ISSN: 0973-7510
E-ISSN: 2581-690X
End-stage renal disease (ESRD) is one of serious complications of idiopathic membranous nephropathy (IMN) & it was reported in about one third of patients. IMN is an autoimmune disease in which autoantibodies target antigens at the level of the glomerular basement membrane. Immunological responses may be possibly involved in the pathogenesis of idiopathic membranous nephropatghy (IMN). Cytokines act as a potent immunomodulators. The present study was conducted to evaluate the role of MIF G173C and TNF-α G308Agenes gene polymorphism in the pathogenesis of IMN. We have investigated single nucleotide polymorphisms of MIF G173C and TNF-α G308A genes in 94 subjects. Forty-six patient had IMN-nephrotic syndrome while 48 subjects were apparently healthy individuals used as a controls ,then the serum level of TNF-á and IL-13 was detected by ELISA technique. The frequencies of MIF C-173C (13.04 vs 4.00%) genotypes and C allele (29.35vs 22.00%) were higher in IMN patients than control group while TNF-α A308A (21.74 vs. 0) genotypes and A allele (38.88 vs 10%) were significantly higher in patient than control groups and associated with higher mean serum concentration of TNF-α (668.33+27.60) versus (45.64+2.38) and IL-13 (36.70+0.55) versus (2.72+0.22), in IMN patients than apparently healthy subjects. AA genotype with TNF-α-G308A allele polymorphism and CC genotype with MIF 173C allele are mainly expressed among IMN patients and susceptibility with disease might be prospected.
Tumor necrosis, Idiopathic membranous
In the 1920-30s , Bradley and Tyson1 were the 1st who defined nephrotic syndrome. The term nephrotic syndrome started to be used as being:”…one of the most striking phenomena of renal disease. The combination of gross oedema, hypoproteinemia, hypercholesterolemia, lipidemia and heavy proteinuria, in the absence of congestive heart failure, is unique and easily recognized.”
The annual incidence of nephrotic syndrome(NS) in adults is three per 100,000 persons, approximately 80% to 90% of NS cases in adults are idiopathic2. Membranous nephropathy is the most common cause in whites, and focal segmental glomerulosclerosis is most common in blacks; each of these disorders accounts for approximately 30% to 35% of NS cases in adults2.
Membranous nephropathy has an insidious onset, usually with (subclinical) proteinuria which leads later to peripheral oedema. It can be diagnosed at any age but its age distribution shows two peaks at 30 to 40 and 50 to 60 years. Of all the individuals affected by membranous nephropathy, 20% stay asymptomatic with non-nephrotic levels of proteinuria (<3.5 g/day) and 80% become clinically manifest, presenting a full blown nephrotic syndrome3.
A pooled analysis of studies of patients with idiopathic MN found a 2:1 predominance of men4 .
MN is most often primary (idiopathic), although it has been associated with hepatitis B antigenemia, autoimmune diseases, thyroiditis, malignancies, and the use of certain drugs such as nonsteroidal antiinflammatory drugs (NSAIDs), gold, penicillamine, and captopril. In the majority of cases of tumor-associated MN, the malignancy has been diagnosed or is clinically apparent at the time that proteinuria is discovered.
IMN is considered an autoimmune disorder in which antibodies against some antigens result in generation of immune complex with subsequent activation of the complement cascade6. Recently, the M-type phospholipase A2 receptor (PLA2R), expressed in podocytes, has been identified as the autoantigen7.
