms and maintains intestinal microbiota. Group B Streptococcus (GBS) ingested by women during pregnancy and labor can reach the amniotic system through the membrane and prevent GBS infection by Streptococcus agalactiae.49 A study conducted by Reid & Bruce on Lactobacillus sp, observed that Lactobacillus GG did not show any significant effect in women suffering from gastrointestinal infections, but if Lactobacillus reuteri RC-14 and Lactobacillus rhamnosus GR1-1 were supplemented with hydrogen peroxide, the risk of gastrointestinal diseases was limited compared to the groups supplemented with 6 × 109 CFU of placebo milk or milk supplements.45
Probiotics for infants to prevent allergic diseases
Food allergies are mainly caused by immunoglobulin E (IgE) antibodies, which manifest through skin and respiratory issues.43 Most common food allergies are due to the consumption of milk, eggs, and peanuts. Among one-year-old infants, 3% exhibit peanut allergy, 9% egg allergy, and 3% milk allergy. Allergy to cow milk is commonly observed and is substituted with amino acid formulas.50 Peanut allergy is significantly lower compared to milk allergy and egg allergy, as it has been noted that early introduction of peanuts can reduce the allergy up to 80% in 4 to 11 month old infants.51 Vitamin D plays an essential role in innate immunity and reduction of food allergies.43 Variability in vitamin D content in breast milk raises the requirement for additional dietary sources, such as vitamin D-fortified infant formula, along with exclusive breastfeeding. An increase in vitamin D concentration reduces inflammatory cytokine secretion.52,53 Supplementation with 5 × 109 CFU of L. rhamnosus GG (ATCC 53103) twice daily ameliorated atopic eczema/dermatitis syndrome.
Osborn and Sinn reported the protective effect of probiotics against eczema in children up to four years old. However, concerns about the non-existent use of allergens in infants suggest that more studies are needed to confirm these findings.49,54 Several studies have reported a significant reduction in infant eczema following supplementation with probiotics, including L. rhamnosus, B. breve, P. propionibacterium, and prebiotic galacto-oligosaccharides.54-56 Microbial species such as Propionibacterium, Streptococcus, Lactococcus, and Escherichia coli along with Lactobacillum and Bifidobacterium help to prevent allergic manifestations in infants born by caesarean section and those fed with standard formula.
Probiotics and birth weight in newborns
Incidences of necrotizing enterocolitis (NEC) account for 10%-50% of mortality in newborns. Since the major symptoms are associated with intestinal disorders, the potential of probiotics from breast milk has not been well explored. However, the determinants of NEC are primarily targeted in relation to an abnormal intestinal microbiome and pathogenesis. The occurrence of NEC-like intestinal injury observed in extremely low weight birth infants (ELBW) is mainly due to the loss of probiotic abundance. Breastfed infants, however, exhibit higher levels of Bifidobacterium species that resist inflammation by secreting short-chain fatty acids and activating cytoprotective genes. Al-Hosni et al. though supplementation of B. infantis and Lactobacillus GG, observed no relation with weight gain, even after 34 weeks, the supplemented probiotics enhanced the growth velocity in ELBW infants. Hartel et al. reported that B. infantis and L. acidophilus alleviated gastrointestinal problems but did not increase the weight of infants.27,57,58 Since low birth weight increases the risk of NEC and nosocomial infections, improving the weight of infants is crucial.59 However, feeding L. sporogenes at 35 × 107 CFU did not increase newborn weight or reduce NEC-related mortality.60,61 Rouge et al. conducted a double-blind, randomized experiment on oral supplements of B. longum BB536 and L. rhamnosus GG and observed that these probiotics did not increase gastrointestinal tolerance to enteral feeding among exceptionally very low birth weight infants, except for those weighing over 1000 g.62 While the use of BB536-LGG probiotics appeared safe in the short term, their long-term effects remain unexamined.
