ISSN: 0973-7510
E-ISSN: 2581-690X
Western Algerian waste line is a dump of any form of pollution. Therefore sampling sites are ports, also close to urban waste which shelter a wide variety of fungal population. After seawater samplings, isolation we screened 235 kind of fungi for their behaviors with (Pb, Zn, Cd and Cu). Fourteen Trichoderma sp. isolates were evaluated for their tolerance to heavy metals stress at different concentrations of (Pb, Zn, Cd and Cu). Trichoderma sp. was exposed to concentrations between (0 – 4000 mg/l). The stress of biomass caused by the presence of heavy metals remains minimal. Maximum optimal growth has been demonstrated in our study at high concentrations of metals. Fungi expand gradually in cultures of metallic solutions. Its fresh weight reached 1, 89 Gr on 200 mg/l and 0,556Gr on 2000 mg/l. In the Zinc (Zn So4) solution the mycelium will grow mostly on 600 mg/l his weight achieved 0,97 Gr. Development in copper (Cu So4) solution is minimal, the fungi has pushed on 2000 mg/l to reach 0,046Gr. The Trichoderma sp. show an important potential of tolerance, it growth and stays different comparing to others fungus; The results emphasized the heavy metals detoxification abilities of Trichoderma sp. Bioremediation strategy could enhance the treatment of contaminated areas.
Marine, Trichoderma sp., biomass, tolerance, heavy metals, western Algeria
Pollution by heavy metals represents an important form of environnemental contamination; particularly pronounced in the summer season, without previous treatment, these waters sprinkled right represents, therefore, the major source of contamination of the marine ecosystem. It is the accumulation of pollutants through the food chain is the origin of the alteration of any natural environment.
The marine environment represents a final receptacle and dump any form of pollution, especially the urban wastewater discharges loaded for heavy metals, which is very bad to all life (Sun and al., 2010; Leitao, 2009; Al Sohaibani and al., 2009 ), paint pigments, electric accumulators and battery and other organic discharges, therefore there is accumulation through the links in the trophic chain (Kok and al., 2001), these pollutants represent the largest source the spread of this scourge after the coastal zones of the Algerian coast and are home to a large fungal population, especially at the port sites. From where we started our study and our water samples.
Heavy metals contaminate soil and seawater, like: Lead, Chromium, Mercury, Uranium, Selenium, Zinc, Arsenic, Cadmium, Silver, Gold and Nikel, they are consistently present in the environment (Zhang et al., 2016), important micronutrients, and necessary for balance from the environment and become toxic they exceed their threshold (Chibuike, 2014).
Metallic pollution is the most answered these days given the industrial development, this form of contamination is non-biodegradable and the substances persist in the water. Existing techniques for metal removal are diverse; Reverse osmosis, electrolysis, chemical precipitation, and phytoremediation, ultra filtration. (Alluri and al., 2007).
Several bioremediation techniques used for contaminated soil and water involved not just in physical and chemical treatments in the biological processes, like a lot of bacteria and fungi engaged for their tolerance and reducing power to heavy metals (Idriss et al., 2004); the following are the reasons for the removal of pollutants or their recovery. In 2010, Yih–Min and al, demonstrated the usefulness of mushrooms (Penicillium sp, Aspergillus niger and Trichoderma viride) could degrade lead phosphate. Another work developed in Taiwan was talking about the immobilization of the Pb, Me and Cd by Aspergillus terreus. One year later Kumar and al., conclude during their work that there is a decrease of Zinc and Cadmium by Aspergillus niger by the phenomenon of biosorption in 2011, the same result is found in 2015 by Tsekova, that has support the resistance of mycelium to Cd as well as Cu, still in the same year in Brazil the work of Cavalcanti luna also talks about the adaptation of Aspergillus sp. to Cu. The best last study was in 2017 Provedano-Priego et al., working on a set of filamentous fungi (Penicillium sp, Aspergillus niger and Trichoderma viride) and the behavior with lead phosphate in the same year bioremediation by bacteria associated with fungi which was the subject of study in China; of Liu and al., 2017; who have studied the behavior related to pollution by PAHs and heavy metals.
Bioremediation of the soil is also a major challenge to solve the pollution problem by indishable substances, Trichoderma sp. is the subject of resolution, and many works are chained in this context. In 2010, the study was designed to test the potential persistence of this plant-beneficial fungus in soils contaminated by mining waste, the persistence of the Trichoderma sp. in those extreme conditions was demonstrated by the study. Three year later; 2013, Abdi and al., are studying the effects of microorganisms in the bioremediation of soil polluted by Zinc in Algeria.
2015, Song and al, still in China the soil study continues; work on trade between Lolium perenne, the Cd and soil and microbial biomass, and conclude that the involvement of fungal inoculate are characterized by the modification of soil acidity, rhizosphere and plants accompanied by Co2 production according to other works, inoculate provide an alternative for improving phytoextraction (Olsson and al., 1996 and Lavelle, 1997).
Among the most concerned species are the Trichoderma sp. are the subject of many studies because they occur under difficult conditions and unusual and stimulates the growth of plants (Vinale and al., 2008), the species of Trichoderma sp. improves the quality of the environment where it is, and sustainably protects against heavy metal pollution, (Téllez Vergas and al., 2017), his job was to study the behavior of Trichoderma sp and Cu and to try to improve its phytotoxic effects in the onion (Allium cepa L.). It is also valid for plants, they have a great ecological role, it has been through several studies, and they contribute to biodegradation and phytoremediation through metabolic activities (kuiper and al., 2004)
Trichoderma species are imperfect filamentous fungi with telomorphs and belong to the order hypocreales in the Ascomycete devision. Trichoderma sp. are among the most frequently isolated soil fungi and are well known for their biocontrol ability against a wide range of plant pathogenic fungi (Howell, 2003), they play in important role in ecology et biodegradation band bioaccumulation of high amounts of various metals from wastewater and soil (Ezzi and Lynch, 2005; Arnand and al., 2006). Fungi has a very fast growth, and extensive on Sabouraud medium at 25°C. The colonies have woolly aspect white at the beginning then appear getting older green clumps, arranged in concentric rings on the culture medium the back remains colorless.
Trichoderma sp. are most frequently isolated from the ground, they are known for their ability to reduce the risk of pollutants and toxic elements throughout the environment (Nonghmaithem, 2016), they tolerate more than one category of metal (Krediks et al., 2007) they play an ecological role in bioremediation, there are non-biodegradable pollutants that enter the food chain, however, metal ions influence the development, sporulation and enzymatic activity of Trichoderma (Papavizas and al., 1985 and Jaworska and Dluzniewska, 2007), and can change enzymes and metabolites (Kredics et al., 2001), Trichoderma sp. have acquired a very dramatic role in the ecological approach.
The isolates of Trichoderma sp. have tolerance to Ni, Cd and other heavy metals, as demonstrated by the study developed in 2016, in India by Nongmaithem and al., who demonstrated the biosorbing potential of Trichoderma sp., in the same thematic still bioremediation by Trichoderma sp. another study was conducted by whole team in Mexico Telléz Vergas and al., had goal in this study to Improve the phytotoxic effect caused by copper in the onion.
The fungus is unable to accumulate large concentrations of heavy metals essentially phytotoxic (Ruiz et al., 2009). A high number of high numbers of heavy metals Cd, Cu, Cr, and Pb are remedied by fungi (Liu et al., 2017).
Isolation and identification of fungus
The fungus studied is taken from wastewater discharges from three ports: Oran, Kharouba and Ghazaouet (Fig.1). The collected samples was transferred into a sterile plastic container, taken immediately to the laboratory and maintained at 4°C for further studies; the only medium used in our study is the Sabouraud; the fungus grows and develops in SDA (Sabouraud Dextrose Agar) solid medium.
