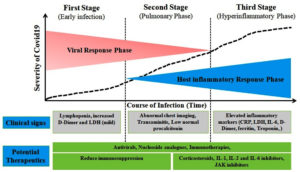
The world has been rocked by the 2019 coronavirus disease (COVID-19), which has significantly changed our way of life. Despite the unusual measures taken, COVID-19 still exists and affects people all over the world. A remarkable amount of study has been done to find ways to combat the infection’s unsurpassed level. No ground-breaking antiviral agent has yet been introduced to remove COVID-19 and bring about a return to normalcy, even though numerous pharmaceuticals and therapeutic technologies have been reused and discovered. The cytokine storm phenomenon is of utmost importance since fatality is strongly connected with the severity of the disease. This severe inflammatory phenomenon marked by increased amounts of inflammatory mediators can be targeted for saving patients’ life. Our analysis demonstrates that SARS-CoV-2 specifically generates a lot of interleukin-6 (IL-6) and results in lymphocyte exhaustion. Tocilizumab is an IL-6 inhibitor that is currently thought to be both generally safe and effective. Additionally, corticosteroids, tumor necrosis factor (TNF)-blockers and Janus kinase (JAK) inhibitors could be effective and dependable methods to reduce cytokine-mediated storm in SARS-CoV-2 patients.
SARS-CoV-2, Cytokine Storm, Nucleoside Analogues, JAK Inhibitors, Diabetes
Riding on the roller coaster of coronavirus evolution, it became evident that the first coronavirus was discovered in domestic poultry and companion animals in the 1930s, and since then, coronaviruses have been recognized as pathogens that caused respiratory, gastrointestinal, liver, and neurologic diseases in animals. Subsequently, seven coronavirus strains/genera came to be known to cause respiratory disease in humans.1,2 Coronaviruses are classified by the International Committee on Taxonomy of Viruses as belonging to the realm Riboviria, order Nidovirales, and subfamily Orthocoronavirinae of the family Coronaviridae.3 The synthesis of a 3′-coterminal nested collection of subgenomic mRNAs by this order during infection is referred to here as the order Nidovirales.4 They are enclosed viruses with a positive-sense single-stranded RNA genome and a nucleocapsid with helical symmetry5 that contain coronaviruses, one of the largest RNA viruses with genomes ranging from about 26 to 32 kilobases. The presence of peplomers, rod-shaped glycoprotein spikes that protrude from their surface and remind us of the solar corona from which their name is derived, was discovered through electron microscopy research.5
In general, coronaviruses 229E and OC43 cause the common cold; the serotypes such as NL63 and HUK1 were found to produce common cold symptoms.5 These coronaviruses that cause respiratory infections of varying degrees showed zoonotic transmissions from animals to humans and then displayed their uncanny ability of horizontal transmissions in humans.3 Serious lower respiratory tract infections, such as pneumonia, have been reported, usually in children, veterans, and immunocompromised people. The World Health Organization (WHO) regards coronaviruses as a vast family of viruses that have the potential to cause a wide range of illnesses in humans or animals. It was the year 2003 when severe acute respiratory syndrome coronavirus (SARS-CoV) was the major cause of an outbreak of severe acute respiratory syndrome (SARS). A mutational RNA-stranded virus known as SARS-CoV-2 caused the recently identified coronavirus, COVID-19, which exhibits clinical signs, to be reported in Wuhan, China in 2019. As a result of the unique SARS-CoV-2 virus’ post-infection worldwide spread, WHO had to announce it as a pandemic on March 11, 2020. Similar to the previous two coronaviruses i.e., SARS-CoV and the Middle East Respiratory Syndrome Corona Virus (MERS-CoV) that have caused life-threatening infections during the past 20 years, this SARS-CoV-2 is a unique beta-coronavirus.6
During COVID-19 progression the body responds aggressively by releasing a lot of pro-inflammatory cytokines, a phenomenon described as a “cytokine storm”.6 The severity of SARS-CoV-2 tends to be directly related to the cytokine storm. An excessive amount of inflammation results from the immune system’s hyperactive response to the SARS-CoV-2 virus. Studies looking at the cytokine profiles of COVID-19 patients revealed a direct correlation between the cytokine storm and lung damage, multiple organ failure, and a poor prognosis for severe COVID-19.4-6 The present review provides the comprehensive scenario not only about the cytokine storm and its clinical manifestations in COVID-19 infected individuals but also presented the treatments given to COVID-19 patients.