Probiotics given to preterm infants
The benefits of probiotic feeding in preterm infants include the reduction of pathogenic species in the bowel reservoir, improved enteral nutrition, reduced dependence on intravenous nutrition, strengthened gut mucosal barrier against bacteria and bacterial products, and upregulation of protective immunity. Probiotics improve nutrition, reduce the incidence of sepsis, and prevent neonatal NEC.63 According to Deshpande et al., Bifidobacteria and Lactobacilli are the preferred probiotic species. Lactobacilli, however, comprise only a small proportion of the gut microbiota. Bifidobacteria are the dominant strains in infancy; however, Lactobacilli and Bifidobacteria together exert a “bifidogenic effect”, encouraging the growth of endogenous lactic acid bacteria.64 This effect is caused by a fermentation process that produces short-chain fatty acids. Any probiotic strain must be administered at the appropriate mass or dose to live and colonize the gut.64 Research has shown that a dose of 106-107 CFU/g of probiotics can effectively colonize the infant gut.
Since preterm newborns must develop early commensal flora, probiotic supplementation should begin as soon as possible, before infections colonize or antibiotics eliminate existing commensals. Satoh et al. recorded the earliest start of probiotic supplementation at four hours after birth. To ensure that gut function recovers after the initial illness with minimal risk of intolerance or translocation, clinical stability is preferred.64,65 The shedding of probiotic organisms in stool often stops 2-3 weeks after supplementation is ceased. Hence, continuous dosing is required to encourage persistent colonization in preterm infants.64-66 Supplementation is stopped once the corrected gestational age of 36-37 weeks is reached.
Infection prevention
Infants with birth weights less than 1000 g are at more risk of NEC and gut infections; therefore, breastfeeding is essential. Practical issues are reported in babies with weak gut motility and poor intravenous feeding. In addition to theoretical disputes about the relationship between intravenous feeding and NEC, physicians use various strategies for early management of these diseases with the help of probiotics.63,67 A 24% reduction in mortality rate was observed (95% CI: 9-39% p < 0.01), and specific attributable mortality due to Staphylococcus epidermidis was 26.7% (95% CI: 23-30.4%; p = 0.01) in probiotic-supplemented individuals.45,68 Administration of Lactobacillus rhamnosus GG at 6 × 109 CFU inhibited pathogens that caused urinary tract infections and NEC. According to Kitajima, daily intake of Bifidobacterium breve at 0.5 × 109 CFU for 28 days aids in alleviating stomach-related problems, such as gas accumulation and vomiting (Figure 3).
NEC prevention
NEC is the most commonly acquired abdominal emergency in preterm newborns. NEC cases can cluster over time and space. Modifying bacterial metabolic processes, such as the fermentation of carbohydrates to produce intramural gases (e.g., hydrogen), occurs before NEC develops. Probiotics play a role in the management and prevention of inflammatory bowel disease and its treatment. Administration of probiotics, such as Lactobacillus or Bifidobacterium, prevents sepsis until discharge from the hospital, thereby preventing intestinal infections (Figure 4).54 Daily intake of Lactobacillus casei (6 × 109 CFU) for 3 days to 6 weeks or until discharge from the hospital limits microbial infections.49 Other Bifidobacterium species, such as B. infantis, B. bifidum, B. longum, and L. acidophilus, reduce NEC-related mortality in preterm infants and improve feed tolerance after administration of 2.5 × 109 CFU daily until discharge from the hospital.
Dosage and safety regulation of probiotics in newborns
The preferred dosage of probiotics (Lactobacillus and Bifidobacterium species) for preventing NEC in infants ranges from 1-18 billion CFU/day, depending on the weight of the infant.69-72 Supplementation with 108-1010 CFU of Lacticaseibacillus rhamnosus GG, 250-750 mg of Saccharomyces boulardii CNCM I-745, and 108-1010 CFU of Lacticaseibacillus reuteri DSM 12246 for 5-7 days reduces diarrhea.73-76 According to Deshpande et al., the general dosage of probiotics should not exceed 1.5 × 108 to
3 × 108 CFU for low birth weight or preterm infants born before 32 weeks. Probiotics and live bacteria also contain dead bacteria and their fragments, which help boost immune responses.77 The probiotics supplement can be administered either within 4 h of birth or when the infant is ready to feed.78 Supplemented probiotics are excreted through stool within 2-3 weeks of administration; therefore, continuous supplementation should be maintained for at least 35 weeks. To date, no study has reported that probiotics cause sepsis after administration.79,80 However, product contamination can cause death. Currently, no studies support the administration of probiotics to surgical infants.81 However, according to Underwood et al., probiotics administration minimizes the risk of sepsis compared with feeding on unpasteurized mother’s milk. Safety studies confirm that probiotics administration does not cause adverse effects on infant health.82 The safest probiotic identified for pregnant women is Lactobacillus rhamnosus, which has been tested in women whose infants were at the risk of developing atopic dermatitis.82,83 Probiotics must meet stringent microbiological standards for quality and purity before administration to patients.77 They can be administered alone or in combination with antibiotics for treating gastrointestinal and genitourinary conditions.84 The Food and Drug Administration (FDA) regulates probiotics as nutritional components and evaluates them before use.