The first one to suggest that renal diseases can be caused by immune reactions was Schick in 1907.8 The autoimmune hypothesis in renal pathology gathered renewed support when in 1949 Langeet al. were able to demonstrate the presence of antibodies against kidney tissue emulsion in a group of patients with chronic glomerulonephritis.9
The term MN reflects the primary histologic change noted on light microscopy: glomerular basement membrane (GBM) thickening with little or no cellular proliferation or infiltration.10
The diagnosis of MN can only be made by renal biopsy. Pathologic sine qua non of MN is the presence of subepithelial immune-complex deposit, which was best demonstrated by electron microscopy.11
A Cochrane review showed that combining an alkylating agent with a corticosteroid has short- and long-term benefits for membranous nephropathy in adults with nephrotic syndrome.12
Idiopathic membranous nephropathy has a generally favorable prognosis, which roughly follows a “rule of thirds”: about one-third of patients have a benign course with a high rate of remission; one-third have ongoing evidence of proteinuria or edema but maintain normal renal function; and somewhat less than one-third of patients progress toward end-stage renal disease within 10 years.12
The cytokines are key factors in modulating the immune response and class switching of antibodies. Cytokines modulate the activity of T and B lymphocytes, regulate cellular activities and the response to pathogens. The polymorphisms in promoter regions of cytokine genes can influence the transcriptional and expression levels of these cytokines.13, 14
Many research groups have established the link between these polymorphisms and various pathologies. For instance, the variant -572C of IL-6 gene and the polymorphism -308A of TNF-α and TNF d2 allele were associated to idiopathic membranous nephropathy in different ethnic groups ,moreover, it is demonstrated that TNF-α-308A can also cause Non Idiopathic Membranous Nephropathy.15
This is a case-control study include 2 groups of people
Subjects for this study, 48 matched controls aged between 22 – 55 years (mean age 34.5; 32 male and 16 female) and 46 patients had IMN-Nephrotic Syndrome, aged between 21 and 58 years (mean age 35.7; 31 male and 15 female) were enrolled. Samples were collected at the Department of Nephrology / Al-Diwanyia Teaching Hospital , Histopathology & Immunology departments of the College of Medicine/ Al-Qadisiya University, between October 2014 and May 2017. Physical examination, hematochemical and urine analysis, 24 h urinary collection for albumin & creatinine, albumin, creatinine and cholesterol plasma levels, 24 h creatinine clearance and daily urinary protein excretion have defined the diagnosis of membranous nephropathy (MN). The diagnosis was confirmed on renal biopsies with microscopic and immunofluorescence techniques. Clinical examination and laboratory data excluded the presence of other alterations that could define secondary membranous nephropathies. In fact these patients were negative for tumors, systemic lupus, viral hepatitis infections or other causes of MN like rheumatoid arthritis. These patients were negative for antinuclear antibodies(ANA), Rheumatoid factor (RF), Hepatitis B and Hepatitis C markers (HBsAg and anti-HCV).
Serum cytokine assay
Serum concentrations of TNF-α IL-13were measure by using ELISA Kit (RayBio) according to the manufacturer’s instructions.
Genotyping
PCR–restriction fragment length polymorphism(RFLP) was used to determine the genotypes of the MIF G-173C and TNF-α G308Ageneswere (RFLP) Table (1). The technique used include: A AccuPower TM PCR PreMix (Bioneer) were used to purified the PCR products , & the next step was visualization of PCR products in an ethidium bromide-stained 1.5% agarose gel using a UV Transilluminator. Finally PCR products were digested with the Nco1 restriction enzymes for TNF-α & AluI restriction enzymes for MIF. Again the digested PCR products were visualized in anethidium bromide-stained 2.5% agarose gel using a UV Transilluminator.
Table (1):
The primer sets and restriction enzymes cloning designed for the Polymerase chain reaction(PCR) – restriction fragment length polymorphism(RFLP) analyses
Gene Variations |
Restriction enzymes |
Primers used for PCR analysis |
|---|---|---|
MIF G173C |
AluI |
F: 5′-CTAAGAAAGACCCGAGGCGA-3′ R: 5′-GGCACGTTGGTGTTTACGAT-3′ |
TNF-α G308A |
Nco1 |
F- 5AGGCAATAGGTTTTGAGGGCCAT-3 R-5 TCCTCCCTGCTCCGATTCCG-3 |
Statistical analysis
Comparing the observed numbers of each genotype which was assessed for both the control group & the patient group with those expected under the HWE(The Hardy– Weinberg equilibrium assumption) for the estimated allele frequency.
Data were presented, summarized and analyzed using two software programs. These were the Statistical Package for Social Science (SPSS) version 20 and Microsoft Office Excel 2010.
Odds ratios (OR) and 95% confidence intervals (CI) for the association between the genotypes, alleles or haplotypes and the risk of IMN were estimated by logistic regression analysis.
P value of d”0.05 was considered to indicate statistical significance. The results were also presented as the mean values ± 1 standard deviation (SD).
Demographic and Biochemical distribution
The distribution of demographic and biochemical features of both IMN patients and control groups are shown in Table (2). In which there is a significant differences between the IMN patients and controls in all the biochemical results.