Cytokine Storm: a general definition
It is a hyperactive immune response marked by the release of interferons, interleukins, tumor necrosis factors (TNFs), chemokines, and other mediators.4 These mediators are a crucial component of an effective innate immune response required for the clearance of pathogens. Cytokine storm alludes to cytokine levels that are harmful to host cells.5 However, it has been very difficult to distinguish between a healthy and a dysregulated inflammatory response in the pathophysiology of severe illness.6 Most of the mediators linked to the cytokine storm have pleiotropic downstream effects and interact regularly with one another in biological activity, which adds to its complexity.7
Similarly, hypercytokinemia or cytokine storm is thought to be the cause of the condition seen in critically ill individuals infected with the SARS-CoV-2 virus in the current COVID-19 global pandemic scenario.7 Acute respiratory distress syndrome, thromboembolic illnesses such acute ischemic strokes brought on by major artery occlusion and myocardial infarction, encephalitis, acute kidney injury, and vasculitis are among the severe signs of COVID-19 that are associated with SARS-CoV-2 infections (Kawasaki-like syndrome in children and renal vasculitis in adult) (Figure 1). Understanding the immunopathogenesis of the cytokine storm in COVID-19 patients may provide new opportunities for early diagnosis and the implementation of therapeutic measures to reduce the risks of morbidity and death brought on by cytokine storm.8
Cytokine storm: Mechanism, Pathogenesis, and Clinical Manifestations
Mechanism of cytokine storm
The inflammatory response is indispensable for the pathogen identification that then leads to the onset of immune cells, helps eliminating these unwanted hostile guests, and enables the tissue repair process.9 As an exception, SARS-CoV-2 triggers a rather prolonged and dysregulated cytokine/chemokine response in many infected individuals, known as the cytokine storm. Spike glycoproteins are extremely immunogenic parts of the coronaviruses9,10; the SARS-CoV-2 peplomer binds angiotensin-converting enzyme (ACE-2) receptors to access human alveolar epithelial cells type II.9 Using the highly flexible three-hinged stalk-domain which acts ‘like a balloon on a string, the spikes appear to hover over the virus-surface and thus can scan for the presence of specific receptors for docking to the alveolar macrophages. The uniform distribution of ACE-2 receptors on the alveolar epithelial type II cell surface, endothelial cells, and renal and intestinal cells of the target organs have been found strongly correlate with the manifestations of clinical symptoms in COVID-19 infection.10,11 Contrary to “secondary cytokine” storms caused by various subsets of T lymphocytes activated at later stages of viral infection or as a side effect of T cell-involving therapies, “primary cytokine” storms are caused by viral infections and are primarily produced by cells such as epithelial, endothelial and alveolar macrophages.12 It’s interesting to note that proinflammatory T cell subsets, such as cytotoxic T cells that express perforin and granulysin and produce IL-17 (T-helper 17 or TH17 cells), were observed to rise, severely harming the lungs’ immune system.12 Besides, an efficient and long-lasting antiviral response is typically exhibited by the natural killer (NK) cells in association with essential players in the immune system, including neutrophils, macrophages, and dendritic cells. NK cells in normal situation deem to kill infected macrophages responsible for cytokine storms, therefore diminished humoral counts may augment the severity of disease in COVID-19 patients.13 During viral infections, these intricate cellular interactions can control the cytokine milieu, initial viral load, and CD4+ T-cell mediated cellular immune responses. Though killing off infected target cells effectively lowers viral load, NK cells also counter the systemic inflammatory response and unwelcome cytokine storm known as “hyperferritinemic syndrome” synonymous with “macrophage activation syndrome” by lysing and eliminating activated inflammatory cells namely neutrophils, dendritic cells, monocytes/macrophages, and T cells.13
Pathogenesis of cytokine storm