In 2014, the US FDA found that contamination of probiotics with parasitic fungus, Rhizopus oryzae caused gastrointestinal mucormycosis in premature infants. Therefore, before using probiotics as dietary supplements, validation through an Investigational New Drug application for review is important. Different regulatory bodies regulate the use of probiotics and their secretory products in dietary supplements and foods, as they do not have universal regulations. In Europe, food safety is regulated by the European Food Standards Agency, which confers a Qualified Presumption of Safety to a list of microbial cultures that require no further assessment for use in infant foods.77 Additionally, in the US, the food sector is regulated by the FDA, which enforces Good Manufacturing Practice guidelines.
A study in a neonatal intensive care unit assessed the feces of infants and observed that even infants who did not receive any external probiotics had probiotic strains (Lactobacillus) in their feces. This indicates that the intestinal tract is continuously exposed to different bacterial strains.84 Certain bacteria, such as Saccharomyces boulardii, cause infections in infants due to cross-contamination from nearby infants.85 According to the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition, administration of probiotics to infants under four months of age reduces gastrointestinal infections.86 The National FDA and local FDA also investigate the safety of probiotic-containing foods.
With the emergence of new bacterial and viral infections, the identification of novel therapeutic and preventive strategies in the field of nutrition has become crucial. The breast milk microbiome plays an important role in regulating infant health by supporting immune development and disease management. This review focuses on utilizing probiotic microorganisms in the formulation of infant foods. Using Google Scholar and PubMed databases, various health benefits of probiotics were documented. Probiotics and microbes in breast milk have long-lasting impacts on the overall health of infants. Although various studies have focused on the development of energy-rich foods for infants, few have emphasized utilization of microbe-infused foods in infant nutrition. This review highlights the less-studied path for identifying probiotics in infant health management. Probiotics reviewed in this study have minimal or no side effects, making them useful in the market, especially for infants and older age groups. Therefore, probiotic therapy could be an alternative microbial therapy to shape future medicines for infant treatment. However, further studies are required to gain a complete understanding of microbes and assess their overall impact on infant health. This will help optimize various therapies and treatments based on probiotics and their importance in infant development.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Zhao Y, Dong BR, Hao Q. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst Rev. 2022;8(8):CD006895.
Crossref - Heikkila MP, Saris PEJ. Inhibition of Staphylococcus aureus by the commensal bacteria of human milk. J Appl Microbiol. 2003;95(3):471-478.
Crossref - Tancredi DJ. Global health: Probiotic prevents infections in newborns. Nature. 2017;548:404-405.
Crossref - Yadav MK, Kumari I, Singh B, Sharma KK, Tiwari SK. Probiotics, prebiotics and synbiotics: Safe options for next-generation therapeutics. Appl Microbiol Biotechnol. 2022;106(2):505-521.
Crossref - Yao M, Lien EL, Capeding MRZ, et al. Effects of term infant formulas containing high sn-2 palmitate with and without oligofructose on stool composition, stool characteristics, and bifidogenicity. J Pediatr Gastroenterol Nutr. 2014;59(4):440-448.
Crossref - Ballard JDO, Morrow AL. Human Milk Composition: Nutrients and Bioactive Factors. Pediatr Clin North Am. 2013;60(1):49-74.
Crossref - Cho HJ, Cho HK. Central line-associated bloodstream infections in neonates. Korean J Pediatr. 2019;62(3):79-84.
Crossref - Bezirtzoglou E, Stavropoulou E. Immunology and probiotic impact of the newborn and young children intestinal microflora. Anaerobe. 2011; 1;17(6):369-74.