Fig. 1. Map of the sampling sites of the western Algerian coast
Purified isolates were obtained by streaking repeatedly colonies in SDA medium and observation under light microscopy. Pure cultures of isolated microorganisms were identified using the keys of Pitt (1979) and Domsch & al., 1980. The cultures were characterized to the genus level on the basis of macroscopic characteristics (colonial morphology, color and appearance of colony, shape) and microscopic characteristics (septation of mycelium, shape, diameter and texture of conidia).
We used the 1ml dilution method of 10-3, 10-4, 10-5 according to Nongmaithem, 2016 are incorporated in large glass kneaded boxes with Sabouraud medium. The suspensions were distributed uniformly over the medium surface by horizontal shaking the incubation is carried out in 7days at a temperature of 28°C±1°C, the colonies are identified with Trichoderma sp .
Colonies invade the whole surface; these green colonies are distinguished from other colonies of different strain.
Fungal strains were maintained and on Sabouraud dextrose agar during 14days at 28°C (Provedano-Priego & al., 2017)
Tolerance to heavy metals
Metal tolerance assay in solid medium
The metal salts used were lead carbonate Pb 2+, copper Cu 2+, and zinc sulfate Zn 2+, Cadmium sulfate Cd 2+. Fungal strains were maintained and on Sabouraud dextrose agar during 14 days at 28°C (Provedano-Priego & al., 2017). For each metal, all Sabouraud plates supplemented with 0, 200, 400, 600, 800, 1000, 2000, 4000 mg/l with metal salts in triplicates and 0,6 cm diameter mycelial plug of Trichoderma sp. was inoculated centrally. Fungal strains were maintained and on Sabouraud dextrose agar during 14 days at 28°C (Provedano-Priego & al., 2017) and the diameter growth (mm) was measured every alternate day. The effect of the heavy metal on the growth of the isolates tested was estimated by measuring the radius of the colony extension (mm) against the control (medium without metal) and the determination of the index of tolerance. The index is defined as the ratio of the extension radius of the treated colony to that of the untreated colony. Isolates showing resistance to Pb2+, Cu2+, Zn 2+ and Cd 2+ were selected for the following experiments. (Ezzouhri et al., 2009), The plates were inoculated by placing 6 mm mycelial discs of 4 days old cultures of the Trichoderma isolates on the agar surface and incubated according (Nongmaithem et al., 2016)
Dual fungal essay antifungal activities under metal stress
Sabouraud plates used were supplemented with metals as described in last paragraph with different concentrations. The control was established by inoculating agar plug instead of mycelial plugs of Trichoderma. Incubation was performed using similar conditions of incubation, and the radial growth of mycelium extension was measured at every alternate day. The antifungal activity was expressed as percentage inhibition of radial growth (PIRG%) as follows.
PIRG (%) = ((R1- R2)/R1) ×100%
Where; R1 indicates the radial growth of mycelium in control plates
R2: indicates the radial growth of mycelium co paired with fungus. The experiment was performed in triplicates (Nongmaithem et al., 2016).
Metal tolerance assay in liquid medium
The resistance of the selected isolates to Pb2+, Cu2+, Zn2+ and Cd2+, was determined by the dilution method. Metal ions were added separately to Sabouraud at concentrations of 0 to 4000 mg/l, the plates were inoculated with 6 mm agar plugs from young fungal colonies, pre-grown on SDA. Three replicates of each concentration and controls without metal were used. The inoculated plates were incubated at 25°C for at least 7 days.
For biomass preparation, a stock solution of heavy metals Pb (NO3)2, Cu So4, ZnSo4 and Cd(No3)2 was prepared by dissolving the appropriate quantity of metal salt, Sabouraud medium 100 ml was made in conical flasks or glass bottle, then appropriate quantities of metals salt were added to Sabouraud medium liquid to get required concentrations (0, 200, 400, 600, 800, 1000, 2000, 4000 mg/l). The non amended solution served as a control without heavy metals additional. The stock solution was sterilized and ready to receive mycelium fragment culture, in the strictest sterile conditions.
The plates inoculated with addition of mycelial disc that measures 6 mm of 4days old cultures of Trichoderma isolates into agar surface and incubated at 28 ± 1°C for 2 or 3 days. Fungus showing maximum radial growth on the media.
Three replicates are executed; the culture is done in (25°±2°C) for 11 days according to Su Yien Ting and after culture solutions are filtred with Millipore following studies established (Su Yien Ting and Jioe, 2016) and (Provedano –Priego et al., 2017) . The biomass extracted is filtred with Whatman filters n° 1 or filter paper and then washed thoroughly with deionized water to remove the growth medium. The Harvested mycelia were oven –dried at 80°C for 48h and the dry weight was measured using a digital weight balance with occurancy of 0, 1 mg.
Statistical analyses
To evaluate the influence of heavy; metals the data was submitted to variance analysis using Statistica soft ware by Tukeys tests the means between treatments were compared by using test probabability for significant difference p< 0,05.
Screening for Heavy Metal growth profile
In this study, marine fungi were submitted to a preliminary experiment to screen their efficiency for ability to growth in cultures in the absence and presence of heavy metals on solid and liquid medium.
On solid medium; four kinds of heavy metals (Pb, Cu, Zn, and Cd) were tried with mycelium of Trichoderma sp. in different concentrations and whitout metals. Among this series for heavy metals only three metals were chosen for completing tests series of heavy metals liquid medium (Pb, Zn ,Cu), the Lead selection for high toxicity for environnement, the Zinc and Copper for their importance for cellular and metabolic activities .
Tolerance of Trichoderma sp. to heavy metals in solid medium
The Trichoderma sp. were identified and selected for further study, based for variability growth and mycelial cultural diameter. The expansion of Trichoderma sp. revelate an abilility to growth in heavy metals at high and various concentrations, the maximum growth rate was observed in Pb rates followed by Cadmium cultural growth rate, then mycelial rates of Zinc, lastly Copper growth rates.
All the diameters of mycelium heavy metals rates are different significantly between them, the diameter of Pb rates growth stay the best diameter and most important remains the highest significantly (p< 0,05) to metals Cu, Zn, Cd, its general overage is 67,93 mm of mycelial growth with 27,58% of inhibition PIRG (Table.1).
The mycelial growth in Copper Cu rates is the most reduced significantly with mycelial diameter rate of 41, 72 mm and 52, 24 % of inhibition PIRG (Fig.2, 3).
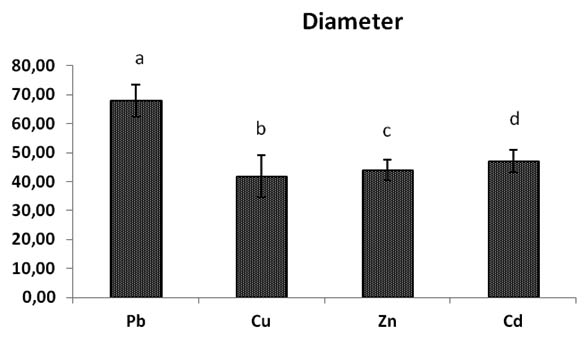
Fig. 2. Effect of heavy metals (Pb, Cu, Zn, Cd) concentrations on Trichoderma sp., Bars indicates standard deviation. Colums of differents treatements with different letters are significant-ly different at P<0,05
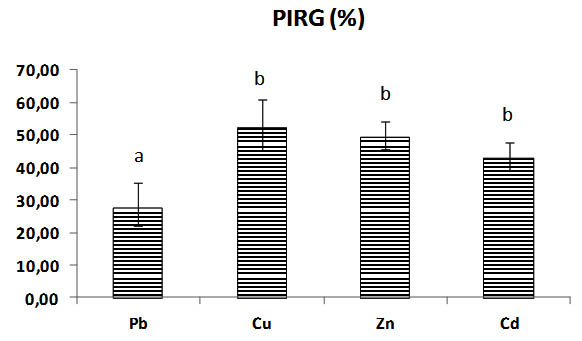
Fig. 3. Percentage of inhibition (PIRG) in solid medium of Trichoderma sp., with heavy metals concentrations (Pb, Cu, Zn, Cd), Bars indicate standard deviation. Colums of differents treatements with different letters are significantly different at P<0,05
The percentage for inhibition of Pb is significantly different to others metals , the inhibition of fungi is minimal and reduced , and important with copper rates the Copper inhibits the mycelial growth significantly p< 0,05 , there is no significant difference with PIRG inhibition of fungi by Zinc and Cadmium rates p >0,05. (Fig. 2, 3), (Table.1).