As a part of the general pathophysiological phenomenon, cellular infection and viral replication lead to the activation of the host-cell inflammasome.14 Due to these aggressive proinflammatory responses, the release of proinflammatory cytokines takes place with the concomitant cell death by a process known as pyroptosis.14 The damaged cells are further triggered to exhibit the amplified inflammatory responses and elevated cytokine release in response to viral infection, a pathophysiological condition termed as cytokine release syndrome (CRS)15 or cytokine storm, deemed partially responsible behind the acute respiratory disease syndrome (ARDS) and multiple organ dysfunction syndrome (MODS) in COVID-19.16 Rapid burst of intracellular virus replication initiate pyroptosis, immune system evasion, and cell lysis, and together these trigger the mass release of pro-inflammatory cytokines and chemokines. Therefore, deterioration of COVID-19 victims’ clinical symptoms may be the result of a combination of cytopathic effects caused directly by the virus infection and immunopathology injury caused by a turbulent cytokine storm.17 In this context, a few studies on cytokine profiles from COVID-19 patients indicated that the cytokine storm correlated positively with pulmonary cell and tissue damage, the unfavorable prognosis of severe COVID-19, and extrapulmonary multiple-organ failure.18 Experimental results from various studies on old and young non-human primates led the viral epidemiologists to postulate that the virus titer could be less significant and instrumental than the uncontrolled inflammatory responses in inflicting deaths of the old non-human primates.16,18 Thus, it may be stated that “cytokine storm” potentially exacerbates the pathophysiological conditions in COVID-19 infected patients.12,19
Clinical manifestations of cytokine storm
A recent series of studies have shown that COVID-19 infected patients had increased levels of inflammatory cytokines, such as interleukin (IL)-1β, IL-2, IL-6 IL-7, IL-8, IL-9, IL-10, IL-18, tumor necrosis factor (TNF)-a, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor, fibroblast growth factor, macrophage inflammatory protein 1, compared to healthy individuals.10 Besides this, amongst the intensive care unit (ICU) patients, circulating levels of three cytokines IL-6, IL-10, and TNF-a also correlated with the severity of infection as reflected by their elevated concentrations compared to mild/moderate cases. ILs such as IL-1, IL-6, TNF, and interferon (IFN)-γ orchestrate the pathological process that leads to vascular permeability, plasma leakage, and disseminated intravascular clotting (DIC).20 This drastic increase in cytokine releases results in an influx of macrophages, neutrophils, and T lymphocytes from the circulation into the adjacent infection site, severely damaging human tissue and unleashing the destabilization effect on endothelial cell-to-cell interactions, damaging on vascular barrier and capillaries diffusion of alveolar damage. It has been reported that the suppression of the usual T-cell activation was caused by IL-629, and TNF-a could induce the T-cell apoptosis via interacting with its receptor TNF receptor 1.21 In a separate animal study, it was observed that the rapid replication of SARS-CoV in BALB/c mice induces the delayed release of IFN-a/β accompanied by the influx of numerous pathogenic inflammatory mononuclear macrophages.22
The accumulated mononuclear macrophages receive many activating signals via the IFN-a/β receptors on their cell surface and produce more monocyte chemo-attractants (such as CCL2, CCL7, and CCL12), resulting in the further accumulation of mononuclear macrophages. Subsequently, these mononuclear macrophages produce higher levels of proinflammatory cytokines (TNF, IL1-β, and IL-6), thereby worsening the disease progression. A mesenchymal stem cell (MSC) therapy has been suggested recently to abrogate the undesirable activation of macrophages and T-lymphocytes given stimulating their appropriate differentiation and thus thwarting the burst of pro-inflammatory cytokine release.23,24 Stem cells have been found to suppress the activities of viruses via Chaf1a-mediated and Sumo2-mediated epigenetic regulation (termed proviral silencing).25 For patients suffering from lung fibrosis and cytokine storm, MSCs-based immunomodulation has been suggested as a suitable therapeutic approach.26 Recently type IV transplantation of MSCs has been suggested as safe and effective in critically ill patients suffering from COVID-19 pneumonia27 though no approved MSC-based approaches have been reported up till now for the prevention and/or medication of COVID-19 patients, though the initial data of clinical trials has been immensely promising.
It has been observed that acute COVID-19 patients admitted to the ICU ward had elevated erythematosus sedimentation rate (ESR), C-reactive protein (CRP), and enhanced IL-6, TNFa, IL-1β, IL-8, IL2R, besides being associated with ARDS, hypercoagulation and disseminated intravascular coagulation (DIC), manifested as thrombosis, thrombocytopenia and gangrene.28,29 The illness due to COVID-19 is characterized by thrombus and inflammation, causes extensive alveolar injury as a result of heightened macrophage activities and cytokine storms. Due to these events, the cell membranes are disrupted, and significant endothelial damage occurs leading to thrombosis.30 Thrombocytopenia is a pathophysiological condition, where there is a reduction in platelet counts. Since platelets are involved in antifungal immune responses, thrombocytopenia may aggravate the risk of mucormycosis infection. Besides, the spikes in mucormycosis cases were correlated to the immunosuppression caused by the administration of corticosteroids and dexamethasone for COVID-19 treatment may trigger cytokine storm and vascular cell damage. Another pathophysiological condition known as secondary haemophagocytic lymphohistiocytosis (sHLH) is a hyperinflammatory syndrome characterized by a fulminant and fatal hypercytokinaemia with multiple-organ failure.30 In adults, sHLH is most commonly triggered by viral infections and occurs in 3.7–4.3% of sepsis cases.31