Crossref - Kuwelker K, Langeland N, Lohr IH, et al. Use of probiotics to reduce infections and death and prevent colonization with extended-spectrum beta-lactamase (ESBL)-producing bacteria among newborn infants in Tanzania (ProRIDE Trial): study protocol for a randomized controlled clinical trial. Trials. 2021;22(1):312.
Crossref - Łubiech K, Twarużek M. Lactobacillus bacteria in breast milk. Nutrients. 2020; 10;12(12):3783.
Crossref - Yan F, Polk DB. Probiotics and immune health. Curr Opin Gastroenterol. 2011;27(6):496.
Crossref - Favaro L, Todorov SD. Bacteriocinogenic LAB Strains for Fermented Meat Preservation: Perspectives, Challenges, and Limitations. Probiotics Antimicrob Proteins. 2017;9(4):444-458.
Crossref - Underwood MA, Umberger E, Patel RM. Safety and Efficacy of Probiotic Administration to Preterm Infants: Ten Common Questions. Pediatr Res. 2020;88(Suppl1):48-55.
Crossref - Bergmann H, Rodriguez JM, Salminen S, Szajewska H. Probiotics in human milk and probiotic supplementation in infant nutrition: a workshop report. Br J Nutr. 2014;112(7):1119-1128.
Crossref - Thomson P, Medina DA, Garrido D. Human milk oligosaccharides and infant gut bifidobacteria: Molecular strategies for their utilization. Food Microbiology. 2018;75:37-46.
Crossref - Moossavi S, Miliku K, Sepehri S, Khafipour E, Azad MB. The Prebiotic and Probiotic Properties of Human Milk: Implications for Infant Immune Development and Pediatric Asthma. Front Pediatr. 2018;6.
Crossref - Lara-Villoslada F, Olivares M, Sierra S, Miguel Rodriguez J, Boza J, Xaus J. Beneficial effects of probiotic bacteria isolated from breast milk. Br J Nutr. 2007;98(S1):S96-S100.
Crossref - Haarman M, Knol J. Quantitative real-time PCR analysis of Fecal Lactobacillus species in infants receiving a prebiotic infant formula. Applied and environmental microbiology. 2006; 72(4):2359-65.
Crossref - Harmsen HJM, Wildeboer–Veloo ACM, Raangs GC, et al. Analysis of Intestinal Flora Development in Breast-Fed and Formula-Fed Infants by Using Molecular Identification and Detection Methods. J Pediatr Gastroenterol Nutr. 2000;30(1):61-67.
- Stark PL, Lee A. The microbial ecology of the large bowel of breast-fed and formula-fed infants during the first year of life. J Med Microbiol. 1982;15(2):189-203.
Crossref - Moubareck CA. Human milk microbiota and oligosaccharides: a glimpse into benefits, diversity, and correlations. Nutrients. 2021;29;13(4):1123.
Crossref - Lokossou GAG, Kouakanou L, Schumacher A, Zenclussen AC. Human Breast Milk: From Food to Active Immune Response With Disease Protection in Infants and Mothers. Front Immunol. 2022;13:849012.
Crossref - Garcia-Ricobaraza M, Garcia-Santos JA, Escudero-Marin M, Dieguez E, Cerdo T, Campoy C. Short- and Long-Term Implications of Human Milk Microbiota on Maternal and Child Health. Int J Mol Sci. 2021;22(21):11866.
Crossref - Peran L, Sierra S, Comalada M, et al. A comparative study of the preventative effects exerted by two probiotics, Lactobacillus reuteri and Lactobacillus fermentum, in the trinitrobenzenesulfonic acid model of rat colitis. Br J Nutr. 2007;97(1):96-103.
Crossref - Silfverdal SA, Bodin L, Olcen P. Protective effect of breastfeeding: an ecologic study of Haemophilus influenzae meningitis and breastfeeding in a Swedish population. Int J Epidemiol. 1999;28(1):152-156.
Crossref - Cesar JA, Victora CG, Barros FC, Santos IS, Flores JA. Impact of breast feeding on admission for pneumonia during postneonatal period in Brazil: nested case- control study. BMJ. 1999;318(7194):1316-1320.
Crossref - Howie PW, Forsyth JS, Ogston SA, Clark A, Florey CD. Protective effect of breast feeding against infection. BMJ. 1990;300(6716):11-16.
