ISSN: 0973-7510
E-ISSN: 2581-690X
Spider flower (Gynandropsis gynandra), also known as “Maman” that is traditionally fermented and consumed as pickles in Malaysia. In this study, the chemical composition, microbiological content and biological activities of the fresh and fermented Maman leaves and stem were evaluated. Approximate analysis was carried out by using AOAC standard methods, microbial content was determined by using total plate count, antioxidant activity was evaluated DPPH (%) and ferric reducing power. The antimicrobial activity evaluated by 96 well micro-titer plates against Salmonella Typhimurium ATCC14028, Escherichia coli ATCC12229 and Staphylococcus aureus ATCC6538. The results demonstrated reduction in the protein, fiber, and carbohydrate contents of Maman sample after fermentation in both leaves and stem, while the content of ash was increased. Fermented Maman contained significantly high (p<0.05) number of anaerobic bacterial count which is 5.5600 log CFU/mL for the stem and 5.4633 log CFU/mL for the leaves. The aerobic bacterial count was reduced significantly after the fermentation by approximately two logs. The antioxidant activity, phenolic compounds, and flavonoids content of fresh Maman was slightly higher than the fermented samples. The fermented Maman samples demonstrated 90% growth inhibition towards selected pathogens, while fresh samples showed less than 50% bacterial growth inhibition. This indicates that natural fermentation process improved the nutrient content and the biological activity of Maman.
Fermented Mamamn, Physicochemical, Antioxidant, Microbial activity, Malaysian fermented foods.
Fermentation is the oldest method of preserving food and improving many favourable food features such as flavour, shelf-life and texture. There are many types of fermented vegetable products around the world such as miso, soy sauce, pickled vegetable and kimchi (Lee, 1997). According to Rhee et al., (2011), fermented foods are those foods which involves the action of microorganisms or enzymes that cause biochemical changes and improve the food properties. Furthermore, acidification of fermented foods may reduce food poisoning and transmission of pathogenic organisms (Fernández et al., 2015). Gynandropsis gynandra (L.) commonly known as spider flower, cat whiskers, African cabbage, and in Malaysia known as “Maman” is considered as one of the medicinal plants which grows in many tropical countries, and in some areas, it is cultivated as an indigenous leafy vegetable. This plant is widely used in various countries like Indo-China, Africa, North and Central America as an alternative ailment for headache, neuralgia, cough, wounds, counter irritants (Kori et al., 2009). The medicinal or healing properties are due to the bioactive components such as phenolic compound, and flavonoid.
In Malaysia Maman is traditionally consumed as pickles and also consumed raw or cooked. The traditional method to prepare fermented Maman is starts by washing the raw materials, remove lower part of the steam, add hot water to the fermentation plastic containers, adding 10% salt and red chilli for flavouring, then incubate at room temperature for 5-6 days before consumption. Maman is a superior source of micronutrients as compared to the leafy vegetables such as cabbage, kale, lettuce, spinach and others (Chweya, 1983). The extractable protein from the leaf of Maman is rich in amino acid such as aspartic and glutamic acids, leucine and lysine (Oke, 1973). Fermentation process is reported to increase the nutrient values and the biological activities of several leafy vegetables such as cabbage (Kusznierewicz et al., 2008). Moreover, fermented Maman is heavily consumed by local people in Malaysia but to the best of our knowledge there are no studies to determine the chemical composition and biological activity changes after the natural fermentation. Therefore, the aims of this study were to determine the physical, chemical properties, and biological activity of fresh and fermented Maman.
Raw material sample
The Maman plants (3 kg) were collected from the Agriculture University Park, Universiti Putra Malaysia (UPM), and transferred to the Biotechnology laboratory, Faculty of Food Science and Technology, UPM for fermentation process and further analysis. The plants were separated into two parts leaves and stem, washed with water to remove soil and the damaged parts. They were then dried and stored at room temperature.
Preparation of fermented samples
Maman plants were separated into two parts leaves and stem, both soaked overnight in clean water. The leaves and steams were subjected to spontaneous fermentation separately in jars containing 10% brine and incubated at room temperature 28 °C for 3 days. The fresh and fermented sample of leaves and stem were dried in the convection dryer at 45 °C for 48 hours. Dried samples were ground using pastel and mortar.
Characterization of fresh and fermented Maman
Determination of pH value
Ten grams of fresh and fermented dry samples were dissolved in 100 mL of water and the pH was determined at room temperature 28 °C using a digital pH meter (JENWAY 3505).
Approximate analysis
The chemical constituents including crude fat, crude protein, crude fiber, ash and moisture, were determined following the standard methods according to AOAC (2012). The carbohydrate contents were calculated by subtracting the total percentage value of other proximate composition with 100 %. Samples were analysed in triplicate.
Colour analysis
The colour analyses for fresh and fermented leaves and stems were carried out following the method as described by Claussen et al., (2007) using the Hunter lab instrument (Minolta R-300 Chromameter, Japan). The rates of lightness (L*), redness (a*) and yellowness (b*) of fresh and fermented sample fractions were measured.