Table (1):
General mycelia growth and inhibition of Trichoderma sp. by different heavy metals (Pb, Cu, Zn, and Cd) at different concentrations (0 to 4000) mg/l in Sabouraud dextrose agar rates
Diameter (mm) |
PIRG (%) |
|
|---|---|---|
Pb |
67,93± 5,56a |
27,58 ± 7,9a |
Cu |
41,72± 7,27b |
52,24 ± 8,67b |
Zn |
43,93 ± 3,54c |
49,21 ± 4,75b |
Cd |
47,08 ± 3,86d |
42,91 ± 4,66b |
For the Pb, the concentrations C1 and C2, show no significant diffence with the control rate p>0, 05.
From C3 there is a significant diameter decrease p<0,05 , the growth mycelium resume his growth at C4, the diameter mycelial growth decrease with increasing concentration until completely inhibition, from C5 to C7; which corresponds at 4000 mg/l of Pb.
The mycelial rates growth of Cu, at concentration C1 and C2 the growth of Trichoderma sp. show a significant development of fungi to compare with control m at C4 the culture of mycelium is inferior significantly to C0 Control p<0,05 .
Fungi in concentrations C5, C6, C7 of Cu, the growth of fungi is inhibited significantly, no growth is detected (fig.4).
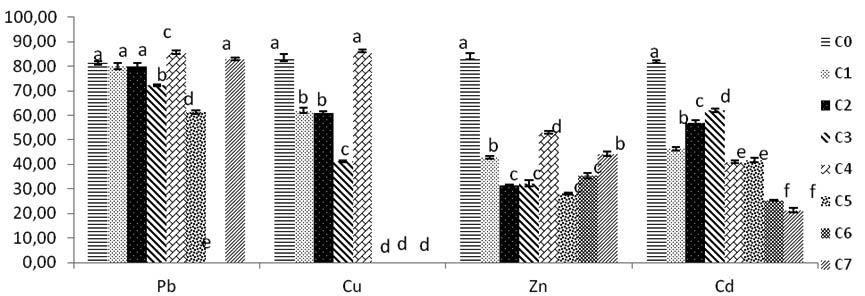
Fig. 4. Growth and tolerance of Trichoderma sp in solid culture SDA added with concentrations of (Pb, Cu, Zn, Cd). C0:0 mg/l, C1:200 mg/l, C2:400 mg/l, C3:600 mg/l, C4:800 mg/l, C5:1000 mg/l, C6:2000 mg/l, C7:4000mg/l. Bars indicate standard deviation. Colums of differents treatements with different letters are significantly different at P<0,05
The mycelia growth is affected by Zinc concentrations. All diameters fungi growths are lower and reduced significantly to compare with control fungi growth who reaches 84 mm, a good growth is observed with C4 Zinc concentration with 53 mm mycelair diameter growth. Except the others rates of remaining concentrations of fungi in C2, C3, C5, C6 which are not significant difference (p >0, 05) (Fig.4).
Inhibition increases with high concentrations from C1 to C3; then it decreases, growth is inhibited at C4 rate Zinc then resume at higher concentrations of zinc but these fluctuations are not significant with C0 control and C4.the inhibition of Trichoderma sp. growth with Zinc concentrations decreases until C7 (Fig. 5).
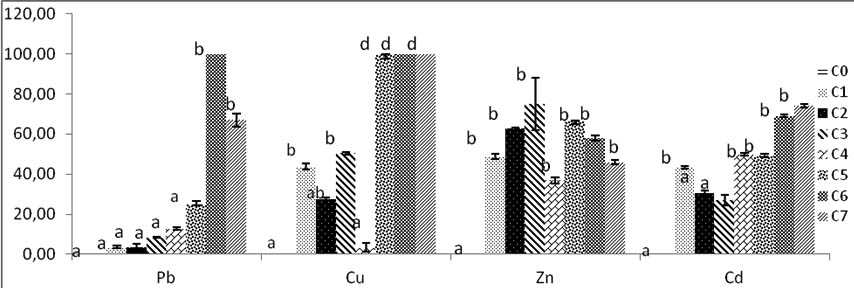
Fig. 5. Percentage of inhibition radial growth (PIRG) of Trichoderma sp by heavy metals in solid culture SDA. C0:0mg/l, C1:200mg/l, C2:400mg/l, C3:600mg/l, C4:800mg/l, C5:1000mg/l, C6:2000mg/l, C7:4000mg/l. Bars indicate standard deviation. Colums of differents treatements with different letters are significantly different at P<0,05
The Cd rates growth shown all diameters in various concentrations significantly different compared with control. The best fungi growth is reported at C3, from this concentration there is mention of a decrease of diameter growth. The inhibition of mycelium by Cd increases with high concentrations rates. The maximal PIRG inhibition rate reported at 4000 mg/l of C7, and significantly high to others concentrations Cd rates p<0, 05 (Fig.5).
Tolerance of Trichoderma sp. to heavy metals in liquid medium
After culture on liquid Sabouraud medium the mycelium is weighed and dried 24h at 80°C, the mass of fungi got before and after culture is weighed is named dry weight, among the metal salts only three were required chosen for their tolerance with Trichoderma sp. to push while working on the most marked concentrations where we have a maximum of positive reponse from 0 to 2000 mg/l (Table. 2).
Table (2):
General weight of biomass before and after dry of Trichoderma sp .with different heavy metals (Pb, Cu, Zn) at different concentrations (0 to 2000) mg/l in medium SDA
F.W (g) |
D.W (g) |
|
|---|---|---|
Pb |
0,93 ± 0,13 a |
0,07 ± 0,01 a |
Cu |
0,60 ± 0,19 a |
0,07± 0,01 a |
Zn |
0,92 ± 0,4 b |
0,11 ± 0,02 b |
F.W: fresh weight.
D.W: dry weight.
After 24h at 80°C, biomass dried and weighed, the dry weight of Zn solution medium is most increase significantly compared to dry weight of fungi in Pb, and Cu solutions after time dry, the weight of biomass change but there is no significant dimininution. The weight of general biomass of fungi in Pb and Cu medium solution is almost the same because there is no significant difference p > 0, 05 between the two (Fig. 6).
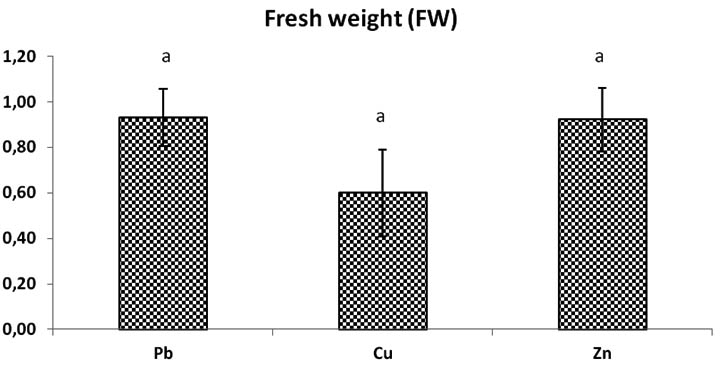
Fig. 6. Total biomass fresh weight (Fw) of Trichoderma sp. in liquid medium at concentrations of heavy metals (Pb, Cu, Zn), Bars indicate standard deviation . Colums of differents treatements with different letters are significantly different at P<0,05
The results of dry weights found are statistically similar to results of biomass of Triochoderma sp. before dry.