Remedies for the cytokine storm
Accumulative studies of clinical trials have observed the ‘cytokine storm’ in critical patients with COVID-19. Correct treatment of hyperinflammation using existing and approved therapies considering the safety aspect has been recommended to check the spiraling mortality. Therefore, appropriately suppressing the cytokine storm would be a significant clinical step to prevent the eventuality.31 Timely clinical intervention to quell the cytokine storm at its early stage by using a regimen of immunomodulators and cytokine antagonists, and the reduction of lung inflammatory cell infiltration, holds the key in ameliorating the recovery and survival rate amongst the critically ill patients.19
Several anti-cytokine drugs or formulations may potentially address the cytokine storm and mitigate the severity of the storms. One such medication that can be used to treat cytokine storms and macrophage activation syndrome (MAS) in autoimmune/autoinflammatory illnesses is a corticosteroid.32,33 If used at the right moment, they may be helpful in the COVID-19 scenario in the more severe forms of CRS to control the systemic inflammatory response and avoid the development of ARDS.33
The prompt use of corticosteroids can result in early improvements such as a decrease in body temperature and an increase in oxygenation.17 The correct administration of glucocorticoids in patients with severe SARS considerably decreased the mortality rate and shortened the hospital stay, according to a retrospective analysis of 401 patients with the illness.16 Studies have revealed, however, that administering corticosteroid medication while a person was infected with the SARS-CoV-2 had unfavorable effects. Early corticosteroid administration to SARS patients increased plasma viral load in non-ICU patients, worsening the illness.17,19 The short-term (3-5 days) use of glucocorticoids has been hypothesized to be acceptable and may be advised for individuals who have excessive inflammation and a steady decline in oxygenation indicators.33 It should be kept in mind that high glucocorticoid doses can weaken the immune system, which can cause a delay in coronavirus clearance.20
TNF blockers
As previously discussed, being one of the major inflammatory factors, TNFs are the key players to trigger cytokine storms. TNFs thus deem to be attractive targets that can subdue the cytokine storm.19 Certain in vivo studies in murine models have demonstrated that TNFs could potentially contribute to acute alveolar injury and cause the impairment of T-cell responses in SARS-CoV-challenged mice.19 It has been demonstrated in mice that either the loss of the TNF receptor or its neutralization renders protection against virus-mediated morbidity and mortality.19 Simultaneously, it also might be noticed that TNF level could not be detected in the serum.17-19 Nevertheless, the clinical efficacy of TNF blockers should be further tested though TNF blockers failed to earn recommendations for the treatments of COVID-19 patients.34 The SARS-CoV-2 spike protein appears to induce a TNF-a-converting enzyme (TACE)-dependent alteration of ACE-2, which allows virus penetration into host cells.35 Adalimumab, a TNF-blocker, is now being tested in a clinical trial for COVID-19 infection (ChiCTR2000030089).
IL-1 family antagonists
The surge of IL-1 family interleukins including IL-1 β, IL-18, and IL-33 were reported previously12 Studies involved in the inhibition of IL-1β to suppress the cytokine storm have got immense importance. The IL-1 β antagonist anakinra was thought to be used for treating the cytokine storm.36 Re-evaluation of data from a phase 3 randomized controlled trial of IL-1 blockade (anakinra) in severe sepsis, showed a significant survival benefit in patients with hyperinflammation, without adverse effects.36 However, presently there is no clinical recommendation for any specific IL-1 family blockers as their effects have not been clinically demonstrated by in vivo systems and clinical trials.
IL-2 and IL-6 Immunotherapy
Unrestrained release of IL-2 like TNF in COVID-19 condition not only leads to the development of fever but also capillary leakage or increased capillary permeability to various proteins showing clinical manifestations of edema, ARDS and renal injury.