Table (2):
Distribution of demographic & biochemical characteristics of IMN patients and controls
Parameters |
Patients =46 (mean ± SE) |
Controls = 48 (mean ± SE) |
P value |
|---|---|---|---|
Age |
35.7+0.73 |
34.5+0.60 |
0.957 |
Male/female |
2.06:1 |
2:1 |
0.943 |
Serum cholesterol (mg/dl) |
479.11+16.63 |
179.72+3.99 |
<0.001 |
Serum total protein (g/dl) |
4.48+0.05 |
7.38+0.07 |
<0.001 |
Serum albumin (g/dl) |
1.88+0.05 |
4.02+0.09 |
<0.001 |
Serum createnin (mg/dl) |
1.86+0.22 |
0.59+0.04 |
<0.001 |
Table (3):
Distribution of MIF G173C and TNF-α-G308A genotypes and alleles in patients and controls group
| Genotype) | Patient’s N = 46 (%) | Controls N = 48 (%) | P value | OR (95% CI) | EF | PF |
|---|---|---|---|---|---|---|
| MIF G-173C | ||||||
| GG | 25 (54.35) | 54.35 (60.00) | 0.803 | 0.794 (0.295-2.132) | — | 0.140 |
| GC | 15 (32.61) | 32.61 (36.00) | 0.798 | 0.860 (0.309 -2.393) | — | 0.092 |
| CC | 6 (13.04) | 13.04 (4.00) | 0.409 | 3.600 (0.408- 31.741) | 0.619 | — |
| G | 65 (70.65) | 70.65 (78) | 0.429 | 0.679 (0.658.-3.296) | — | 0.228 |
| C | 27 (29.35) | 29.35 (22) | 0.429 | 1.473 (0.303-1.520) | 0.228 | — |
| TNF-α-G308A | ||||||
| GG | 21 (45.65) | 20 (80) | 0.006 | 0.210 (0.067-o.656) | — | 0.658 |
| GA | 15 (32.61) | 5 (20) | 0.287 | 1.935 (0.608-6.160) | 0.363 | — |
| AA | 10 (21.74) | 0 (0) | 0.011 | 14.671* (0.822- 261.837 | 0.889 | — |
| G | 57 (63.33) | 45 (90) | <0.001 | 0.181 (0.066-0.499) | — | 0.717 |
| A | 35 (38.88) | 5 (10) | <0.001 | 5.526 (2.002.-15.254) | 0.717 | — |
Distribution of MIF G173C, TNF-α G308A Genotypes and Alleles in Patient and Control Group
Distribution of MIF G-173C, TNF-α G308A polymorphism was detected by PCR-RFLP technique, at this locus there’re three genotype; for MIF G-173C GG, GC and CC with band sizes 100 pb, 100/263 pb and 363 pb respectively Figure (1) , and for TNF-α G308A GG, GA and AA with band sizes 87 pb, 87/107 pb and 107 pb respectively, Figure (2). The frequency distribution of genotypes and alleles of MIF G-173C, TNF-α G308A in patient and control groups are summarized in Table (3). Single nucleotide polymorphisims (SNPs) were genotyped in the MIF and TNF-α genes in a group of patients with IMN to determine the correlation between the genotypes/allotypes and their clinical features , this the most important aim of our study.
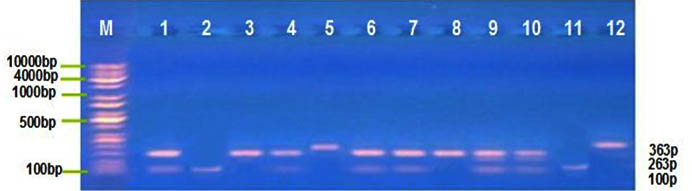
Fig. 1. MIF G173C electrophoresis after restriction digestion with Alu1
Lane (M): DNA molecular size marker (KAPA Universal Ladder), 2,11= Homozygous for wild type genotype (100bp), Lane 3.5.12= Homozygous for mutant genotype (363bp), Lane 1,4,6,7,8,9,10=heterozygous genotype (100/263bp).
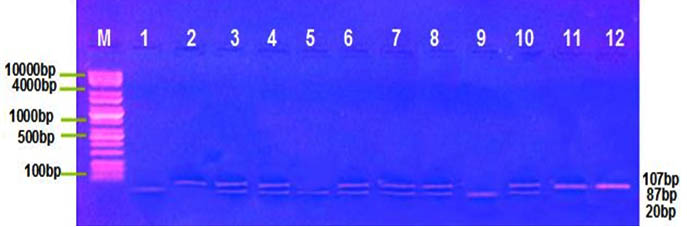
Fig. 2. TNF-α G308A electrophoresis after restriction digestion with Nco1
Lane (M): DNA molecular size marker (KAPA Universal Ladder), Lane 1,5,9,= Homozygous for wild type genotype (87bp), Lane 2,11,12= Homozygous for mutant genotype (107bp), Lane 3,4,6,7,8, 10= heterozygous genotype (87/107bp).