The biomass growth in Pb solutions medium decreases significantly until C4 which corresponds at 800 mg/l , the biomass believes more at C5; 1000 mg/l and exceeds the weight registered at different lower concentrations all different compared to control C0.
After drying biomass 48h at 80°C; the dry weight down significantly, the lowest dry weight raised at C2 (Fig.8). The medium Cu solution inhibits the growth of biomass apart for solution with high concentration 1000 mg/l; at this concentration proliferation reaches its maximum and exceeded the control biomass. The dry weight of this series of Cu medium culture is maximal at 1000 mg/l almost the same as the witness .on the other hand the dry weight is reduced significantly all over concentrations after dry. In Zn medium solution, the weight of biomass unregistered for C5 is similar to control weight without difference, the Trichoderma sp. biomass is different and very reduced compared to control biomass for C2, C3, C4 at these levels, weights is similar between them without comparison, P|>0,05 (Fig.8).
Table (3):
Results of the variance analysis (Test F, ANOVA) of; D, PIRG, F.w and D.w of Trichoderma sp.
F (Metal x Concentration) |
F (Concentration) |
F (Metal) |
|
|---|---|---|---|
1,62N.S |
7,12** |
7,69** |
D |
13,68** |
4,68** |
7,5** |
PIRG |
4,99** |
10,18** |
5, 84* |
F.W |
1,10N.S |
20,34** |
3,24* |
D.W |
D : Diameter ; PIRG:Percentage of inhibition of radial growth in solid medium ; F.W :Fresh weight ; D.W : Dry weight , (* : significant (p< 0,05) ; ** : Very significant (p<0,001) ; ns : Not significant df (Metal) = 3.92; df (Concentration) = 7.88; df (Metal x Concentration) = 31.64
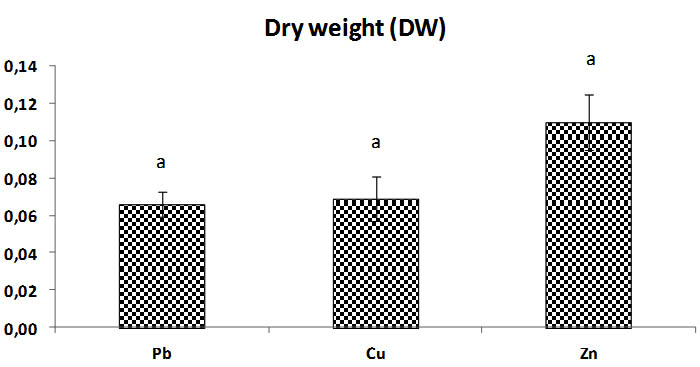
Fig. 7. Total biomass dry weight (Dw) of Trichoderma sp. at concentrations of heavy metals (Pb, Cu, Zn), Bars indicate standard deviation. Colums of differents treatements with different letters are significantly different at P<0,05
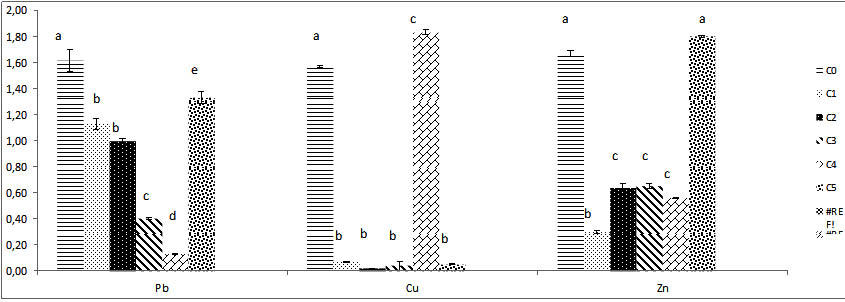
Fig. 8. The different results of biomass fresh weight of Trichoderma sp. under vari-ous concentrations of heavy metals ( Pb, Cu, Zn) .Colums of differents treatements with different letters are significantly different at P<0,05
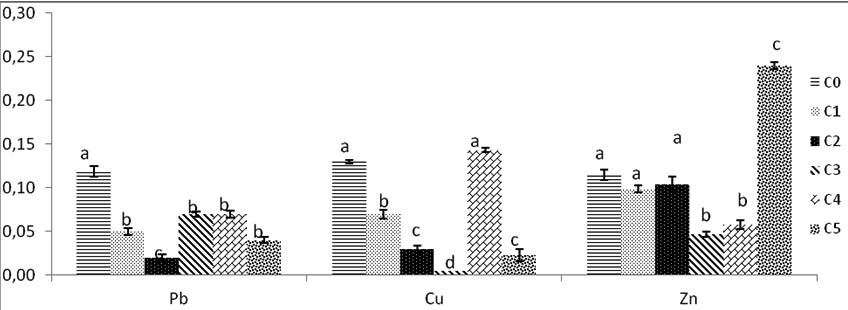
Fig. 9. Weight dry of biomass of Trichoderma sp. after drying fresh weight 48h at 80°C under various concentrations of heavy metals ( Pb, Cu, Zn). Colums of differents treatements with different letters are significantly different at P<0,05
After exposure to heat, the fresh weight decreases in volume and weight, it is reduced after dry but remains high significantly compared to control. The weight of C1 and C2 remain similar to control no difference between them.
Bioremediation is an important solution for elimination and reduction of various polluants from contamined sites , soil land industrial wastewater , with the help of biological organisms such as fungus , bacteria, and plants by degradation ; certain fungal, algal and bacterial are capable of accumulation (Kumar and al., 2011), especially the filamentous fungi, Trichoderma sp. isolated from wastewater studied . Through our final results in this study the tolerance profile was evaluated. The level resistance capacity was studied in medium solid and liquid; the mycelial growth was presented as follows Pb>Cd>Zn>Cu (Fig.2).
Lead is considered one of the most toxic and dangerous heavy metals, that caused problems pollution in marine environnement, and many modifications in mycelium and induce production of melanin chlamydospore development, a fugal reponse based in biological adaptation for fungi, cells to synthesize some enzymes for lead uptake (Ezzouhri, 2009 and Provedano and al., 2017).
Bioremediation strategy converting the contaminant metals into theirbless toxic forms , fungal stains was able to tolerate Pb lead and transform metallic lead into lead phosphate ;mineral phases , this stable minerals could reduce the bioavailability of lead, (Provedano and al., 2017)
Biomineralisation of lead phosphates (e.g pyromorphyte) by fungi is a well known process (Leitao, 2009).
Trichoderma sp. were identified with selected culturals variability existed among the isolates different characteristics with changement of color caused aspect sporulation in different heavy metals cultures raised , the Trichoderma sp. pushed well and fits with different concentrations of Pb(No3)2 , the maximum radial growth was shown at 800 mg/l (C4) with 85,67mm and 4000 mg/l (C7) with 81,33, the optimum growth enregistred similar to diameter control equivalent to 83mm , it tolerated high concentration of lead. The effect of lead tolerance was tested on Sabouraud medium liquid added with metal salts. The inhibition is from 1000 mg/l to 2000 mg/l (Fig.4), the same tolerance results are observed with another fungus the endurance of Pb of each fungal strain was recorded by inoculating them separately in lead amended plates. A. niger RH 17 and A. niger RH 18 showed the highest resistance, being 6000 and 7000 mg/l , respectively, warranting them to be successful candidates for metal detoxification. The colony diameter of A. niger RH 17 after 7 days was 48.4 mm, while in another study (Price et al., 2001) then study of Povedano and al., in 2017 Penicellium Chrysogenum, Aspergillus niger and Trichoderma viride could tolerate high concentration Pb , able to resist concentrations up to 8mM of lead , Trichoderma viride tolerate this concentration.