A prominent inflammatory cytokine, IL-6, is raised in the serum of COVID-19 patients and is involved in inflammatory cytokine responses.34 In COVID-19 patients admitted to the ICU, the aberrant increase in the number of CD14+ CD16+ inflammatory monocytes capable of producing IL-6 was noticed.34 A potential IL-6 antagonist is tocilizumab, a recombinant humanized monoclonal antibody directed toward the IL-6 receptor. Tocilizumab binds to the membrane-associated and humoral IL-6 receptor thereby suppressing the Janus activated kinase (JAK)-signal transducer and activator of transcription (STAT) signaling pathway and production of downstream inflammatory molecules.37 Zhou et al. reported that GM-CSF produced by hyperactivated TH1 cells and IFNγ in lung cells promoting the production of monocytes through the release of GM-CSF could potentially be the therapeutic targets towards the treatments of COVID-19 patients.38
JAK Inhibitors
The possibility of treating CS using cytokine downstream inhibitors, such as JAK inhibitors, is also being investigated. Baricitinib, fedratinib, and ruxolitinib were among the authorized medications found by Stebbing et al.39 for myelofibrosis, rheumatoid arthritis, and other conditions. These medications may prevent clathrin-mediated endocytosis, which prevents viral cell infection. Members of the NAK family, such as AP2-associated protein kinase 1 (AAK1)40 and cyclin G-associated kinase (GAK), are the targets of these medications; inhibition of these enzymes has been demonstrated to decrease viral infection in vitro. These medications are being investigated for the treatment of CS because they are specific inhibitors of JAK-STAT signaling and have anti-inflammatory characteristics. The disruption of AAK1 may therefore prevent the virus from entering cells and from virus particles assembling inside of cells.41 The AP2-associated protein kinase 1 (AAK1) enzyme is one of the recognized endocytosis regulators.41 According to reports, baricitinib can bind the cyclin G-associated kinase, another regulator of viral endocytosis, and suppress AAK1 activity when used in therapeutic doses.41
Due to its low plasma protein binding and negligible interaction with CYP enzymes and drug transporters, baricitinib presents great potential for combination therapy.42 Due to its limited interaction with the relevant CYP drug-metabolizing enzymes, baricitinib may also be used in combination with the direct-acting antivirals (Lopinavir or ritonavir and remdesivir), which are now being utilized to treat COVID-19 epidemic. Baricitinib and related direct-acting antivirals may lessen virus multiplication, infectivity, and the abnormal host inflammatory response. Subject to adequate clinical testing, baricitinib has been deemed useful in treating SARS-CoV-2 infections.43 The justification for utilizing JAK inhibitors during a SARS-CoV-2 infection is that many cytokines—which immune system cells release to activate one another—need JAKs to do their jobs.44 According to Schett et al.45 JAK inhibitors specifically can block the cytokine IL-6, which is produced by alveolar cells in the lungs. High levels of IL-6 have been associated with acute lung injury in the past with SARS-CoV-1. Table lists the pharmacotherapies used to treat diabetes and its complications as well as COVID-19 infection.
Table:
Pharmacotherapies used during COVID-19 infection and associated complications.
| No | Therapeutics/Drugs | Target | Reference |
|---|---|---|---|
| 1 | Adalimumab | TNF-α | Gheblawi et.al.36 |
| 2 | Anakinra | IL-1 | Shakoory et.al.37 |
| 3 | Tocilizumab | IL-6 receptor | Riegler et.al.38 |
| 4 | Baricitinib | JAK-STAT signaling | Stebbing et.al.40 Espinosa, et.al.43 |
| 5 | Fedratinib | JAK-STAT signaling | Stebbing et.al.40 |
| 6 | Ruxolitinib | JAK-STAT signaling | Stebbing et.al.40 |
| 7 | Metformin | AMP-activated protein kinase; glucagon-like peptide-1 (GLP-1) hormone | Carrasco-Sánchez et.al.46 Chen et.al.47 |
| 8 | Sitagliptin | Dipeptidyl peptidase 4 (DPP4) | Solerte et.al.52 |
| 9 | Glucocorticoids | Immunosuppression | Gillespie et.al.52 |
| 10 | Tofacitinib | JAK-1 and JAK-3 | Jacobs et.al.56 |
| 11 | Aspirin | Antiplatelet drug | Ghasemzadeh et al.30 |
| 12 | Clopidogrel | Ghasemzadeh et al.30 | |
| 13 | Ticlopidine and Ticagrelor | Ghasemzadeh et al.30 |
Therapeutics for specific treatment of patients with both COVID-19 and diabetes
Insulin therapy is determined by the severity of COVID-19, and patients are closely watched, even though it has been suggested for diabetic people with severe COVID-19.45 In one study, patients who were given insulin had poor clinical outcomes than those who were given metformin.46 Despite the evidence of better results in diabetic patients with COVID-19 receiving metformin, this medicine should be stopped if patients develop respiratory distress, renal dysfunction, or cardiac failure as a result of acidosis.45 In the CORONADO research, Cariou et al.47 showed that the usage of metformin was lower in patients who died as well as other treatments such as insulin therapy, renin–angiotensin–aldosterone system (RAAS) blockers, β-blockers, and loop diuretics were related to a fatality on the seventh day.58 They hypothesized this observation might be linked to the existing comorbidities and diabetic issues in those who died because those individuals had more recurrent therapy with insulin and other multiple medicines.48 A recent study found that patients with COVID-19 had considerably greater postprandial glycemic variations and exposures to hyperglycemia when evaluated by continuous glucose monitoring.49 Excessive stress and increased release of hyperglycemia-related hormones including catecholamine and glucocorticoids may be triggered by COVID-19 illness in a diabetic individual. These hormones produce unpredictable glucose fluctuation in the blood and raise blood glucose levels (Figure 2). Periodic blood sugar monitoring has to be part of the therapy strategy as a result.49
