ISSN: 0973-7510
E-ISSN: 2581-690X
The health status of the individuals is determined by the microflora inhabiting the gastrointestinal tract. One way to restore the balance of the gastrointestinal microflora is the use of probiotics on the Lactobacillus strains. Probiotic bacteria must have a set of properties that allow them to compete with pathogens and opportunistic pathogens in the gut. Antimicrobial activity, antibiotic susceptibility, tolerance to bile and salt of Lactobacillus strains (Lactobacillus casei 3 B-RKM 0008, Lactobacillus plantarum 8RA-3 pl B-RKM 0015, Lactobacillus sakei 24a B-RKM 0559) we studied. The results showed that L. casei 3 B-RKM 0008 and L. plantarum 8RA-3 pl B-RKM 0015 showed antagonism toward conditionally pathogenic microorganisms, including Escherichia coli ATCC 25922 B-RKM 447, Staphylococcus aureus 209P B-RKM 0057, Serratia marcescens 221F B-RKM 0059, and Candida albicans ATCC 885-653 Y-RKM 0475. Strains of Lactobacillus were resistant to gentamicin, ofloxacin, ciprofloxacin, levofloxacin, cefotaxime, ceftriaxone, ceftazidime, amicacin. Lactobacillus strains to be able to grow in the bile (0.5-20%) and salt (2-6%). These results suggest that these strains may be used in the future as probiotic cultures for manufacturing of novel synbiotic product.
Lactobacillus, Antimicrobial activity, Antibiotic, Bile, Salt.
The microbial ecology in the gastrointestinal tract influences many functions in our body. These are digestion, absorption of nutrients, detoxification. It is finally affecting the functioning of immune system. Hence, the balance in microbiota of gut is focused to provide the colonization resistance against infectious agents and to promote anti-allergicprocesses and to reduce hypersensitivity 1.
Probiotics have been defined as viable microorganisms that, when administrated in adequate amounts, exert beneficial effects in the prevention and treatment of specific pathologic conditions. Studies have suggested that they enhance gut barrier function, normalize intestinal milieu, synthesize antibacterial substances, and stimulate immunity 2-3. Also, probiotics such as these have good safety and tolerability profiles, and side effects are uncommon 4.
Lactobacilli are an important part of the normal flora commonly found in the mouth, gastrointestinal tract and female genitourinary tract 5. Because they produce organic acids, hydrogen peroxide and bacteriocins, many strains of lactobacilli show antagonistic activity toward pathogenic and conditionally pathogenic microorganisms. Recently, increasing attention has been given to their probiotic, health-promoting capacities, among which their antagonistic potential against pathogens plays a key role 6-7.
Within the latter genus several species are currently used as probiotics, including Lactobacillus acidophilus, Lactobacillus casei/paracasei, Lactobacillus fermentum, Lactobacillus johnsonii, Lactobacillus paracasei, Lactobacillus plantarum, Lactobacillus reuteri, Lactobacillus rhamnosus and Lactobacillus salivarius 8.
Probiotic potential included survival in gastrointestinal simulated juice, antagonistic and bacteriocin activity, acid pH and bile tolerance, antibiotic resistance to antibiotics, adhesion ability and others 9-11.
The genera Lactobacillus, Lactococcus, Leuconostoc, Pediococcus, and Enterococcus have been associated with more than 300 different bacteriocins. The inhibition spectra of the bacteriocins produced by these lactic acid bacteria can be broad or narrow, but in general the bacteriocins exhibit inhibition against closely related Gram-positive bacteria, although several bacteriocins have been shown to be active against certain Gram-negative bacteria, including pathogenic species of Escherichia and Salmonella 12-14. Bacteriocins might warrant serious consideration as alternatives to traditional antibiotics. These molecules exhibit significant potency against other bacteria, including antibiotic-resistant strains 15. Resistance to antimicrobial drugs (antibiotics) is a common characteristic in the world of lactic acid bacteria 16. Moreover, Lactobacilli, Pediococci and Leuconostoc spp. have been reported to be highly resistant to vancomycin and some Lactobacilli have high resistance to bacitracin, cefoxitin, ciprofloxacin, fusidic acid, streptomycin, sulphadiazine, teicoplanin, vancomycin, erythromycin, gentamicin and kanamycin 17-20. Lactic acid bacteria may serve as reservoirs of antibiotic resistance genes potentially transferable to human pathogens. Hence, there is a growing interest in the possible role of lactic acid bacteria as vectors of antibiotic resistance determinants 21. Tolerance to NaCl, gastric acid and bile has thus become important selection criterion for probiotic strains 22-23. Different stress factors might considerably affect viability or performance of the Lactobacillus strains 24.
The objective of this study was to identify the probiotic properties of Lactobacillus strains to develop the synbiotic product, based on Lactobacillus strains and plant extracts for Kazakh population.
Bacterial strains
Lactobacillus casei 3 B-RKM 0008, Lactobacillus plantarum 8RA 3-pl B-RKM 0015, Lactobacillus sakei 24a B-RKM 0559 and Indicator microorganisms, such Escherichia coli ATCC 25922 B-RKM 447, Staphylococcus aureus 209P B-RKM 0057, Serratia marcescens 221F B-RKM 0059, and Candida albicans ATCC 885-653 Y-RKM 0475, were purchased from Republican Collection of Microorganisms, Ministry of Education and Science of the Republic of Kazakhstan (Astana, Kazakhstan).
Validation of Lactobacillus by Culture and Biochemical tests
Culture and Biochemical properties of all the strains Lactobacillus were done by Gram staining, Catalase and Oxidase test 25-28.