Sun and Shao in 2007, talk about resistance of Penicellium sp to Lead , the hyphae grown at 4milliMol this fungi accumulate Pb in cellule structure , had intracellular dispositifs , Both fungal strains showed maximum Pb removal at 1000 mg/l metal concentration. And supports for resistance phenomene exhibited by fungis which translates by extra cellular bioaccumulation which provide strength and tolerance according to Shao study in 2007. In our study lead diameter of Trichoderma sp. decrease significantly at 1000 mg/l and bounces 4000 mg/l, was highly efficient in lead absorption since it absorbed 90% of the contaminating solution
Pb(NO3)2, after 48 hours. Heavy metals 2007, Shao and Sun suggested that intracellular accumulation is an important mechanism for fungi from deep sea sediment to resist heavy metals. These fungi are a potential treatment for heavy metal pollution, otherwise the fresh weight of the mycelium decreases before and after drying with the increase of concentration, the highest weight of Pb enregistred is of 1, 33g , the fungi tolerate solution concentration of last concentration in this series test heavy metals treatment fixed at 1000 mg/l , the fungi grown all over , even after drying the biomass is clearly absorbent to Lead salt accumulated , into fungi cells, generally the microorganisms evaluated exhibited higher reduction of biomass when cultivated in the presence of metal ions , inhibition of growth and active metal process accumulation (Leyval and al., 1997., Pepper, 2000., Malik, 2004., Lopez, Vazquez., 2003 ).
The Cadmium tolerance represented in figure indicated the strong capacity of Trichoderma sp. at C3 corresponding to 600 mg/l , a diameter growth enregistreted is 62 mm after this concentration the Cadmium; become toxic and slow down the growth of culture mycelium of Trichoderma sp., at 4000 mg/l the inhibition is maximal the fungi can’t growth at this high concentration , according to our results Trichoderma sp. resist to Cd until 600 mg/l (Fig. 4) , in 2016 by Nongmaithem and al., study the fungi achieved 73, 99% , biomass production decline with increasing metal concentration at 150 ppm. This tests show the ability to remove the cadmium for culture rate medium depends of cellular growth and metabolism action; reduction of production in biomass indicate the effects of various concentrations salts metals on fungi reaction and total Cadmium retained by fungi . Many species of fungi showed a potential for removal, among them Fusarium sp., Penicellium sp. et Aspergillus flavus and Trichoderma konjinji , Trichoderma harzianum isolated from polluted industrial area have a capacity to remove metal ions in aqueous solution , the removal metal ions cell do not depend of metabolism (Kapoor and Viraraghavan., 1997., Gharieb and Gadd., 2004., Gogsungur, and al., , 2005., Acosta and al., 2007., Massaccesi and al., 2002) , an other study in 2011 supports the Trichoderma harzianum; capacity for removal cadmium with a high level tolerance to growth in the presence of high Cd concentrations. This fungi used in this study is able to grow at high concentrations, can be used in bioremediation. Li and al., 2007 talk about ability to growth in Cadmium concentration above 5miliMol in strain Pseudomonas , this organism was able to remove 99% of Cadmium, Lopez and Vazquez demonstrated that the presence of Cadmium; inhibited the growth cells of Trichoderma atroviride in 50% to concentrations lower than 1miliMol, the Trichoderma harzianum isolate showed an other way a high resistance to Cadmium in concentrations 1mililMol, 2miliMol, 3miliMol, the literature shows that microorganisms like fungi have a capacity to accumulate polyphosphate used in bioremediation technology (Keasling and Hupf., 1996 and Keasling and al., 1999., Torres and al., 1998., Moreno, and al., 2001., Van Loosdrecht and al.,1997).
The genus of Trichoderma is able to modify the rhizosphere of plants, and product an important substance organic acid such gluconic acid furamic and citric acid approved in study of Kapazak and al., 2014. An other study appropriate talking about Aspergillus niger to remove and accumulate Cadmium and Zinc the removal was less significant by accumulated biomass in 2011; the similar results were reported by Ahmed and al., in 2006, where Aspergillus niger and Penicellium sp. were tested for tolerance and high remove action to Cr and Ni .
Copper is an important co-factor enzymatic processes, represents the third most abundant transition metal found in living organisms (Brandolini and al., 2002). The growth of all fungi tested was decreased after addition of Copper in comparison with Zinc. The blue color of the isolates’ mycelia on agar media amended with copper salts is of Cu ions to in medium rate.
The Trichoderma viridie turned blue on agar media at all concentration according to Ezzouhri study in 2011, similar morphologic and physic characteristics were deferred in our study the mycelia agar rate color also turn in blue in presence of CuSo4 ions . Trichoderma have resistance and high capacity to biosorb Cadmium and other heavy metals (Volesky, 1990; Zafar et al., 2007; Lopez-Errasquin and Vasquez, 2003).
In study of Levinstakete in 2002 growth of T.viride and P. Chrysogenum were slowed down at 1,5 and 2 miliMol Cr in the medium. Metals such as Zn, Cu, are essential to biological actions, their toxicity may be presented differently Trichoderma sp. tolerate Copper at 800 mg/l maximum, up to this concentration the fungi is inhibited and don’t growth, the growth diameter decrease with increase metal concentration; the same remarks have been mentioned on the whole series on liquid medium heavy metals solution the fungi don’t have capacity to have a good growing activity but , the mycelium biomass achieved the maximum in 800 mg/l solution concentration , the biomass before and after drying always stays at the pick of its growth at 800 mg/l corresponding to C4 in representation (Fig. 6, 7) , the structure of T. viride, so it was excluded and the study was carried out to optimize the Cr (VI) removal efficiency by a marine isolate named T. viride. Micro-organisms isolated from industrial processes and polluted environments with high metal concentrations exhibit considerable tolerance to these elements. This tolerance may be due to abiotic factors (pH, temperature, nutriments in the environment or growth media). Growth parameters, sporulation, diameter biomass and physical details represent a set used to determine level of toxicity of Copper an other type of heavy metals In study of Téllez-Vergaz and al., 2017; who is interested to evaluate metal toxicity in plants talk about parameters growth of plants; stem elongation number of roots and (Ait Ali and al., 2002; Shi et al., 2010), survival oignon biomass depends for CuSo4 concentration.
Trichoderma sp. isolated from polluted sea water sites exhibit a potential tolerance and a good capacity to accumulate Cu from metal contaminated sediment, the growth was not affected at 100 ppm, according to Iskandar and al., in 2011, 120 ppm was able to slow down the growth of Trichoderma asperellum isolate. Trichoderma sp. was affected in our study to 200 mg/l equivalent to 200 ppm and inhibited mycelial growth at 43, 85% and fungi is inhibited to high concentration at 1000 to 4000 mg/l , absence of growth , the biomass of Aspergillus niger had reduced at 2miliMol in presence of copper , this fungus was able to grow at concentrations up to 300 ppm in the culture medium (Tsekova and Tedorova, 2002) exactly at the result of our study which determines the resistance potential and capacity growing to 800 ppm or 800 mg/l for Trichoderma sp.
The microorganisms reduce the concentration of Cu and sequester a substance poly phosphate, a biodegradation of Cu and heavy metals is dependent to metabolism of polyphosphate, this polymer provides resistance to many heavy metals, in various microorganisms; yeasts, bacteria and filamentous fungi.(Keasling, 1997, 2000., Lima and al., 2003, Alvarez, Jerez., 2004., Remonsellez and al., 2006 ).
The CuSo4 accumulated in fungi cell , which does interest to evaluate potential effect to ameliorate the toxic effects of Cu onion plants (Hajierghari, 2010, Cacciola and al., 2015, Nonghmaithem and al., 2016) , growth inhibition is the principal effect of Cu reported and the first symptom of toxicity of Cu to inhibit photosynthesis by accumulation on the leaves (Geremias et al., 2011), following Luo and al., 2016; this study interested many species selected for capability tolerance to heavy metals and bio accumulation Cu above 1000 ppm .
In Trichoderma harzianum showed that presence of Cadmium reduced the polyphosphate activity. The hydrolisis of organic phosphorus acid phosphatase protect structure cell of fungi and help to resist at high concentration (Gadd, 2010).