Microbiological analysis
Microbial count was carried out to determine the aerobic and anaerobic microorganisms present in the fresh and fermented Maman. Briefly, 1 gm of each sample was added to 9 mL of peptone water (0.1 g/L). Then, 0.1 mL was inoculated onto nutrient agar for aerobic count and on MRS agar for anaerobic count using spread plate method. The plates were incubated for 24 to 48 h at 37 °C. Plates containing 25 to 250 colonies were enumerated, and the bacterial count is express as log 10 CFU/ml.
Antioxidant activity
The antioxidant activity of fresh and fermented Maman was determined by DPPH and FRAPS methods, while the phenolic content and flavonoids were determined by proper methods. One gram (1 g) of powder Maman was extracted with 50 mL of methanol at room temperature for one hour. The sample mixture was continuously mixed for 30 minutes (150 RPM) in orbital shaker at room temperature. Then, the extraction was filtered using vacuum filtration and stored at 4 °C for 2 weeks. The total phenolic content of the extract was determined using the Folin-Ciocalteau method (Wong et al., 2006). The total phenolic content of fresh and fermented Maman was calculated using gallic acid as standard. In addition, total flavonoid content was determined following the aluminum chloride colorimetric assay as described by Sulaiman and Balachandran (2012) and quantified using quercitin as standard. The radical scavenging assay were carried out using the DPPH method as described by Chan et. al (2007). Ferric reducing power (FRP) was measured using the potassium ferricyanide assay (Siow and Hui 2013) with gallic acid as standard.
Antibacterial activity
Antibacterial activity of fresh and fermented Maman was determined against food-borne pathogenic bacteria including Salmonella Typhimurium ATCC14028, Escherichia coli ATCC12229, and Staphylococcus aureus ATCC6538. Selected bacteria were growing aerobically at 37 °C for 24 hour in nutrient broth. The 96 wells micro-titer method was carried out for antibacterial activity as described by Muhialdin et al., (2016) with some modifications. Briefly, the pathogenic bacteria was incubated in nutrient broth overnight. The aqueous extract of the fresh and fermented powder samples was placed in micro-titer plates and 100 uL of nutrient broth containing 106 of each pathogens was added to the wells. The plates were incubated at 37 °C for 24 hours and the optical density was measured at 600 nm by using ELISA reader.
Statistical analysis
All data collected were recorded as mean ± standard deviation for triplicate determination and analysed statistically by analysis of variance (ANOVA) using Minitab version 14 (MIniatb Inc., NSW, Australia).
Fermented Maman pH value
The pH value of Maman leaves decreased significantly after fermentation from 5.46±0.02 to 4.70±0.56 while the pH of stem was slightly decreased from 5.38±0.04 to 5.29±0.01 (Figure 1). During the fermentation carbohydrates are metabolized by lactic acid bacteria which subsequently decreased the pH value. Lactic acid bacteria are naturally present on majority of plants and are the most importance bacteria in food fermentation (Woodford, 1985). The result showed that the production of acid was higher during the fermentation of leaves in comparison to the fermented stems. This is due to the higher nutrient contents stored in plant leaves, for example carbohydrates content is higher in leaves as compared to the stems. According to Daeschel et al. (1987), the growth of lactic acid bacteria that present on plants is restricted by availability of nutrients, temperature and competition with other microorganisms. During the fermentation process, LAB converts carbohydrates into carbon dioxide and organic acids which subsequently reduced the pH value and causing acidic environment that inhibits the growth of other microorganisms. Therefore, fermented foods are significantly safe for direct consumption and have long shelf life.
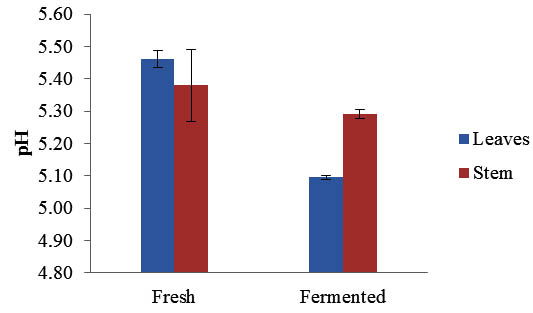
Fig. 1. pH value of Maman leaves and stem before and after fermentation
Maman proximate analysis
The proximate analysis of fresh and fermented Maman was carried out to evaluate the effects of fermentation on Maman. The results demonstrated the significant changes of the chemical composition after the fermentation for 3 days (Figure 2). The moisture content of fresh Maman leaves was 84.34 ± 0.33% and slightly increased to 88.12 ± 0.07% after fermentation. However, the moisture content of fresh Maman stems was slightly reduced from 87.79 ± 0.41% to 86.35 ± 0.06% (p<0.05). This is could be due to movement of water in and out from brine into the plant, where the water moves from the brine into the leaves pores (hypotonic) as a result of water pressure which was lower on the leaves than the brine. Likewise, the water pressure in the stems (hypertonic) was higher than brine and resulted in the water moving out to the brine. However, high water activity and brine conditions are preferred by LAB and can improve the fermentation (Chen, 2013; FAO, 1998). The chemical composition of Maman including ash, protein, fat, fibre and carbohydrate showed significant differences before and after the fermentation (Figure 2). It is observed that all the constituents of Maman were decreased after fermentation process for the leaves and stem except for ash. The increment of ash content after fermentation is due to the addition of salt in brine used in the preparation of fermented Maman. The reduction in crude fiber values could be due to the activities of microorganisms that are known for the bioconversion lignocelluloses into protein. This agrees with the findings of Hwei-Ming et al. (1994) and Balagopalan (1996). The decrease carbohydrate content could be attributed to the utilization of the soluble dietary fibre and carbohydrate as source of energy by the fermenting microorganisms for growth and metabolism. According to Tortora et al. (2001), the carbohydrate breaks down during fermentation by lactic acid bacteria.