Antagonistic activity
Antagonistic activity of Lactobacillus strains was detected by agar well diffusion method on Muller Hinton agar, previously inoculated with 0.1 ml of a 24 h broth culture of indicator microorganisms. The indicator bacteria E. coli ATCC 25922, S. aureus 209P, S. marcescens 221F were incubated in Nutrient Broth (NB, HiMedia, Mumbai, India) at 37°C, and C. albicans ATCC 885-653 in Sabouraud Dextrose Broth (SDB, HiMedia) at appropriate temperature for 24 h. The 100 µl of Lactobacilli inoculum were loaded into each well (diameter 5 mm). Plates were incubated at 37°C for 48 hours. After incubation, all plates were examined for the presence of zone of inhibition around the Wells 29.
Bacteriocin-producing activity
Study of bacteriocin production by Lactobacillus used the agar diffusion method and the same indicator strains as above. To 15 ml of 0.7% semiliquid Man, Rogosa, Sharp (MRS, HiMedia) agar cooled to 50°C was added 1 ml of indicator culture (108 CFU/ml) (McFarland standard set, HiMedia). After solidification, three holes per plate of 5-mm diameter were excavated for each tested Lactobacillus strains and 35 µl of supernatant were added to each well. Supernatants of Lactobacillus strains were prepared as follows: 1 ml of lyophilized culture was added to 20 ml of liquid medium MRS and incubated for 16 h at 37°C, after which 1 ml of the broth from the cell suspension was subcultured into 20 ml of liquid MRS medium and incubated for a further 16 h. Thereafter, the cells were removed by centrifugation at 3,095×g for 5 min (Eppendorf Centrifuge 5810 R, Germany). Supernatant was added to the first well. To eliminate inhibitory activity due to organic acids, the pH of the supernatant was adjusted to pH 6.0 with 1 M NaOH and that solution was added to the second well in a volume of 35 µl. Supernatant with pH 6.0 was added to the third well, along with 1 mg/ml of catalase (Sigma, USA) to remove hydrogen peroxide. The plates were incubated for 1 day. A positive result for the presence of bacteriocin in the supernatant was the presence of an inhibition zone of the indicator strain around the third well 30.
Antibiotics susceptibility
The susceptibility of the Lactobacillus strains were tested against different antibiotics classes including Penicillin, Cephalosporine, Tetracycline, Glycopeptides, Quinolone, Lincozamids, Carbapenems, Aminoglycoside, Macrolides and others preparations. The bacterial suspension (108 CFU/ml) was inoculated onto MRS agar (HiMedia, India) plates using swabbing technique. Then antibiotics disks were deposited on the plates. The susceptibility / resistance to these antibiotics was examined after incubation at 37°C for 24 h the inhibition zones around the disks 31.
Testing of NaCl tolerance
For the determination of NaCl tolerance of the Lactobacillus strains three test tube containing MRS broth (HiMedia, India) were adjusted with different concentration 2%, 4%, 6%, 10% of NaCl. After sterilization, each test tube inoculated with 1% (v/v) frech over night culture of Lactobacillus strains and incubated at 37°C for 24 h. After 24 h of incubation their growth were determined by observing their turbidity. Maximum growth were indicated as double positive sign (++), normal growth as single positive sign (+) and no growth were indicated as negative sign (-) 32.
Testing of bile tolerance
The Lactobacillus strains were inoculated in MRS broth (HiMedia, India) supplemented with 0.5%, 1%, 3%, 5%, 10%, 20% of bile bovine (Samson-Med, Russia) along with a control for each of the strains. The cultures were observed for growth for a period of 1 day 33.
Statistical analysis
Our experiments were performed in triplicate and results elaborated as mean ± standard error of the mean of three experiments. The statistical significance was assessed by Student¼s t test. Results were considered significant at p<0.05.
Three strains of Lactobacillus sakei 24a B-RKM 0559, isolated from Kazakh national product kazy, Lactobacillus casei 3 B-RKM 0008, isolated from human fecal, and Lactobacillus plantarum 8RA 3-pl B-RKM 0015, isolated from commercial preparation «Lactobacterin» were studied. All the strains of Lactobacillus were validated for their morphological and biochemical characteristics: Gram positive rods (Figure 1), No-spore, Catalase and Oxidase negative test. Colonies on medium MRS agar (HiMedia, India) are smooth, round, shiny, color cream, with straight edge, convex profile, texture thick, diameter 1.0-3.0 mm.
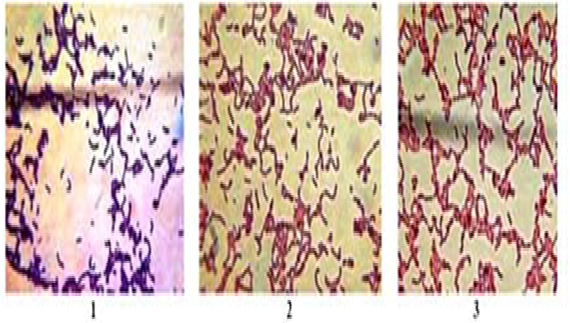 Fig. 1. Morphology of Lactobacillus strains under light microscope after Gram¼s reaction, 100×1 – L. casei 3 B-RKM 0008, 2 – L. plantarum 8RA-3 pl B-RKM 0015, 3 – L. sakei 24à Â-RKM 0559
Fig. 1. Morphology of Lactobacillus strains under light microscope after Gram¼s reaction, 100×1 – L. casei 3 B-RKM 0008, 2 – L. plantarum 8RA-3 pl B-RKM 0015, 3 – L. sakei 24à Â-RKM 0559Antagonistic activity
In recent decades, the selection of microbial molecules and/or bacterial strains able to produce antagonistic molecules to be used as antimicrobials and preservatives has been attracting scientific interest, in order to eliminate or reduce chemical additives34. The antagonistic activity of the three Lactobacillus strains (L. sakei240, L. plantarum 8RA-3 pl, L. casei 3) was screened against E. coli “!! 25922 -R“ 0447, S. aureus209 -R“ 0057, S. marcescens221F -R“ 0059 and C. albicans “!! 885-653 Y-R“ 0475 were determined by measuring the zone of inhibition. L. casei 3 B-RKM 0008 and L. plantarum 8RA-3 pl were able to inhibit all the testing pathogenic microorganisms such as E. coli, S. aureus, S. marcescens and C. albicans. Whereas, L. sakei 24a failed to show effect over C. albicans. The strain L. casei 3 B-RKM 0008 had high antagonistic activity toward all investigated test strains; the diameter of the zones of inhibition was range 10-13 mm. The effect of the three strains of Lactobacillus could be understood from the Figure 2.