MIF G-173C were genotyped in 257 patients with IMN-NS by Vivarelli et al, Italy16 and he was found that the frequency of the C allele (high producer) was higher in patients than in controls. Similarly, Berdeli et al17 investigated the MIF G-173C SNP in 214 patients with IMN-NS and found that the frequencies of the GC genotype and C allele were higher in the patients than in the control subjects. Although, in our study there was no statistical significance in the MIF G-173C genotypes/allotypes, but the CC genotype has obviously suggests as an etiological factor for IMN-NS, as it had an OR of 3.6 and Etiologic Fraction (EF) of 0.619, In contrast, the GG genotype had rather preventive role as it had Protective Fraction (PF) of 0.140 and low OR (0.794). With The possibility of G allele may be protective, whereas the C allele may increase susceptibility to membranous nephropathy. This may be due to the potential effects of macrophage migration inhibitory factor on the natural immune response due to the inhibition of immune cell activation, which is regulated by glucocorticoids18, as MIF is expressed in glomerular parietal and visceral epithelial cells and in tubular epithelial cells in the kidney[19], which plays important physiological roles in the regulation of macrophage function, and lymphocyte immunity, as well as a pathogenetic role in some immunologically induced kidney diseases20
Alteration in cellular immunity and pro-inflammatory cytokines may contribute to kidney injury. One of these cytokines is TNF-α, a 17-kd protein encoded in the major histocompatibility complex locus on chromosome 6. It is produced in response to various stimuli, not only by infiltrating monocytes-macrophages but also by glomerular and mesangial cells with pro-inflammatory activities21.
TNF-α showed a strong association at genotypic level (OR = 14.671), as well as at allelic level (OR = 2.002), which demonstrates that this may be one of the risk factors for IMN and may be affect the steroid response.22 Polymorphism at position 308 of the TNF-α promoter, representing G to A base transitions, has been linked to increased TNF transcription23. Earlier studies have shown increase of TNF-α synthesis and gene expression in patients with IMN and focal glomerular sclerosis.24
Serum cytokine
Mean serum concentration of the IL-13 (36.70+0.55) versus (2.72+0.22) and TNF-α (668.33+27.60) versus (45.64+2.38); was significantly higher in the IMN patients group in comparison to control group, with a p-value of (<0.001) table (4). These results observe a significant association between concentration of TNF-α and NS , this may be due to its important role in pathophysiology of IMN as the TNF-α expression in renal disease has been found in both resident cells and infiltrating monocytes-macrophages25, and It acts in a paracrine way to recruit monocytes and macrophages to the glomerular region ,besides acting with other mediators to increase vascular permeability26.
Table (4):
Serum concentration of TNF-α and IL-13 in patients and control group
| Cytokine | Control(n=48) | Patients(n=46) | P-value | |||
|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | |||
| TNF-α | 45.64 | 2.38 | 668.33 | 27.60 | <0.001 | |
| IL-13 | 2.72 | 0.22 | 36.70 | 0.55 | <0.001 | |
Printza et al., reported that serum IL-13 levels were significantly higher in the active stage of IMN compared with the two remission phases and that although IL-13 levels were even lower than in the active stage, they remained elevated during both remission phases compared with the controls.27
There is significantly higher Concentration of IL-13 and TNF-α in IMN patients in comparison to control group. This provides strong evidence that pro-inflammatory cytokines play a major role in the pathogenesis of IMN. TNF-α-G308A polymorphism with AA genotype and A allele are mainly expressed among IMN patients and susceptibility with disease might be prospected whereas G allele might serve as protective factors for the disease.
ACKNOWLEDGMENTS
It is a great pleasure to thank the many people who made this research possible. First of all, I would like to express my gratitude to all patients & controls who participated in the study & I wish to thank all my colleagues for their constant.
- S. E. BRADLEY and C. J. TYSON, The nephrotic syndrome, N. Engl. J Med., 1948; 238: 260-266.
- Hull RP, Goldsmith DJ. Nephrotic syndrome in adults. BMJ. 2008; 336(7654):1185–1189
- L. H. Beck, Jr. and D. J. Salant, Membranous nephropathy: recent travels and new roads ahead, Kidney Int., (2010).
- S.L. Hogan, K.E. Muller, J.C. Jennette, R.J. FalkA review of therapeutic studies of idiopathic membranous glomerulopathy Am J Kidney Dis, 1995; 25: pp. 862-875
- Glassock RJ. Diagnosis and natural course of membranous nephropathy. Semin Nephrol 2003; 23:324.
- Honkanen E, von Willebrand E, Teppo AM, et al. Adhesion molecules and urinary tumor necrosis factor-alpha in idiopathic membranous glomerulonephritis. Kidney Int. 1998; 53:909–917.