The Aspergillus niger was considered to be also a greatest potential biotechnological and industrial uses , the microbial cells are used in biotransformation process, and the biomass was an excellent biosorbent to heavy metals ions (Remonsellez and al., 2006, Gadd, 2010) in the literature the microorganisms and filamentous fungi such as Aspergillus , Mucor, Trichoderma, Rhizopus , product various metabolites able to reduce pollution heavy metals, and a potential to remove Cu ions (Adrio and Demain., 2003, Bennet, 2010, Djiksterhuis and Wosten., 2013).
The Zinc is an essential element for organisms, become toxic at high concentration. The major part of fungus group were able to grow at 12, 5 miliMol and more higher concentration. The aspect morphological were affected at high concentration ; many descriptifs details change ; after effect of high Zn concentration, with secretion of substance colored in yellow , who which shows stress response in Zn solid medium rates . In the present study the concentration decreasing comparing to mycelial diameter growth control. The Trichoderma sp. growth at differentes concentrations ; the fungi diameter increase more and more until 800 mg/l who matches to 53 mm of diameter growth, followed by diameter growth enregistred at 4000 mg/l, the fungi tolerate and resist the most high concentration , the Trichoderma sp. was inhibited at 1000 mg/l; le Zn is toxic and slows down the mycelium expansion, a resumption of development is noticed; the fungi bounces and become thik but with zone of inhibition detected which marks the circumference yellow; Fomina et al., 2005 reported that heavy metals accumulated accompanied with excretion of different substances organics acids such as lactic acid, oxalic acid, acetic acide. The damages were related to cells membrane and cytoplasm caused by metals ions. the properties of growing fluctuations caused by fungi accumulate Zn in cell wall selected for resistance to heavy metals; the zinc trained fungus according to study for (Kumar and al., 2011) observed A, niger solubilize Zn in malt grown solution. Kok and al., in 2001 talk gets good results proves that Aspergillus niger have metal tolerance against Zn and Cd, and support that various filamentous fungi like Trichoderma sp. slected for our study were an excellent product in bioremediation activity.
Levinskaite (2001) demonstrated that the growth rate of Penicillium atratmentosum , 25SL decreased slowly as the Zn ions concentration increased up to 40 miliMol. In Zn solution liquid medium the biomass increasing with high concentration solution for all fungus such as Trichoderma sp. growth at 1000 mg/l and uptake Zn from metallic solution after drying the weight dry stay the most important at 1000 mg/l. the removal of Zn is egual than the Cu; Price et al., talk in 2001 about A.niger growing to concentration up to 0,1miliMol Zn the same profile with copper is mentioned.
The Trichoderma sp. increases with increasing metal solution concentration, this fungi proliferates and absorbed des Zn ions from solution ; Price in 2001 talk about Cu and Zn removal by Aspergillus niger at 0,1miliMole solution concentration. Similar results shown that Aspergillus niger growth in solutions of Zn So4 and could remove Zinc from solutions the A.niger removed 0,533 mg in 0, 01 solution ZnSo4. Das in 2008 precise that specific Zn uptake decreased with the increase in biomass living; the process followed in second mate kinetics biosorption. The biomass uptake cadmium was higher than zinc, and in dual system combination solution the uptake of zinc is the higher according to Kok 2011, an other similar ascertainment was mentioned approving the higher capacity removing zinc from wastewater that better than fungus grown in culture media amended with zinc (Price, 2001), Aspergillus niger was grown in solution in which Zn increased from 10 too 500 ¼m.
In Lead medium solutions, the biomass of Trichoderma sp. decreased with increase metal concentration, the fungi weakness does not absorb until solution at 1000 mg/l equivalent to 1,33 g , from here this large significant decrease is related to absorption process, the ions. Accumulated depend of cell density present in medium culture; the adsorption capacity were expressed as Cd>Pb>Cu (Fig.8) for Aspergillus flavus with study of Kok and al., but results given by actual study shown the capacity of biomass absorption is Pb>Zn>Cu.
All test series experience, used ph: 5, 6 according to Roy and Pan, 2005. For an optimum uptake was fast at ph: 6 maximum. Because the tolerance may be also influenced by abiotic factors such as PH, temperature and nature of nutriment and environnement for growing. The absorption of heavy metals depends on cell density present in medium culture following the results found in work of Iqbal; 2005. Who talking about mycelia growth occurs in for pallets under submerged conditions in liquid medium with agitation. Finally Trichoderma sp. growth in all heavy metals but to different degrees he is one of the Trichoderma species who has demonstrated in study of Tellez Vergaz and al., in 2017 a great amelioration in stress in onion plants due to excess of Cu, the Trichoderma sp. supports excess and highest concentration proven in our study , for Cd the last study for Nonghmithem and al., in 2016 exibits the Trichoderma isolates to Ni and Cd ; it’s through his study that we can support our hypothesis on Cd tolerance to some concentrations, the increase Cd concentrations affecting germination and growth , even at higher concentrations the stress of fungi increase , the regression is unregistered up to 800 mg/l, the zinc also is assimilated and absorbed already he enters in cellular needs and metabolism activities, Ting and Jioe demonstrated in 2016 that isolates of Trichoderma sp. in their study of tolerance to metals ; the Zn is tolerated at 100 ppm at this concentration the diameter growth was 80 mm, at 200 mg/l or 200 ppm in present study the diameter was 43 mm , this fungi is generally able to tolerate metals via cellular adaptation, for Ting and Jioe the fungi has an exceptional tolerance to Al up to 500 ppm and Pb which is the subject of our study; they mentioned a tolerance of Pb up to 300 ppm, according to our results le Trichoderma sp. assimilate and resist to lead even at 4000 mg/l , it is considered the best metal assimilated and absorbed by Trichoderma in many studies .
Our findings indicate that fungal populations isolated from heavy metal-contaminated sites and sea water has the ability to resist higher concentrations of metals. The Trichoderma sp. have a better tolerance to all metals, a comparative level of metal resistance was also shown in the present study concludes that Trichoderma sp. have an ability to resist higher concentrations of Pb, it grows up to 4000 mg/l. The tolerance and the resistance of the isolates depended much more on the fungus tested than on the sites of its isolation, the Trichoderma sp. potential metal biosorbents is mentioned through the results obtained shown a great tolerance with success each heavy metals cited at the front, especially lead, and pushes in liquid solution up to 1000 mg/l, the process is based on their capacity uptake with fungal expansion, the fungi develop metal resistance and performance. Trichoderma sp. can be exploited as potential metal biosorbents and used as potent bioremediation.
- Arnand, P., isar J, Savan S, Saxena PK, Bioaccumulation of copper by Trichoderma viride. Bioresour technol. 2006; 97: 1018-1025.
- Chibuike, Gu., Obiora, Sc., Heavy metals polluted soils: effect on plants and bioremediation methods. Appl. Environ. Soil Sci., 2014; 2014, 1-12.
- Harman, G.E., Howell, C.R., Viterbo, A ., Chet, I., Lorito, M., Trichoderma species-opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol 2004; 2: 43-56.
- Howell, CR., Mechanisms employed by Trichoderma species in the biological control of plant diseases; the history and evolution of current concept. Plant Dis. 2003; 87; 4-10.
- Ezzi, MI, Lynch JM, Biodegradation of cyanide by Trichoderma spp. And Fusarium spp. Enzyme Microb Technol; 2005; 36: 849-854.
- Idris, R., Trifonova, R., Puschenreiter, M., Wenzel, W.W., Sessitch, A., Bacterial communities associated with flowering plants of the Ni hyperaccumulator Thlaspi goesingenese. Appl. Environ. Microbiol. 2004; 70: 2667-2677.
- Kuiper, I., Lagendijk, E.I., Bloemerg, G.V., Lugtenberg, B.J.J., Rhizoremediation: a beneficial plant-microbe interaction. Mol. Plant Microbe In. 2004; 17: 6-15.