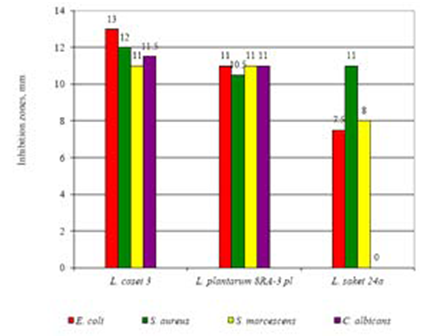 Fig. 2. Inhibition spectrum of Lactobacillus sp
Fig. 2. Inhibition spectrum of Lactobacillus spBacteriocin-producing activity
One important attribute of lactic acid bacteria is their ability to produce antimicrobial compounds such as organic acids, diacetyl, hydrogen peroxide, ethanol, reuterin and bacteriocins or bactericidal proteins. In recent years, interest in bacteriocins has grown substantially due to their potential usefulness as natural food preservatives in addition to promoting good health35. We investigated bacteriocin-producing activity of culture supernatants of Lactobacillus strains. Figure 3 illustrates typical results of study of bacteriocin production of L. sakei 24a toward E. coli.
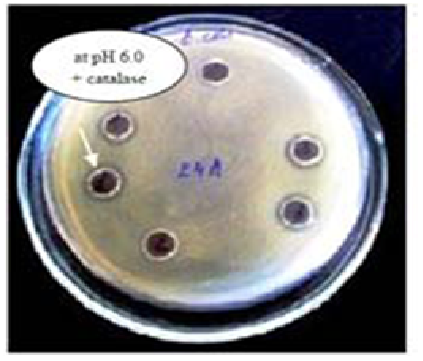 Fig. 3. Bacteriocin activity of L. sakei 24a Â-RKM 0559
Fig. 3. Bacteriocin activity of L. sakei 24a Â-RKM 0559It is noteworthy that all the isolates exerted extracellular antimicrobial activity against E. coli ATCC 25922 B-RKM 0447, S. aureus 209 B-RKM 0057, S. marcescens 221F B-RKM 0059 and C. albicans ATCC 885-653 Y B-RKM 0475. Supernatants of Lactobacillus strains no showed antagonism only without addition of alkali, and in front of bacteriocin-producing activity manifested by the addition of catalase, which indicates the presence of bacteriocins in these Lactobacillus strains (Table 1).
Table (1):
Bacteriocin-producing activity of Lactobacillus strains.
| Test-cultures | Variant | Bacteriocin-producing activity of LAB (mm) | ||
|---|---|---|---|---|
| L. casei 3 B-RKM 0008 | L. plantarum 8RA-3 pl B-RKM 0015 | L. sakei 240 B-RKM 0559 | ||
| E. coli* | control | 7.0±1.08 | 6.0±0.58 | 8.0±0.71 |
| + NaOH pH 6,0 | – | – | – | |
| рН 6,0 +catalase | 6.0±0.13 | 6.0±1.0 | 8.0±0.58 | |
| S. aureus** | control | 7.0±0.71 | 6.0±0.58 | 8.0±0.91 |
| + NaOH pH 6,0 | – | – | – | |
| рН 6,0 +catalase | 6.0±0.58 | 6.0±1.0 | 9.0±0.58 | |
| Ser. marcescens | control | 6.0±0.41 | 6.0±0.91 | 6.0±0.58 |
| + NaOH pH 6,0 | – | – | – | |
| рН 6,0 +catalase | 6.0±0.58 | 7.0±0.41 | 6.0±1.0 | |
| C. albicans*** | control | 7.0±0.91 | 7.0±0.82 | 6.0±1.0 |
| + NaOH pH 6,0 | – | – | – | |
| рН 6,0 +catalase | 7.3±0.33 | 7.0±0.58 | 6.0±0.41 | |
*Р˂0,02
**P˂0,5
***P˂0,1
Antibiotics susceptibility
Medical treatment with antibiotics can lead to the elimination of essential intestinal microflora and make it an easy target for pathogens. In order to prevent and restore the equilibrium in the gastrointestinal tract it is important to study the susceptibility of the lactobacilli strains to the action of a variety of antibiotics, used in the clinical practice 36. The susceptibility of Lactobacillus strains were tested against 24 of the most frequently used in medical practice antibiotics with different mechanisms of action. The results of the studies antibiotics susceptibility are summarized in Table 2.