- Beck LH, Jr, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009; 361:11–21
- Schick B., Die Nachkrankheiten des Scharlach, Jb. Kinderheilk., 1907; 65: 132-173.
- K. Lange, M. M. Gold, D. Weiner, and V. Simon, AUTOANTIBODIES IN HUMAN GLOMERULONEPHRITIS, J Clin. Invest, 1949; 28: 50-55.
- Fogo AB, Lusco MA, Najafian B, Alpers CE. AJKD Atlas of Renal Pathology: Membranous Nephropathy. Am J Kidney Dis 2015; 66: e15
- J.C. Jennette, R.H. Heptinstall (Eds.), Heptinstall’s pathology of the kidney (6th ed.), Lippincott Williams & Wilkins, Philadelphia, PA (2007), pp. 205-252
- Chen Y, Schieppati A, Chen X, et al. Immunosuppressive treatment for idiopathic membranous nephropathy in adults with nephrotic syndrome. Cochrane Database Syst Rev. 2014; 10: CD004293
- Thibaudin, D., Thibaudin, L., Berthoux, P., Mariat, C., Filippis, J.P., Laurent, B., Alamartine, E. and Berthoux, F. (2007) TNFA2 and D2 Alleles of the Tumor Necrosis Factor Alpha Gene Polymorphism Are Associated with Onset/ Occurrence of Idiopathic Membranous Nephropathy. Kidney International, 71, 431-437.
- Bantis, C., Heering, P.J., Aker, S., Siekierka, M., Kuhr, N., Grabensee, B. and Ivens, K. Tumor Necrosis Factor-á Gene G-308A Polymorphism Is a Risk Factor for the Development of Membranous Glomerulonephritis. American Journal of Nephrology, 2006; 26: 12-15. http://dx.doi.org/10.1159/000090706
- Wilson, A.G., Symons, J.A., McDowell, T.L., McDevitt, H.O. and Duff, G.W. Effects of a Polymorphisms in the Human Tumour Necrosis Factor á Promoter on Transcriptional Activation. Proceedings of the National Academy of Sciences of the United States of America, 1997; 94: 3195-3199.
- Vivarelli M, D’Urbano LE, Stringini G, Ghiggeri GM, Caridi G, Donn R, Tozzi A, Emma F, De Benedetti F: Association of the macrophage migration inhibitory factor “173*C allele with childhood nephrotic syndrome. Pediatr Nephrol 2008; 23: 743–748
- Berdeli A, Mir S, Ozkayin N, Serdaroglu E, Tabel Y, Cura A. Association of macrophage migration inhibitory factor – 173C allele polymorphism with steroid resistance in children with nephrotic syndrome. Pediatr Nephrol, 2005; 20: 1566–1571
- Zhang W. et al. Effect of MDR1 gene polymorphism on progression of end-stage renal disease. Acta. Pharmacol. Sin. 2007; 28: 579–583
- Lolis E. Glucocorticoid counter regulation: macrophage migration inhibitory factor as a target for drug delivery. Curr. Opin. Pharmacol. 2001 ; 1: 662–668
- WilsonA. Effects of a polymorphism in the human tumor necrosis factor alpha promoter on transcriptional activation. Proc. Natl. Acad. Sci. USA. 1997; 1:3195–9.
- Lionaki S, Siamopoulos K, Theodorou I, et al. Inhibition of tumour necrosis factor alpha in idiopathic membranous nephropathy: a pilot study. Nephrol Dial Transplant. 2009; 24: 2144–2150.
- Matsumoto K. Spontaneous and LPS-stimulated release of tumor necrosis factor-alpha by peripheral blood monocytes in patients with focal glomerular sclerosis. Nephron. 1995; 70:118–119.
- Abott F. et al. Interleukin-1beta stimulates human mesangial cells to synthesize and release interleukins-6 and -8. Kidney int. 1991; 40(4):597–605.
- Gruss H. Molecular, structural and biological characteristics of the tumor necrosis factor ligand superfamily. Int. J. of clin. & lab. Res. 1996 ; 26(3):143–59.
- Daniel V. et al. T-lymphocyte populations, cytokines and other growth factors in serum and urine of children with idiopathic nephrotic syndrome. Clin. Nephrol. 1997; 47(5):289–297.
- Florit EA, Ubeda-Aranda I, Delgado-Conde P, et al. Membranous glomerulonephritis, psoriasis and etanercept. A chance or casual association? Nefrologia. 2012; 32:228–232.
- Printza N. et al. IL-18 is correlated with type-2 immune response with steroid sensitive nephrotic syndrome. Cytokine 2008; 44(2):262-8.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