- Kumar, P., Tewari, R.K., Sharma, P.N., Modulation of copper toxicity induced oxidative damage by exess supply of iron in maize plants. Plant Cell Rep. 2011; 27: 399-409. doi:http://dx.doi.org/10.1007/s00299-007-0453-1.
- Nongmaithem, N., Roy, A., Bhattacharya, P.M., Screening of Trichoderma isolates for their potential of biosorption of nikel and cadmium. Braz. J. Microbiol. 2016; 47: 305-313. http:// doi.org/10. 1016.01.008.
- Papavizas, GC. Trichoderma and Gliocalium : biology, ecology and potential for biocontrol. Ann Rev Phytopathol, 1985; 23: 23-54.
- Jaworska, M., Dluzniewska J., The effect of manganese ions on developement and antagonism of Trichoderma isolates. Polish environ Stud, 2007; 16(4): 549-553.
- Vinale, F., Sivasithamparan, K., Ghisalberti, E.l., Marra, R., Woo, S.L., Lorito, M., Trichoderma –plant-pathogen interactions. Soil. Biol. Biochem, 2008; 40: 1-10. Doi:
http://dx.doi.org/10.1016/j.soilbio.2007.07.002 - Olsson, P. A., Chalot, M., Baath, E., Finlay, RD.,Soederstroem, B., Ectomycorrhizal mycelia reduce bacterial activity in a sandy soil. Fems microbiol. Ecol. 1996; 21: 77-86.
- Ruiz, E., Rodriguez, L., Alonso-Azcarate, J., Effects of earthworms on metal uptake of heavy metals from polluted mine soils by different crop plants. Chemosphere 2009; 75: 1035-1041.
- Lavelle, P., Fungal activities and soil process: adaptive strategies thet determine ecosystem function. Adv. Ecol.Res. 1997; 27: 93-132.
- Cao, L., Jiang, M., Zeng, Z., Du A., Tan H., Liu Y., Trichoderma atroviride F6 improves phytoextraction efficiency of mustard (Brassica juneca(L.) Coss. Var. foliosa Bailey) in Cd Ni contamined soils, 2008. Chemosphere, 2008; 71; 1769-1773.
- Kok, K-H., Karim, M.I.A., Ariff, A., Bioremoval of Cadmium, Lead, and Zinc using Non –living biomass of Aspergillus flavus. Pakistan Journal of Boiolgical Sciences 2001; 4(7): 849-853.
- Sun, Y-M., Horng, C-Y., Chang, F-L., Cheng, Li-C., Tian, W-X., Biosorption of lead, Mercury, and Cadmium ions by Aspergillus terreus immobilized in a natural matrix. Polish Journal of Microbiology . 2010; 59(1): 37-44.
- Song N., Ma, Y., Zhao, Y., Tang, S., 2015. Elevated ambient carbon dioxide and Trichoderma inoculum could enhance cadmium uptake of Lolium Perenne explained by changes of soil biomass. Applied soil ecology; journal home page.http:// WWW.elsevier . com/locate/apsoil.
- Zhang, C., Lai, C., Zeng, G.M., Huang, D-L., Yang, Y., Zhou, Y-Y., Cheng, M., Efficacy of carbonaceous nanocomposites for our sorbing ionizable antibiotic sulfamethazinefrom aqueous solution. Water Res. 2016; 95: 103-112.
- Cavalcanti Luna, M-A, Vieira, E. R., Okada, K., Campos-Takaki, G.M., Elesbao do Nacimento, A., Copper-induced adaptation, oxidative stress and its tolerance in Aspergillus niger UCP1261. Electronic journal of biotechnology, 2015; 19(418-427).
- Provedano –Priego, C., Martin-Sanchez, I., Jroundi, F., Sanchez-castro, I., Fngal biomineralzation of lead phospahtes on the surface of lead metal. Minerals engineering, 2017; 106:46-54.
- Leitao, A.L., Potential of penicillium species in the bioremediation field. Int. J. Environ; Res. Public. Health, 2009; 6: 1393-1417.http:// dx.org/10.3390/ijerph6041393.
- Su Yien Ting, A., Jioe, E., In vitro assessement of antifungal activities of antagonist fungi towards pathogenic Ganoderma boninense under metal stress. Biological control, 2016; 96: 57-63.
- Tsekova, K., dentchev, D., Todorova., Effect of copper and Cadmium on filamentous fungi belonging to geneus Aspergillus. Biotechnology and biotechnological equipements, 2015; 13:1, 77-80, DOI:10.1080/13102818.1999.10819024.
- Téllez vergas, J., Rodriguez –Monroy, M., Meyer, M.L., Montes-Belmontes., R., Sepulveda – Jiménez., 2017. Trichoderma asperellum ameliorates phytoyoxic effects of copper in onion (Allium cepa L.).
- Liu, S-H., Zeng, G-M., Niu Y-Q., Liu, Y., Zhou, L., Jiang L-H; Tan, X-F., Xu, P; Zhang, C., Cheng M., Bioremediation mechanisms of co;bined pollution of PAHs qnd heavy metals by bacteria and fungi: A mini review. Bioresource technology, 2017; 224: 25-33.
- Alluri, H-K., Ronda, S-R., Setalluri, V-S, Singh, J., bondili., Suryanarayana, V and Venkateshwar. P., Biosorption: An eco-friendly alternative for heavy metal removal. African Journal of Biotechnology, 2007; 6(25), pp. 2924-2931. Available online at
http://www.academicjournals.org/AJB - Al Sohaibani Saleh, A., Heavy metal tolerant filamentous fungi from municipal sewage for bioleaching. Asian journal of biotechnology, 2011; 3(3): 226-236.
- Abdi, N; Belouchrani, A; Mameri, N; Mouhouche, B; Contribution to the study of the effect of microorganisms in the bioremediation of the soil polluted by zinc. The 4th international congress water, wastre environnement (EDE4), Agadir, 2013;Marocco.
- Domsch, KH; Gams, W; Anderson, TH., Compendium of soil fungi. Ed. IHW- verlag, 1980; 1: 859.
- Pitt, JI. 1979. The genus Penicillium and its teleomorphic states. Eupenicillium and Talaromyces. Ed. Academic Press. p. 634.
- Ait Ali, N., Bernal, M. P., Tolerance and bioaccumulation of copper in phragmites australis and Zea mays. Plant Soil, 2002; 239: 103-111.doi:http:::dx.doi.org/10.1023/A:1014995321560.
- Geremias, R., Fattorini, D., Fevere , V.T., Pedrosa, R,C., Bioaccumulation and toxic effects of copper in commom onion Allium cepa L. Chem. Ecol., 2010; 26: 19-26 doi:http://dx.doi.org/10.1080/02757540903468144.
- Iskandar , N.L., Zainudin, N.A., Tan, S.G., Tolerance and biosorption of copper (Cu) and lead (Pb) by filamentous fungi isolated from a fresh water ecosystem. J. Environ .Sci . 2011; 23: 824-830. doi:http://dx.doi. org/ 10.1016/S1001-0742 (10) 604755.
- Kumar, A., Brisht, B. S., Joshi., Zinc and Cadmium removal by acclimated Aspergillus niger: Trained fungus for biosorption. International Journal of environnement sciences and Reaserch, 2011; 1(1), pp.27-30.
- Ahmed, I., Ansari, M.I., and Aqil, F., Biosrption of Ni, Cr and Cd by metal tolerant Aspergillus niger and Penicillium sp , using singleand multi-metal solutions, Indian journal of experimental biology, 2006; 44(1): 73-76.
- Fomina, M., Hillier, S., Charnock, J.M., Melville, K., Alexander, I.J., Gadd., G.M. Role of oxalic acid over-extraction in toxic metal mineral transformations by Beauveriacaledonica 2005.
- Volesky, B., Advances in biosorption of metals: selection of biomass types. FEMS Microbiol. Rev. 1994; 14: 291-302.