Table (2):
Antibiotic susceptibility of the Lactobacillus strains.
| Class | Antibiotics | Quantity | Antibiotic susceptibility | ||
|---|---|---|---|---|---|
| L. sakei 24а В-RKM 0559 | L. plantarum 8RA 3-pl В-RKM 0015 | L. casei 3 В-RKM 0008 | |||
| Penicillin | Ampicillin | 25 µg/disc | S | S | S |
| Amoxycillin | 25 µg/disc | S | S | S | |
| Piperacillin | 30 µg/disc | S | S | S | |
| Carbenicillin | 100 µg/disc | S | S | S | |
| Quinolone | Ciprofloxacin | 30 µg/disc | S | S | R |
| Ofloxacin | 5 µg/disc | S | S | R | |
| Levofloxacin | 5 µg/disc | S | S | R | |
| Lincosamid | Lincomicin | 15 µg/disc | S | S | S |
| Carbapenem | Meropenem | 10 µg/disc | S | S | S |
| Imipenem | 10 µg/disc | S | S | S | |
| Glycopeptid | Vancomycin | 30 µg/disc | R | S | R |
| Cephalosporine | Cefazolin | 30 µg/disc | S | S | S |
| Cefuroxime | 30 µg/disc | S | S | S | |
| Cefamandol | 30 µg/disc | S | S | S | |
| Cefotaxime | 30 µg/disc | R | S | S | |
| Ceftriaxone | 30 µg/disc | R | S | S | |
| Ceftazidime | 30 µg/disc | R | R | S | |
| Aminoglycoside | Amicacin | 30 µg/disc | S | R | R |
| Gentamicin | 30 µg/disc | S | R | S | |
| Macrolide: | Erythromycin | 15 µg/disc | S | S | S |
| Clarithromycin | 30 µg/disc | S | S | S | |
| Azithromycin | 30 µg/disc | S | S | S | |
| Tetracycline: | Doxycycline | 30 µg/disc | S | S | S |
| Others: | Linezolid | 30 µg/disc | S | S | S |
R – Resistant, S -Sensitive
L. casei 3 B-RKM 0008 was resistant to all antibiotics from the group Quinolone (ciprofloxacin, ofloxacin, levofloxacin). L. sakei 240 B-RKM 0559 was resistant to all antibiotics from the group Cephalosporine (cefotaxime, ceftriaxone, ceftazidime) with the exception of cefazolin, cefuroxime, cefamandol. From the group of Aminoglycoside the strain L. plantarum 8RA-3 pl B-RKM 0015 was resistant to gentamicin and amicacin. The both strains L. sakei 240 B-RKM 0559 and L. casei 3 B-RKM 0008 were resistant to vancomycin.
Bile and salt tolerance
The ability of Lactobacillus strains to tolerate the effect of different concentrations of bile (0.5-20%) and NaCl (2-10%) after incubation for 24 h tested. The results were shown in Table 3.
Table (3):
Bile and salt tolerance of the Lactobacillus strains.
| Tolerance |
Lactobacillus strains | ||
|---|---|---|---|
| L. casei 3 B-RKM 0008 | L. plantarum 8RA-3 pl B-RKM 0015 | L. sakei 240 B-RKM 0559 | |
| Bile | |||
| 0.5% | ++ | ++ | ++ |
| 1% | ++ | ++ | ++ |
| 3% | ++ | ++ | ++ |
| 5% | ++ | ++ | ++ |
| 10% | ++ | ++ | ++ |
| 20% | – | – | + |
| Salt | |||
| 2% | ++ | ++ | ++ |
| 4% | + | + | + |
| 6% | + | + | – |
| 10% | – | – | – |
++, good growth
+, visible growth
-, no growth
From the data in Table 3, all the Lactobacillus strains (L. sakei240, L. plantarum 8RA-3 pl, L. casei 3) were able to survive over the range of 0.5-20% w/v supplementation of bile in MRS broth. The growth of the strains declined with increased supplementation of 20% bile. However, the strain L. sakei 240 B-RKM 0559 showed good growth at 20% bile concentration.
As for NaCl tolerance, all the Lactobacillus strains were able to tolerate 2-6% NaCl except L. sakei 240 B-RKM 0559 which unable to grow at 6%. As for 10% NaCl, all the Lactobacillus strains were unable to grow.
The intestine is an extremely complex living system that participates in the protection of the host through a strong defense against aggressions from the external environment. This defensive task is based on 3 constituents that are in permanent contact and dialog with each other: the microflora, mucosal barrier, and local immune system 37. The use of probiotics is increasing in popularity for the prevention and treatment of intestinal infection and disease.
Animal models and human clinical trials indicate that probiotics may reduce intestinal inflammation and alleviate symptoms of colorectal cancer 38. One of the main components of probiotics are bacteria of the genus Lactobacillus 39-40. Numerous publications have demonstrated the safety and efficacy of probiotics based on Lactobacillus and Bifidobacteria 41-42. Moreover, probiotic strains selected by probiotic and technological properties: tolerance to low pH, bile salt, antimicrobial potential, auto-aggregation ability, microbial adhesion to solvents, tolerance to high temperature and osmotic pressure 43-47.
It is known that the most effective strains that are typical for the region, which can be adapted to the specific macroorganism. Thus, creation and prescription of probiotic preparations should be dependent on the regional group of the population with a special phenotype, genotype, lifestyle, choice of food, environmental conditions 48-49.
The aim of this work was evaluation the probiotic potential of Lactobacillus strains
(L. sakei 240 B-RKM 0559, L. casei 3 -RKM 0008, and L. plantarum 8RA 3-pl B-RKM 0015) for creation combined product for Kazakh population.