Figure 2. COVID-19 and diabetes have reciprocal effects. Diabetic individuals with COVID-19 infection have serious repercussions as a result of several coexisting diseases that increase the risk. SARS-CoV-2 may cause hyperglycemia during hospitalization due to its affinity for β-cells. Critical metabolic diseases, such as diabetic ketoacidosis, can be caused by β-cell destruction, leading to cytokine storm, and a counter-regulatory hormone response.50
Inhibitors of sodium-glucose transporter-2 should be used with caution because these drugs may lead to ketoacidosis and poor fat metabolism.50 Furthermore, glucagon-like receptor-1 (GLP-1R) analogues should be used with caution because they can cause diarrhoea, nausea, vomiting, and headaches.52 Sitagliptin, a highly selective dipeptidyl peptidase 4 (DPP4) inhibitor, was utilized as an additional oral medication for patients with type-II diabetes and COVID-19 in a recent multicenter, retrospective, case-control, and observational trial. In this trial, sitagliptin medication was linked to lower mortality, better clinical outcomes, and a higher number of hospital discharges.52 DPP4 and ACE2, two of the most important coronavirus receptor proteins, are well-known metabolic signal transducers that regulate inflammation, and glucose homeostasis. Furthermore, glucose-lowering medicines like DPP4 inhibitors, which are commonly used in type 2 diabetes patients, have been shown to alter the biological activities of a variety of immunomodulatory substrates.53 Several therapies for COVID-19 have been proposed by researchers. For instance, the benefits of ACEI and ARBs for renal and heart health in people with diabetes have already been reported.54 However, as previously indicated, the use of ACEI and ARBs in COVID-19 patients with diabetes should be carefully considered. Hyperglycemia is a known side effect of glucocorticoids in both diabetic and non-diabetic people. But even though these substances can worsen insulin resistance, reduce insulin sensitivity, and lead to severe hyperglycemia, they have been used to treat severely ill patients to suppress the high levels of cytokines and c-reactive peptides that are frequently seen in those patients. In clinical trials, no research has indicated that they can reduce mortality or impede virus clearance.53
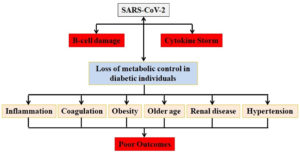
The elevations of IL-6 and IL-10 in COVID-19 are very consistent. The IL-6 receptor is targeted by IL-6, and the receptor recruits JAK, which activates the signal transducer and activator of transcription 3 via a cascade signal.55 Some experts think that tofacitinib, a small molecule medication that targets JAK1 and JAK3, could be used to treat COVID-19 and that tofacitinib was effective in treating COVID-19 patients suffering from ulcerative colitis.56 Because IL-10 can impede the activity of NF-kB to downregulate the synthesis of IL-6, we might think of high levels of IL-10 as negative feedback to counteract the increase in IL-6.57 When utilizing any strategy to modulate cytokine dysregulation, we should closely monitor the laboratory index to avoid over-treatment. For example, if tocilizumab can be administered to lower IL-6 levels, control of IL-6 levels every two days to keep it at a safe level could be investigated in the future.58 People with comorbidities, such as cardiovascular illness, hypertension, and diabetes, have been found to have severe cases of COVID-19. Diabetes has been demonstrated in a growing number of studies to be a significant risk factor for the severity of a variety of different infections. Diabetic patients’ dysregulated immune response has a key role in worsening severity. Diabetes is one of the comorbidities linked to COVID-19-related mortality and morbidity. Cardiovascular diseases, obesity, and hypertension, as well as dysregulated immune response, altered ACE2 expression levels, and endothelial dysfunction, may aggravate the risk of COVID-19 infection in diabetic individuals.
People’s awareness and opinions are likely to influence a large number of safety strategies and, in turn, clinical study findings. As a result, it’s crucial to investigate COVID-19’s unique characteristics in diabetics and treat comorbidities that come with COVID-19 infection, especially among the elderly who have existing critical diseases. Except for corticosteroids, there is little information in the COVID-19 literature about the efficacy and safety of the other prospective treatments. The advantages, duration, dose, and timing of corticosteroids are still up for discussion, and clinical evidence is needed to support the other less promising treatments.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
RD and UR conceptualized the study, performed formal analysis and wrote the manuscript. UR reviewed and edited the manuscript. Both authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Su S, Wong G, Shi W, et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24(6):490-502.
Crossref - Weiss SR, Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol Mol Biol Rev. 2005;69(4):635-664.