Crossref - Victora C, Vaughan JP, Lombardi C, et al. Evidence for protection by breast-feeding against infant deaths from infectious diseases in brazil. Lancet. 1987;330(8554):319-322.
Crossref - Pisacane A, Graziano L, Mazzarella G, Scarpellino B, Zona G. Breast-feeding and urinary tract infection. J Pediatr. 1992;120(1):87-89.
Crossref - Ladd N, Ngo T. The use of probiotics in the prevention of necrotizing enterocolitis in preterm infants. Proc (Bayl Univ Med Cent). 2009;22(3):287-291.
Crossref - Kalliomaki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001;357(9262):1076-1079.
Crossref - Bode L, McGuire M, Rodriguez JM, et al. It’s alive: microbes and cells in human milk and their potential benefits to mother and infant. Adv Nutr. 2014;5(5):571-573.
Crossref - Laforest-Lapointe I, Arrieta MC. Patterns of Early-Life Gut Microbial Colonization during Human Immune Development: An Ecological Perspective. Front Immunol. 2017;8:00788.
Crossref - Asnicar F, Manara S, Zolfo M, et al. Studying Vertical Microbiome Transmission from Mothers to Infants by Strain-Level Metagenomic Profiling. mSystems. 2017;2(1):e00164-16.
Crossref - Le Doare K, Holder B, Bassett A, Pannaraj PS. Mother’s milk: a purposeful contribution to the development of the infant microbiota and immunity. Frontiers in immunology. 2018;9:361.
Crossref - Marcobal A, Sonnenburg JL. Human milk oligosaccharide consumption by intestinal microbiota. Clin Microbiol Infect. 2012;18(Suppl 4):12-15.
Crossref - Biagi E, Quercia S, Aceti A, et al. The Bacterial Ecosystem of Mother’s Milk and Infant’s Mouth and Gut. Front Microbiol. 2017;8:1214.
Crossref - Obadia B, Guvener ZT, Zhang V, et al. Probabilistic Invasion Underlies Natural Gut Microbiome Stability. Curr Biol. 2017;27(13):1999-2006.e8.
Crossref - Plaza-Diaz J, Fontana L, Gil A. Human Milk Oligosaccharides and Immune System Development. Nutrients. 2018;10(8):1038.
Crossref - Makhseed M, Raghupathy R, Azizieh F, Omu A, Al-Shamali E, Ashkanani L. Th1 and Th2 cytokine profiles in recurrent aborters with successful pregnancy and with subsequent abortions. Hum Reprod. 2001;16(10):2219-2226.
Crossref - Collado MC, Cernada M, Neu J, Perez-Martinez G, Gormaz M, Vento M. Factors influencing gastrointestinal tract and microbiota immune interaction in preterm infants. Pediatr Res. 2015;77(6):726-731.
Crossref - Chassard C, de Wouters T, Lacroix C. Probiotics tailored to the infant: a window of opportunity. Curr Opin Biotechnol. 2014;26:141-147.
Crossref - Song MW, Kim KT, Paik HD. Probiotics as a Functional Health Supplement in Infant Formulas for the Improvement of Intestinal Microflora and Immunity. Food Rev Int. 2023;39(2):858-874.
Crossref - Indrio F, Neu J. The intestinal microbiome of infants and the use of probiotics. Curr Opin Pediatr. 2011;23(2):145-150.
Crossref - Reid G, Bruce AW. Probiotics to prevent urinary tract infections: the rationale and evidence. World J Urol. 2006;24(1):28-32.
Crossref - Depoorter L, Vandenplas Y. Probiotics in Pediatrics. A Review and Practical Guide. Nutrients. 2021;13(7):2176.
Crossref - Weizman Z, Asli G, Alsheikh A. Effect of a Probiotic Infant Formula on Infections in Child Care Centers: Comparison of Two Probiotic Agents. Pediatrics. 2005;115(1):5-9.
Crossref - Szajewska H, Mrukowicz JZ. Probiotics in the Treatment and Prevention of Acute Infectious Diarrhea in Infants and Children: A Systematic Review of Published Randomized, Double-Blind, Placebo-Controlled Trials. J Pediatr Gastroenterol Nutr. 2001;33:17-25.