The present study showed that L. casei 3 -RKM 0008, L. sakei 24a and L. plantarum 8RA 3-pl B-RKM 0015 inhibited growth of pathogenic bacteria, such E. coli, S. aureus, Ser. marcescens. Therefore they are able to prevent the growth of other pathogenic microorganisms in gut system. Furthermore, Georgieva et al. (2015) and also reported antagonistic effect against S. aureus, E. coli, B. cereus and C. albicans 50. L. casei 3
B-RKM 0008 and L. plantarum 8RA 3-pl B-RKM 0015 were able to inhibit of pathogenic microflora of the urogenital tract with protection against C. albicans as demonstrated by previous studies 51-52.
Bacteriocin production, along with the production of for example, lactic acid, hydrogen peroxide, and lysozyme, relates to antagonism 53-54. L. casei 3 B-RKM 0008, L. plantarum 8RA 3-pl -RKM 0015 and L. sakei 24a B-RKM 0559 possessed antagonistic activity against Gram-positive bacteria (S. aureus), Gram-negative bacteria (E. coli, Ser. marcescens) and Yeast (C. albicans). Earlier studies reported that bacteriocins not all of lactic acid bacteria are inefficient to inhibit E. coli because the outer membrane hinders the site for bacteriocin action 55-56.
The Bacteriocin activity of the Lactobacillus strains, especially L. sakei 240 B-RKM 0559 against S. aureus shoded a maximum inhibition zone of 9.0±0.58 mm at @” 6,0 + catalase. For comparison, Mbawala et al. (2013) in his work to get a maximum inhibition zone of Lactobacillus spp. 4.5±0.1 mm 57. Thereby it was established that Lactobacillus strains had antagonistic activity against pathogenic bacteria and yeast.
Antimicrobial resistance in microbes poses a global and increasing threat to public health 58. The Lactobacillus strains were found resistant to clinically relevant antibiotics to cure infections. Almost all of the tested strains (L. casei 3 B-RKM 0008, L. plantarum 8RA-3 pl B-RKM 0015, L. sakei 240 B-RKM 0559) were resistant to ciprofloxacin, ofloxacin, levofloxacin, vancomycin, cefotaxime, ceftriaxone, ceftazidime, amicacin and gentamycin.
Similar results resistance to ciprofloxacin and gentamycin were also observed by Pithva et al. (2014), resistance to ofloxacin – by Hyacinta et al. (2015), resistance to amicacin and levofloxacin – by Sharma et al. (2016) where they examined antibiotic resistance of Lactobacillus strains 59-61. But, Haghshenas et al. (2016) shoded that the Lactobacillus plantarum 15HN was sensitive to vancomycin and gentamycin 62. Antibiotics resistance of Lactobacillus could also be regarded as a beneficial property. A resistant probiotic strain that is co-administered with an antibiotic may reduce the gastrointestinal side effects related to antibiotic treatment 63.
Bile tolerance is one of the essential properties required for lactic acid bacteria to survive in the small intestine and to be functionally effective intestine 64. For a probiotic strain to be able to perform effectively in the gastrointestinal tract, it must overcome the antimicrobial challenge posed by bile. In this study, three Lactobacillus strains were tested for bile tolerance. These observations do not agree with those reported by Park et al. (2006) who showed that four strains Lactobacillus acidophilus were slightly suppressed over time and showed bile resistance at 3-5% ox gall 65. Ours results indicate that Lactobacillus strains selected for probiotic tolerant to bile at a concentration of up to 20%. Tolerance to bovine bile for Lactobacillus spp. has been studied by Elcioglu et al. (2014) and Khagwal et al. (2014) 66-67.
While sodium chloride is growth inhibitory to several other types of bacteria, the probiotic organisms withstand high salt concentration in the human gut 68. All the Lactobacillus strains had good growth up to 2-6% concentration in the culture medium, with the exception of L. sakei 240 B-RKM 0559. Our results have the similarities with the findings of Pundir et al. (2013) that were tolerable to 1-6% NaCl 69. Bhardwaj et al. (2016) demonstrated that no growth even for a single Lactobacillus strain was noticed at the sodium chloride concentration of 6.5% 70. Wang et al. (2016) estabilished that increase in the salt concentrations above 6% resulted in decrease of bacterial density L. plantarum ATCC 14917 71. Tolerance to NaCl is important because the Kazakh national food such kurt, kazy, shuzhuk contain a lot of salt. From the obtained results we can conclude that Lactobacillus strains (L. sakei 240 -RKM 0559, L. casei 3 -RKM 0008, and L. plantarum 8RA 3-pl -RKM 0015) showed potential as a probiotic owing to its antibiotic resistance, antimicrobial potential and tolerance to bile and salt.
In this study, we studied of probiotic potential of Lactobacillus strains (L. sakei 24a B-RKM 0559, L. casei 3 B-RKM 0008, and L. plantarum 8RA 3-pl B-RKM 0015). These strains are therefore good candidates for development of biological products for the Kazakh population in view of geographical region-specificity.
ACKNOWLEDGMENTS
This work was financially supported by the Scientific Committee of the Ministry of Education and Science of Republic of Kazakhstan (Project number GF 0982).
- Mishra, A., Sharma, K.P. Isolation and characterization of probiotic microorganism from fermented dairy products. GERF Bulletin of Biosciences, 2014; 5 (1): 10-4.
- Zeng, J., Jiang, J., Zhu, W., Chu, Y. Heat-killed yogurt-containing lactic acid bacteria prevent cytokine-induced barrier disruption in human intestinal Caco-2 cells. Ann. Microbiol., 2016; 66: 171-8.
- Iqbal, M.Z., Qadir, M.I., Hussain, T., Janbaz, K.H., Khan, Y.H., Ahmad, B. Review: probiotics and their beneficial effects against various diseases. Pak. J. Pharm. Sci., 2014; 27(2): 405-15.