- Hall, J. L., Cellular mechanisms for heavy metals detoxification and tolerance. J. Exp. Bot. 2002; 53: 1-11.
- Kapoor, A., Viraraghavan, T., Fungi biosorption – an alternative treatment option for heavy metal bearing wastewaters: Areview. Bioresour. Technol., 1995; 53: 195-206.
- Keasling, J.D., hupf, G.A. Genetic manipulation of polyphosphate metabolism affects cadmium tolerance in Escherichia coli. Appl. Environ. Microbiol. 1996; 87: 743-746.
- Keasling, L.D., Van dien, S-J., Trelstad, P., Renninger, N., McMahon, K., Application of polyphosphate metabolism to environnemental and biotechnological problems. Biochemestry. 1999; 65: 324-331.
- Malik, A., Metal bioremediation through growing cells. Environ. Inter. 2004; 30: 261- 275.
- Lopez, E.E., Vazquez, C. Tolerance of uptake of heavy metals by Trichoderma atroviride isolated from sludge. Chemosphere, 2003; 50: 137-143.
- Moreno, J. L., Garcia, C., Lei, L., Falchini , L., Pietramellara, G., Nannipieri, P., The ecological dose value (ED50) for assessing Cd toxicity on ATP content and dehydrogenase and urease activities of soil. Soil boil. Biochem. 2001; 33: 483- 489.
- Gharieb, N.M., Gadd, G.M., Role of gluthatione in detoxification of metal (loid) by Saccharomyces cervisiae. Biometals, 2004; 17: 183-188.
- Li, Z., yuan, H., Hu, X., Cadmium –resistance in growing Rhodotorula sp . Y11. Bioresour Technol, 2007; 99: 1339-1344.
- Gæksungur, Y., zren, S., G{venc , U., Biosorption of cadmium and lead ions by ethanol treated waste baker’s yeast biomass. Bioresour. Tecnol, 2005; 96: 103-109.
- Van Loosdrecht, M.C., Smolders, G.H., Kuba, T., Heijnen, J.J., Metabolism of microorganisms responsible for enhanced biological phosphorus removal from wastewater. anton. Leeuwenhoek , 1997; 71: 109-116.
- Acosta , I., Moctezuma-zàrate, M.G., Cardenas , J.F., Gutiérrez, C., Biosorption of cadmium (ii) in aqueous solutions by fungal biomass. Rev. Inform. Technol. 2007; 18: 9-14.
- Massaccesi, G., Romero, M., Cazau, M., Bucsinszky, A., Cadmium removal capacities of filamentous soil fungi isolated from industrially polluted sediments, in La Plata (Argentina) world J. Microbiol. Biotechnol, 2002; 18: 817-820.
- McGrath, J.W., Quinn, J.P., Intracellular accumulation for polyphosphate by the Candida humicola G-1 in reponse to acid ph . Appl. Environ.Microbiol. 2000; 66: 4068-4073.
- Das, N., Charumathi, D., Vimala. R., Effect of preatretement on Cd2+ Biosorption by mycelial Biomass of Pleurotus florida. African journal of biotechnology. 2007; 6: 2555-2558.
- Price, M.S., Classen, J.J., Payne, G.A., Aspergillus niger absorbs copper and Zinc frow swine wastewater. Biossource technology, 2001; 77: 41-49.
- Djiksterhuis, J, Wçsten HAB., Development of Aspergillus niger. CBS-KNAW fungal biodiversity centre. Colloids Surf A 2013; 74.
- Tsekova, K ., Todorava, D., 2002. Copper (II) accumulation and superoxide dismutase activity during growth of Aspergillus niger B-77, ZNaturforsh, 57. 319-22.http://dx.doi.org/10.1515/znc-2002-3-421.
- Keasling J.D., Regulation of intracellular toxic metalsand other cations by hydrolysis of polyphosphate .Ann NY Acad Sci ., 1997; 829; 242-9.
- Remonsellez, F., Orell, A., Jerez, C.A., 2006. Copper tolerance of the thermoacidofolic archaeon Sulfolobus metacillus. Possible role of polyphosphate metabolism. Microbiology, 152:29-66.http://dx.doi.org/10.1099/mic.028241-0.
- Bennet, J. W., 2010. An overview of the genus Aspergillus. In Machida, M Gomi, K., editors,Aspergillus molecular biology and genomics . Linton, Uk: Caister Academic Press, P.1-16.
- Adrio, J.L., Demain AL., 2003. Fungal biotechnology .Int microbiol,6:191-9.http://dx;doi.org/10.1007/s10123-003-0133-0.
- Cacciola, S.O., Puglisi, I., Faedda, R., Sanzaro, V., Pane, A., Lo Piero, A., Evoli, M., Petrone, G., Cadmium induces cadmium-tolerantgene expression in the filamentous fungus Trichoderma harizatum. Mol. Biol. Rep. 2015; 42: 1559-1570.doi:http://dx.doi.org/10.1007/s11033-015-3924-4.
- Zafar, S., Aqil, F., Ahmad, I., Metal tolerance and biosorption potential of filamentous fungi isolated from metal contaminated agricultural soil. Bioresour Technol. 2007; 98: 2557-2561.
- Leyval, C., Turnau, K., Haselweter, K., Effect of heavy metals bearing wastewaters: A review. Bioresour. Technol. 1997; 53: 195-206.
- Ezzouhri, L., Castro, E., Maya, M., Espinola, F., Lairini, K., African journal of microbiology reaserch, 2009; 3(2): Pp.35-048.
- Levinskaite, L., Effect of heavy metals on the individual development of two fungi from the genus Penicillium. Biologija., 2001; 1: 25-30.
- Volesky B., Biosorption and biosorbents: In Biosorption of heavy metals. CRC Press, Florida, 1990; pp. 3-44.
- Ahmed, I., Zafar, S., Ahmad, S., Heavy Metal Biosorption potential of Aspergillus and Rhizopus sp. isolated from wastewater treated soil. Appl. Sci. Environ. Mgt. 2005; 9(1) 123 – 126.
- Sun, F., Shao, Z., 2007. Biosorption and bioaccumulation of lead by Penicillium sp. Psf-2. isolated from the deep sea sediment of the Pacific Ocean. Extremophiles (2007) 11:853–858. DOI 10.1007/s00792-007-0097-7.
- Brandolini, V., Tedeschi, P, Capece, A., Maietti, A., Mazzotta, D., Salzano, G., Paparella A., Romano, P., 2002. Saccharomyces cerevisiae wine strains differing in copper resistance exhibit different capability to reduce copper content in wine. W. J. Microbiol. Biotechnol. 18: 499- 503.
- Roy A., Pan S., Effect of fungistasis on germinability of wild and mutant isolates of Trichoderma harizianum and Glicladium virens. Mycolplant Pathol. 2005; 35(2): 319-323.
- Gadd, G.M., Metals, minerals and microbes, Geomicrobiology and bioremediation: 2010; 156: 609-43. http: //dx. doi. org/ 10/1099/mic.0.037143-0.
- Torres, M., Goldberg , J., jensen, T.E, Heavy metals uptake by polyphosphate bodies in living and killed cells of Plectonema boryanum (cyanophyce). Microbios, 1998; 96: 141-147.
- Lima, MABD, Nascimento, AED, Souza, WD, Fukushima, K Campos –Takaki GMD, Effects of phosphorus on polyphosphate accumulation by Cunninghamella elegans. Braz J Microbiol. 2003; 34: 363-72.
- Pepper, I.L., Microbial réponses ton environnementaly toxic cadmium. Microb. Ecol. 2000, 38: 358-364.
- Hajieghari, B., Effects of some metal containing compounds and fertilizers on mycoparasite Trichoderma species mycelia growth response. Afr. J. Biotechnol. 2010; 9: 4025-4033. doi: hhtp://dx.doi.org/ 10.5897/AJB2010.000-3282.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