- Di Gioia D, Aloisio I, Mazzola G, Biavati B. Bifidobacteria: their impact on gut microbiota composition and their applications as probiotics in infants. Appl Microbiol Biotechnol. 2014;98(2):563-577.
Crossref - De Greef E, Hauser B, Devreker T, Veereman-Wauters G, Vandenplas Y. Diagnosis and management of cow’s milk protein allergy in infants. World J Pediatr. 2012;8(1):19-24.
Crossref - Hearty AP, McCarthy SN, Kearney JM, Gibney MJ. Relationship between attitudes towards healthy eating and dietary behaviour, lifestyle and demographic factors in a representative sample of Irish adults. Appetite. 2007;48(1):1-11.
Crossref - Tan THT, Ellis JA, Saffery R, Allen KJ. The role of genetics and environment in the rise of childhood food allergy: Role of genetics and environment in food allergy. Clin Exp Allergy. 2012;42(1):20-29.
Crossref - Viljanen M, Savilahti E, Haahtela T, et al. Probiotics in the treatment of atopic eczema/dermatitis syndrome in infants: a double-blind placebo-controlled trial. Allergy. 2005;60(4):494-500.
Crossref - Savilahti E, Kukkonen K, Kuitunen M. Probiotics in the Treatment and Prevention of Allergy in Children. World Allergy Organ J. 2009;2(5):69-76.
Crossref - Sestito S, D’Auria E, Baldassarre ME, et al. The Role of Prebiotics and Probiotics in Prevention of Allergic Diseases in Infants. Front Pediatr. 2020;8:583946.
Crossref - Osborn DA, Sinn JK. Probiotics in infants for prevention of allergic disease and food hypersensitivity. Cochrane Database Syst Rev. 2007;4.
Crossref - Al-Hosni M, Duenas M, Hawk M, et al. Probiotics-supplemented feeding in extremely low-birth-weight infants. J Perinatol. 2012;32(4):253-259.
Crossref - Mugambi MN, Musekiwa A, Lombard M, Young T, Blaauw R. Probiotics, prebiotics infant formula use in preterm or low birth weight infants: a systematic review. Nutr J. 2012;11(1):58.
Crossref - Viswanathan S, Lau C, Akbari H, Hoyen C, Walsh MC. Survey and evidence based review of probiotics used in very low birth weight preterm infants within the United States. J Perinatol. 2016;36(12):1106-1111.
Crossref - Sari FN, Dizdar EA, Oguz S, Erdeve O, Uras N, Dilmen U. Oral probiotics: Lactobacillus sporogenes for prevention of necrotizing enterocolitis in very low-birth weight infants: A randomized, controlled trial. Eur J Clin Nutr. 2011;65(4):434-439.
Crossref - Kurath-Koller S, Neumann C, Moissl-Eichinger C, et al. Hospital Regimens Including Probiotics Guide the Individual Development of the Gut Microbiome of Very Low Birth Weight Infants in the First Two Weeks of Life. Nutrients. 2020;12(5):1256.
Crossref - Rouge C, Piloquet H, Butel MJ, et al. Oral supplementation with probiotics in very-low-birth- weight preterm infants: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2009;89(6):1828-1835.
Crossref - Millar M, Wilks M, Costeloe K. Probiotics for preterm infants? Arch Dis Child Fetal Neonatal Ed. 2003;88(5):F354-F358.
Crossref - More K, Hanumantharaju A, Amrit A, Nimbalkar SM, Patole S. Use of Probiotics for Preventing Necrotizing Enterocolitis in Preterm Infants: A Survey of Current Practices Among Indian Neonatologists. Cureus. 2024;16(11).
Crossref - Crittenden R, Bird AR, Gopal P, Henriksson A, Lee Y, Playne MJ. Probiotic Research in Australia, New Zealand and the Asia-Pacific Region. Curr Pharm Des. 2005;11(1):37-53.
Crossref - Gardiner GE, Casey PG, Casey G, et al. Relative Ability of Orally Administered Lactobacillus murinus To Predominate and Persist in the Porcine Gastrointestinal Tract. Appl Environ Microbiol. 2004;70(4):1895-1906.
Crossref - Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110(2):285-291.