- Panwar, H., Calderwood, D., Grant, I.R., Grover, S., Brian, D. Green Lactobacilli possess inhibitory activity against dipeptidyl peptidase-4 (DPP-4). Ann. Microbiol., 2016; 66: 505–9.
- Prema, P., Viji, P. Antibacterial activity of a probiotic Lactobacillus plantarum against urinary tract infection causing pathogens. World Journal of Pharmaceutical Research, 2015; 4(4): 2032-41.
- Dec, M., Puchalski, A., Urban-Chmiel, R., Wernicki, A Screening of Lactobacillusstrains of domestic goose origin against bacterial poultry pathogens for use as probiotics. Poultry Science, 2014; 93(10): 2464-72.
- Xiaoming, L., Wenyu, L., Qiuxiang, Z. et al. Screening of lactobacilli with antagonistic activity against enteroinvasive Escherichia coli. Food Control., 2013; 30(2): 563-8.
- Guidone, A., Zotta, T., Ross, R.P., Stanton, C., Rea, M.C., Parente, E., Ricciardi, A. Functional properties of Lactobacillus plantarum strains: A multivariate screening study. LWT – Food Science and Technology, 2014; 56: 69-76.
- Mahasneh, A.M., Hamdan, S., Mahasneh, S.A. Probiotic Properties of Lactobacillus Species Isolated from Local Traditional Fermented Products. Jordan Journal of Biological Sciences, 2015; 8(2): 81-7.
- Reis, N.A., Saraiva, M.A.F., Duarte, E.A.A., de Carvalino, E.A., Vieira, B.B., Evangelista-Barreto, N.S. Probiotic properties of lactic acid bacteria isolated from human milk. Journal of Applied Microbiology, 2016; 121(3): 811-20.
- Tulumoglu, S., Kariptas, E., Erdem, E., Idil, O., Berksov, E.A. Investigation of probiotic properties of lactobacilli bacteria isolated from human gastrointestinal tract. Journal of Food Safety and Food Quality, 2014; 65(6): 151-63.
- Chin, Y.Z., Velu, S., Bakar, F.A., Nor-Khaizura, M.R. Characterization and the influence of milk solids-not-fat on the bacteriocin produced by Lactococcus lactis subsp. lactis S20 isolated from Chinese sauerkraut, a traditional fermented vegetable. Ann. Microbiol., 2016; 66: 673-84.
- Van Heel, A.J., Montalban-Lopez, M., Kuipers, O.P. Evaluating the feasibility of lantibiotics as an alternative therapy against bacterial infections in humans. Expert. Opin. Drug Metab. Toxicol., 2011; 7(6): 675-80.
- Masuda, Y., Ono, H., Kitagawa, H., Ito, H., Mu, F., Sawa, N., Zendo, T., Sonomoto, K. Identification and Characterization of Leucocyclicin Q, a Novel Cyclic Bacteriocin Produced by Leuconostoc mesenteroides TK41401. Appl. Environ. Microbiol., 2011; 77(22): 8164-70.
- Cotter, P.D., Ross, R.P., Hill, C. Bacteriocins – a viable alternative to antibiotics? Nat. Rev. Microbiol. 2013; 11: 95-105.
- Korhonen, J. Antibiotic Resistance of Lactic Acid Bacteria. Kuopio: University of Eastern Finland, 2010; pp 5-6.
- Shazali, N., Foo H.L., Loh, T.C., Choe, D.W., Rahim, R.A. Prevalence of antibiotic resistance in lactic acid bacteria isolated from the faeces of broiler chicken in Malaysia. Gut Pathogens, 2014; 6(1): 1-7.
- Cherkashin, A., Chobert, J.M., Efimochkina, N., Sheveleva, S., Haertlé, T. Antibiotic Resistance of lactic Acid Bacteria Used in Russian Dairy and Probiotic Products. European Researcher, 2013; 44(3-2): 654-9.
- Sukmarini L., Mustopa, A.Z., Normawati, M., Muzdalifah, I. Identification of Antibiotic-Resistance Genes from Lactic Acid Bacteria in Indonesian Fermented Foods. Hayati Journal of Biosciences, 2014; 21(3): 144-50.
- Owusu-Kwarteng, J., Tano-Derrah, K., Akabanda, F., Jespersen, L. Technological properties and probiotic potential of Lactobacillus fermentum strains isolated from West African fermented millet dough. BMC Microbiology, 2015; 15: 261.
- Bulajic, S., Mijacevic, Z. Antimicrobial susceptibility of lactic acid bacteria isolated from sombor cheese. Acta Veterinaria, 2011; 61(2-3): 247-58.
- Presti, I., D’Orazio, G., Labra, M. et al. Evaluation of the probiotic properties of new Lactobacillus and Bifidobacterium strains and their in vitro effect. Applied Microbiology and Biotechnology, 2015; 99(13): 5613-26.
- Fessard, A., Bourdon, E., Payet, B., Remize, F. Identification, stress tolerance, and antioxidant activity of lactic acid bacteria isolated from tropically grown fruits and leaves. Canadian Journal of Microbiology, 2016; 62(7): 550-61.
- Ferrando, V., Quiberoni, A., Reinhemer, J., Suarez, V. Resistance of functional Lactobacillus plantarum strains against food stress conditions. Food Microbiology, 2015; 48: 63-71.
- Netrusov, A.I. Workshop on microbiology. Moscow: Academia, 2005; pp 531-532.
- Tajabadi, N., Mardan, M., Saari, N., Mustafa, S., Bahreini, R., Abdul Manap, M.Y. Identification of Lactobacillus plantarum, Lactobacillus pentosus and Lactobacillus fermentum from honey stomach of honeybee. Brazilian Journal of Microbiology, 2013; 44(3): 717-22.