Crossref - Bhaskar S, Sinha A, Banach M, et al. Cytokine storm in COVID-19-immunopathological mechanisms, clinical considerations, and therapeutic approaches: the REPROGRAM consortium position paper. Front Immunol. 2020;11:1648.
Crossref - Woo PC, Huang Y, Lau SK, Yuen KY. Coronavirus genomics and bioinformatics analysis. Viruses. 2010;2(8):1804-1820.
Crossref - Brian DA, Baric RS. Coronavirus genome structure and replication. Curr Top Microbiol Immunol. 2005;287:1-30.
Crossref - Paules CI, Marston HD, Fauci AS. Coronavirus infections-more than just the common cold. Jama. 2020;323(8):707-708.
Crossref - Vaninov N. In the eye of the COVID-19 cytokine storm. Nat Rev Immunol. 2020;20(5):277.
Crossref - Weaver LK, Behrens EM. Weathering the storm: improving therapeutic interventions for cytokine storm syndromes by targeting disease pathogenesis. Curr Treatm Opt Rheumatol. 2017;3(1):33-48.
Crossref - Richardson P, Griffin I, Tucker C, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395(10223):e30-e31.
Crossref - Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
Crossref - Ding Y, He LI, Zhang Q, et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203(2):622-630.
Crossref - Shimabukuro-Vornhagen A, Godel P, Subklewe M, et al. Cytokine release syndrome. J Immunother Cancer. 2018;6(1):56.
Crossref - Ghasemzadeh M, Ghasemzadeh A, Hosseini E. Exhausted NK cells and cytokine storms in COVID-19: Whether NK cell therapy could be a therapeutic choice. Hum Immunol. 2022;83(1):86-98.
Crossref - Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420-422.
Crossref - Iannaccone G, Scacciavillani R, Del Buono MG, et al. Weathering the cytokine storm in COVID-19: therapeutic implications. Cardiorenal Med. 2020;10(5):277-287.
Crossref - Tay MZ, Poh CM, Renia L, MacAry PA, Ng LF. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363-374.
Crossref - Song P, Li W, Xie J, Hou Y, You C. Cytokine storm induced by SARS-CoV-2. Clin Chim Acta. 2020;509:280-287.
Crossref - Wang H, Ma S. The cytokine storm and factors determining the sequence and severity of organ dysfunction in multiple organ dysfunction syndrome. Am J Emerg Med. 2008;26(6):711-715.
Crossref - Ye Q, Wang B, Mao J. The pathogenesis and treatment of theCytokine Storm’in COVID-19. J Infect. 2020;80(6):607-613.
Crossref - Okabayashi T, Kariwa H, Yokota SI, et al. Cytokine regulation in SARS coronavirus infection compared to other respiratory virus infections. J Med Virol. 2006;78(4):417-424.
Crossref - Patterson BK, Seethamraju H, Dhody K, et al. CCR5 inhibition in critical COVID-19 patients decreases inflammatory cytokines, increases CD8 T-cells, and decreases SARS-CoV2 RNA in plasma by day 14. Int J Infect Dis. 2021;103:25-32.
Crossref - Channappanavar R, Fehr AR, Vijay R, et al. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19(2):181-193.
Crossref - Uccelli A, de Rosbo NK. The immunomodulatory function of mesenchymal stem cells: mode of action and pathways. Ann N Y Acad Sci. 2015;1351(1):114-126.
Crossref - Ben-Mordechai T, Palevski D, Glucksam-Galnoy Y, Elron-Gross I, Margalit R, Leor J. Targeting macrophage subsets for infarct repair. J Cardiovasc Pharmacol Ther. 2015;20(1):36-51.
Crossref - Yang BX, Farran CA, Guo HC, et al. Systematic identification of factors for provirus silencing in embryonic stem cells. Cell. 2015;163(1):230-245.
Crossref - Golchin A, Seyedjafari E, Ardeshirylajimi A. Mesenchymal stem cell therapy for COVID-19: present or future. Stem Cell Rev Rep. 2020;16(3):427-433.
Crossref - Leng Z, Zhu R, Hou W, et al. Transplantation of ACE2-mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11(2):216-228.
Crossref - Zhang Y, Ma ZF. Impact of the COVID-19 pandemic on mental health and quality of life among residents in Liaoning Province, China: A cross-sectional study. Int J Environ Res Public Health. 2020;17(7):2381.
Crossref - Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39(5):405-407.
Crossref - Ghasemzadeh M, Ahmadi J, Hosseini E. Platelet-leukocyte crosstalk in COVID-19: How might the reciprocal links between thrombotic events and inflammatory state affect treatment strategies and disease prognosis? Thromb Res. 2022;231:179-194.