Crossref - Pessoa-Silva CL, Miyasaki CH, de Almeida MF, Kopelman BI, Raggio RL, Wey SB. Neonatal late-onset bloodstream infection: attributable mortality, excess of length of stay and risk factors. Eur J Epidemiol. 2001;17(8):715-720.
Crossref - Hunter C, Dimaguila MAVT, Gal P, et al. Effect of routine probiotic, Lactobacillus reuteri DSM 17938, use on rates of necrotizing enterocolitis in neonates with birthweight < 1000 grams: a sequential analysis. BMC Pediatr. 2012;12:142.
Crossref - Kanic Z, Turk DM, Burja S, Kanic V, Dinevski D. Influence of a combination of probiotics on bacterial infections in very low birthweight newborns. Wien Klin Wochenschr. 2015;127(Suppl 5):S210-215.
Crossref - Kutylowksi J, Yahia N. Types, Frequency, Duration, and Dosage of Probiotics to Prevent Necrotizing Enterocolitis in Preterm Infants Among Countries. Adv Neonatal Care. 2019;19(3):188-197.
Crossref - Mihatsch WA, Vossbeck S, Eikmanns B, Hoegel J, Pohlandt F. Effect of Bifidobacterium lactis on the incidence of nosocomial infections in very-low-birth-weight infants: a randomized controlled trial. Neonatology. 2010;98(2):156-163.
Crossref - Reid G, Gadir AA, Dhir R. Probiotics: Reiterating What They Are and What They Are Not. Front Microbiol. 2019;10:424.
Crossref - Rosenfeldt V, Michaelsen KF, Jakobsen M, et al. Effect of probiotic Lactobacillus strains on acute diarrhea in a cohort of nonhospitalized children attending day-care centers. Pediatr Infect Dis J. 2002;21(5):417-419.
Crossref - Szajewska H, Kolodziej M, Gieruszczak-Bialek D, Skorka A, Ruszczynski M, Shamir R. Systematic review with meta-analysis: Lactobacillus rhamnosus GG for treating acute gastroenteritis in children – a 2019 update. Aliment Pharmacol Ther. 2019;49(11):1376-1384.
Crossref - Szajewska H, Guarino A, Hojsak I, et al. Use of Probiotics for the Management of Acute Gastroenteritis in Children: An Update. J Pediatr Gastroenterol Nutr. 2020;71(2):261-269.
Crossref - Fleming PF, Berrington JE, Jacobs SE. Addressing safety concerns of probiotic use in preterm babies. Early Human Development. 2019;135:72-74.
Crossref - Deshpande GC, Rao SC, Keil AD, Patole SK. Evidence-based guidelines for use of probiotics in pretermneonates. BMC Med. 2011;9:92.
Crossref - Rao S, Esvaran M, Chen L, et al. Probiotic supplementation for neonates with congenital gastrointestinal surgical conditions: guidelines for future research. Pediatr Res. 2023;93(1):49-55.
Crossref - Sakurai Y, Watanabe T, Miura Y, et al. Clinical and Bacteriologic Characteristics of Six Cases of Bifidobacterium breve Bacteremia Due to Probiotic Administration in the Neonatal Intensive Care Unit. Pediatr Infect Dis J. 2022;41(1):62.
Crossref - Hamprecht K, Goelz R. Postnatal Cytomegalovirus Infection Through Human Milk in Preterm Infants: Transmission, Clinical Presentation, and Prevention. Clin Perinatol. 2017;44(1):121-130.
Crossref - Reid G. Safe and efficacious probiotics: what are they? Trends Microbiol. 2006;14(8):348-352.
Crossref - van den Nieuwboer M, Claassen E, Morelli L, Guarner F, Brummer R j. Probiotic and symbiotic safety in infants under two years of age. Benef Microbes. 2014;5(1):45-60.
Crossref - Grev J, Berg M, Soll R. Maternal probiotic supplementation for prevention of morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2018;12(12):CD012519.
Crossref - Onubi OJ, Poobalan AS, Dineen B, Marais D, McNeill G. Effects of probiotics on child growth: a systematic review. J Health Popul Nutr. 2015;34:8.
Crossref - Xiao L, Ding G, Ding Y, et al. Effect of probiotics on digestibility and immunity in infants: A study protocol for a randomized controlled trial. Medicine (Baltimore). 2017;96(14):e5953.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.