- Holt, J.H. et al. Bergey‘s Manual of determinative bacreiology, 9 th ed. Moscow: Mir, 1997; pp 574-6.
- Viswanathan, S., Preethi, G., Veilumuthu, P., Amuthan, M., Rajesh, R., Suba, P. Probiotic Studies in Colostrum of Buffalo. Global Veterinaria, 2015; 14(2): 199-04.
- Sharma, P.K., Roy, R., Sathiavelu, M., Arunachalam, S. Study of Probiotic and Antioxidant activity of Lactobacillus spp. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 2013; 4(4): 809-19.
- Yang, E., Fan, L., Doucette, C., Fillmore, S. Antimicrobial activity of bacteriocin-producing lactic-acid bacteria isolated from cheeses and yogurts. AMB Express a Springer Open Journal, 2012; 73: 134-9.
- Sahraoui, Y., Fayolle, K., Leriche, F., Fleche-Mateos, A., Sadoun, D. Antibacterial and technological properties of Lactococcus lactis ssp. Lactis KJ660075 strain selected for its inhibitory power against Staphylococcus aureus for cheese quality improving. Journal of Food and Technology, 2015; 52(11): 7133-42.
- Hoque, M.Z., Akter, F., Hossain, K.M., Rahman, M.S.M., Billah, M.M., Islam, K.M.D. Isolation, Identification and Analysis of Probiotic Properties of Lactobacillus Spp. From Selective Regional Yoghurts. World Journal of Dairy and Food Sciences, 2010; 5(1): 39-46.
- Subhashini, Bioprospecting of Lactic Acid Bacteria for Potentiality as Probiotics. International Journal of Microbiological Research, 2014; 5(2): 90-7.
- Arena, M.P., Silvain, A., Normanno, G., Grieco, F., Drider, D., Spano, G., Fiocco, D. Use of Lactobacillus plantarum Strains as a Bio-Control Strategy against Food-Borne Pathogenic Microorganisms Frontiers in Microbiology, 2016; 7: 464.
- Princewill-Ogbonna, I.L., Ojimelukwe, P.C. Bacteriocins from lactic acid bacteria inhibit food borne pathogens. IOSR Journal Of Environmental Science, Toxicology And Food Technology, 2014; 8(1): 50-6.
- Nemska,V., Georgieva, N., Danova, S. Initial characterization and antibiotic susceptibility of lactic acid bacteria isolated from traditional dairy products. Scientific works of University of food technologies, 2015; 62: 510-3.
- Bourlioux, P., Koletzko, B., Guarner, F., Braesco, V. The intestine and its microflora are partners for the protection of the host: report on the Danone Symposium. American Journal of Clinical Nutrition, 2003; 78(4): 675-83.
- Zhang, M., Fan, X., Fang B., Zhu, C., Zhu, J., Ren, F. Effects of Lactobacillus salivarius Ren on cancer prevention and intestinal microbiota in 1, 2-dimethylhydrazine-induced rat model. Journal of Microbiology, 2015; 53(6): 398–05.
- Hijova, E., Kuzma, J., Strojny, L. et al. Effect of Lactobacillus plantarum LS/07 on intestinal bacterial enzyme activities in the prevention of cancer, atherosclerosis and dysbiosis. Acta Veterinaria Beograd, 2016; 66 (3): 294-03.
- Buzas, G.M. Probiotics in gastroenterology – from a different angle. Orvosi Hetilap, 2013; 154(8): 294-04.
- Borriello, S.P., Hammes, W.P., Holzapfel, W., Marteau, P., Schrezenmeir, J., Vaara, M., Valtonen, V. Safety of probiotics that contain lactobacilli or bifidobacteria. Clin. Infec. Dis., 2003; 36: 775-80.
- Guarner, F., Malagelada, J.R. Gut flora in health and disease. Lancet., 2003; 361(9356): 512-9.
- Tsend-Ayush, Ch., Ganina, V.I. The probiotic properties of lactic acid bacteria isolated from domestic dairy products Mongolia. Engineering and technology of food production, 2013; 1:1-6.
- Xing, J., Wang, F., Xu, Q. et al. Screening of potential probiotic lactic acid bacteria based on gastrointestinal properties and perfluorooctanoate toxicity. Applied Microbiology and Biotechnology, 2016; 100(15): 6755-66.
- Prema, P. In vitro antagonistic activity of a probiotic Lactobacillus plantarum against water borne pathogens. International journal of Pharmacy and Pharmaceutical Sciences, 2013; 5(4): 175-8.
- Ng, S.Y., Koon, S.S., Padam, B.S., Chye, F.Y. Evaluation of probiotic potential of lactic acid bacteria isolated from traditional Malaysian fermented Bambangan (Mangifera pajang). Cyta-Journal of food, 2015; 13(4): 563-72.
- Oh, Y.J., Jung, D.S. Evaluation of probiotic properties of Lactobacillus and Pediococcus strains isolated from Omegisool, a traditionally fermented millet alcoholic beverage in Korea. Lwt-Food Science and Technology, 2015; 63(1): 437-44.
- Arumugam, M., Harrington, E.D., Foerstner, K.U., Raes J. Enterotypes of the human gut microbiome. Nature, 2011; 473: 174-80.
- Boyle, R.J., Tang M.L.K. The role of probiotics in the management of allergic disease. Clin. Exp. Allergy., 2006; 36: 568-76.
- Georgieva, R., Yocheva, L., Tserovska, L. et al. Antimicrobial activity and antibiotic susceptibility of Lactobacillus and Bifidobacterium spp. intended for use as starter and probiotic cultures. Biotechnology and Biotecnological equipment, 2015; 29(1): 84-91.