Crossref - Ramos-Casals M, Brito-Zeron P, Mariette X. Systemic and organ-specific immune-related manifestations of COVID-19. Nat Rev Rheumatol. 2021;17(6):315-332.
Crossref - Karki R, Sharma BR, Tuladhar S, et al. Synergism of TNF-a and IFN-g triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell. 2021;184(1):149-168.
Crossref - Wan S, Yi Q, Fan S, et al. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). MedRxiv. 2020.
Crossref - Jamilloux Y, Henry T, Belot A, et al. Should we stimulate or suppress immune responses to COVID-19? Cytokine and anti-cytokine interventions. Autoimmun Rev. 2020;19(7):102567.
Crossref - Zhou YH, Qin YY, Lu YQ, e al. Effectiveness of glucocorticoid therapy in patients with severe novel coronavirus pneumonia: protocol of a randomized controlled trial. Chin Med J. 2020;10.
- Gheblawi M, Wang K, Viveiros A, et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020;126(10):1456-1474.
Crossref - Shakoory B, Carcillo JA, Chatham WW, et al. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of the macrophage activation syndrome: Re-analysis of a prior Phase III trial. Crit Care Med. 2016;44(2):275.
Crossref - Riegler LL, Jones GP, Lee DW. Current approaches in the grading and management of cytokine release syndrome after chimeric antigen receptor T-cell therapy. Ther Clin Risk Manag. 2019;15:323.
Crossref - Zhou Y, Fu B, Zheng X, et al. Aberrant pathogenic GM-CSF+ T cells and inflammatory CD14+ CD16+ monocytes in severe pulmonary syndrome patients of a new coronavirus. biorxiv. 2020.
Crossref - Stebbing J, Phelan A, Griffin I, et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20(4):400-402.
Crossref - Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. Jama. 2020;323(20):2052-2059.
Crossref - Pu SY, Xiao F, Schor S, et al. Feasibility and biological rationale of repurposing sunitinib and erlotinib for dengue treatment. Antiviral Res. 2018;155:67-75.
Crossref - Espinosa JM. Down syndrome and COVID-19: a perfect storm? Cell Rep Med. 2020;1(2):100019.
Crossref - Rubin R. Baricitinib is first approved COVID-19 immunomodulatory treatment. JAMA. 2022 Jun 21;327(23):2281.
- Schett G, Manger B, Simon D, Caporali R. COVID-19 revisiting inflammatory pathways of arthritis. Nat Rev Rheumatol. 2020;16(8):465-470.
Crossref - Carrasco-Sanchez FJ, Lopez-Carmona MD, Martinez-Marcos FJ, et al. Admission hyperglycaemia as a predictor of mortality in patients hospitalized with COVID-19 regardless of diabetes status: data from the Spanish SEMI-COVID-19 Registry. Ann Med. 2021;53(1):103-116.
Crossref - Cariou B, Hadjadj S, Wargny M, et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020;63(8):1500-15.
- Chen Y, Yang D, Cheng B, et al. Clinical characteristics and outcomes of patients with diabetes and COVID-19 in association with glucose-lowering medication. Diabetes Care. 2020;43(7):1399-407.
Crossref - Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259-260.
Crossref - Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110-118.
Crossref - Hsia DS, Grove O, Cefalu WT. An update on SGLT2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2017;24(1):73.
Crossref - Filippatos TD, Panagiotopoulou TV, Elisaf MS. Adverse effects of GLP-1 receptor agonists. Rev Diabet Stud. 2014;11(3):202-230.
Crossref - Solerte SB, D’Addio F, Trevisan R, et al. Sitagliptin treatment at the time of hospitalization was associated with reduced mortality in patients with type 2 diabetes and COVID-19: a multicenter, case-control, retrospective, observational study. Diabetes Care. 2020;43(12):2999-3006.
Crossref - Drucker DJ. Coronavirus infections and type 2 diabetes-shared pathways with therapeutic implications. Endocr Rev. 2020;41(3):bnaa011.
Crossref - Gillespie EL, White CM, Kardas M, Lindberg M, Coleman CI. The impact of ACE inhibitors or angiotensin II type 1 receptor blockers on the development of new-onset type 2 diabetes. Diabetes Care. 2005;28(9):2261-2266.
Crossref - Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295.
Crossref - Jacobs J, Clark-Snustad K, Lee S. Case report of a SARS-CoV-2 infection in a patient with ulcerative colitis on tofacitinib. Inflamm Bowel Dis. 2020;26(7):e64.
Crossref - Rojas JM, Avia M, Martin V, Sevilla N. IL-10: a multifunctional cytokine in viral infections. J Immunol Res. 2017;2017.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.