- Abramov, V., Khlebnikov, V., Kosarev, I. et al. Probiotic Properties of Lactobacillus crispatus 2,029: Homeostatic Interaction with Cervicovaginal Epithelial Cells and Antagonistic Activity to Genitourinary Pathogens. Probiotics & Antimicro. Prot., 2014; 6: 165-76.
- Enitan, A., Adeyemo, J., Oqunbanwo, S.T. Influence of growth conditions and nutritional requirements on the productionof hydrogen peroxide by lactic acid bacteria. African journal of microbiology research, 2011; 5(15): 2059-66.
- Ha, M.Y., Kim, S.W., Lee, Y.W., Kim, M.J., Kim, S.J. Kinetics analysis of growth and lactic acid production in pH-controlled batch cultures of Lactobacillus casei KH-1 using yeast extract/corn steep liquor/glucose medium. J. Biosci. Bioeng., 2003; 96(2): 134-40.
- Parfenov, A.I., Bondarenko, V.M. With a century of experience has given us the knowledge of symbiotic intestinal microflora. Therapeutic archives, 2012; 2: 5-10.
- Jena, P.K., Trivedi, D., Chaudhary, H., Sahoo, T.K., Seshadri, S. Bacteriocin PJ4 active against enteric pathogen produced by Lactobacillus helveticus PJ4 isolated from gut microflora of wistar rat (Rattus norvegicus): partial purification and characterization of bacteriocin. Appl Biochem Biotechnol., 2013; 169(7): 2088-100.
- Dec, M., Puchalski, A., Urban-Chmiel, R., Wernicki, A. Screening of Lactobacillus strains of domestic goose origin against bacterial poultry pathogens for use as probiotics. Poultry science, 2014; 93(10): 2464-72.
- Mbawala, A., Mahbou, P.Y., Mouafo, H.T., Tatsadjieu, L.N. Antibacterial activity of some lactic acid bacteria isolated from a local fermented milk product (pendidam) in Ngaoundere, Cameroon. Journal of animal and plant sciences, 2013; 23(1): 157-66.
- Shousha, A., Awaiwanont, N., Sofka, D., Smulders, F.J.M., Paulsen, P., Szostak, M.P., Humphrey, T., Hilbert, F. Bacteriophages Isolated from Chicken Meat and the Horizontal Transfer of Antimicrobial Resistance Genes. Applied and Environmental Microbiology, 2015; 81(14): 4600-06.
- Pithva, S., Shekh, S., Dave, J., Vyas, B.R.M. Probiotic Attributes of Autochthonous Lactobacillus rhamnosus Strains of Human Origin. Applied Biochemistry and Biotechnology, 2014; 173(1): 259-77.
- Hyacinta, M., Hana, K.S., Andrea, B., Barbora, C. Bile tolerance and its effect on antibiotic susceptibility of probiotic Lactobacillus candidates. Folia Microbiologica, 2015; 60(3): 253-7.
- Sharma, P., Tomar, S.K., Sangwan, V. Antibiotic Resistance of Lactobacillus sp. Isolated from Commercial Probiotic Preparations. Journal of Food Safety, 2016; 36(1): 38-51.
- Haghshenas, B., Haghshenas, M., Nami, Y. Probiotic Assessment of Lactobacillus plantarum 15HN and Enterococcus mundtii 50H Isolated from Traditional Dairies Microbiota. Advanced Pharmaceutical Bulletin, 2016; 6(1): 37-47.
- Belkacem, B., Mebrouk, K. Antibiotic Resistance of Some Lactobacilli Isolated from the Digestive Tract of Broiler in Western Algeria. Journal of Animal and Veterinary Advances, 2011: 10(14): 1859-62.
- Nguyen, T.M.L., Nguyen, T., Pham, V.N. Probiotic properties of Lactobacilli isolated from Vietnam traditional fermented foods. International Journal of Renewable Energy and Environmental Engineering, 2015; 3(1): 6-12.
- Park, S.C., Hwang, M.H., Kim, Y.H. et al. Comparison of pH and Bile Resistance of Lactobacillus acidophilus Strains Isolated from Rat, Pig, Chicken, and Human Sources. World Journal of Microbiology and Biotechnology, 2006; 22 (1): 35–37.
- Elcioglu, O., Kunduhoglu, B. Probiotic characteristics of natural lactobacilli isolated from traditional kargi tulum cheese. Italian Journal of Food Science, 2014; 26 (1): 31-40.
- Khagwal, N., Sharma, P.K., Sharma, D.C. Screening and evaluation of Lactobacillus spp. For the development of potential probiotics. African Journal of Microbiology Research, 2014; 8(15):1573-9
- Debashis Halder and Shyamapada Mandal Curd Lactobacilli with Probiotic Potentiality. Translational Biomedicine, 2015; 6(28): 1-6.
- Pundir, R.K., Rana, S., Kashyap, N. Kaur, A. Probiotic potential of lactic acid bacteria isolated from food samples: an in vitro study. Journal of Applied Pharmaceutical Science, 2013; 3(3): 85-93.
- Bhardwaj, A., Puniya. M., Sangu, K.P.S., Kumar, S., Dhewa, T. Isolation and biochemical characterization of Lactobacillus species isolated from dahi. J. Dairy Sci. Technol., 2014; 2: 10-4.
- Wang, P.P., Wu, Z., Wu, J., Pan, D.D., Zeng, X.Q., Cheng, K.M. Effects of Salt Stress on Carbohydrate Metabolism of Lactobacillus plantarum ATCC 14917. Current Microbiology, 2016; 73(4): 491-7.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