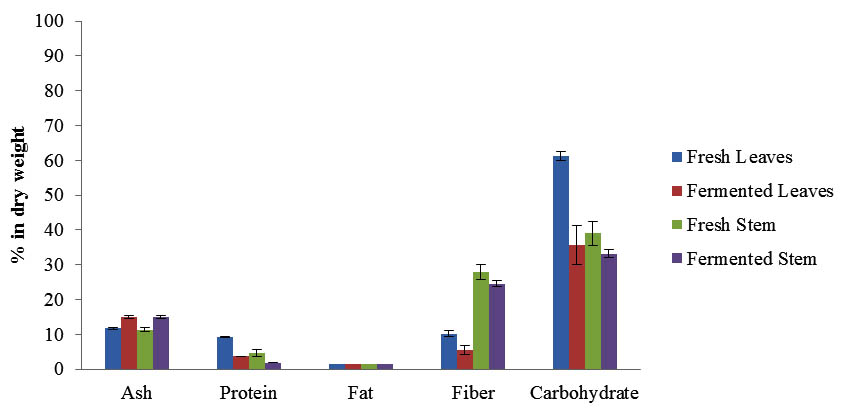
Fig. 2. Proximate analysis of fresh and fermented Maman
The protein content was significantly reduced after the fermentation of the Maman leaves and steam due to high proteolytic activity of LAB that includes extracellular and intracellular enzymes (Matthews et al., 2004). During fermentation, LAB secretes protease enzymes to digest the large proteins and convert it to low molecular bioactive peptides and amino acids that are utilized as an energy sources for LAB metabolism activity. The generated bioactive peptides are known to have several biological activities such as antimicrobial, anticancer, anti-hypertension, and anti-inflammation (Daliri et al., 2017). Furthermore, during fermentation the soluble proteins may be diffused from the leaves and steams into the brine (Daeschel et al., 1987). The fat content was very low for the Maman leaves and steam before and after fermentation and no significant differences were observed. Leafy herbs have very low fat content especially for fresh leafs that is ranged between 0.41 and 0.84 % of the plant composition (Oulaï et al., 2016).
Colour analysis
Colour analysis of fresh and fermented Maman was measured by using Minolta R-300 chromameter measured. Lightness L*, and redness a*, yellowness b* values of Maman stem and leaves increased significantly (p<0.05) after fermentation except for yellowness b* values of stem was reduced (Table 1). The colour of leaves and steam observed to be considerably darker after fermentation. The fermented Maman became darker due to the presence of acids which interacts with chlorophyll. In addition, the incubation temperature during fermentation can cause the darker color of the fermented Maman (FAO, 1998).
Table (1):
Color analysis, L* (lightness); B) a* (redness); C) b* (yellowness) values for the fresh and fermented Maman
| Stem | Leaves | |||
|---|---|---|---|---|
| Fresh | Fermented | Fresh | Fermented | |
| L | +77.78±0.07a | +80.29±0.31b | +36.96±0.13c | +43.17±0.40d |
| a | -4.39±0.33a | +0.77±0.11b | -13±0.36c | +1.54±0.19d |
| b | +5.84±0.40a | +2.49±0.40b | +16.89±0.98c | +23.77±0.26d |
Results are expressed in means ± standard deviation (n=3)
a,bLetters indicate significant difference at p < 0.05 between column for stem
c,dLetters indicate significant difference at p < 0.05 between column for leaves
Microbial count
The total aerobic and anaerobic counts of fresh and fermented Maman showed significant changes (Table 2). The aerobic bacteria were higher for the fresh samples in the stem and the leaves, while the anaerobic bacteria count was low. The fermentation of Maman increased the anaerobic count and reduced the aerobic bacteria. Several factors can determine bacterial counts in the fermented products including the anaerobic conditions during fermentation, the high competition of the microflora, and the production of secondary substances by certain microorganisms that inhibit the growth of others (Hibbing et al., 2010). The anaerobic bacteria are mostly lactic acid bacteria isolated on the selective media MRS agar. The fresh sample of Maman contained low anaerobic count for stem and leaves (3.19 log CFU/mL) (3.19 log CFU/mL) respectively, while fermented Maman showed a higher anaerobic bacterial count. LAB are naturally present in the plants and they are responsible of spontaneous fermentation fermentation process improve the quality and nutritional value of plants raw material. LAB produces several secondary metabolites to inhibit the growth of other microorganisms such as aerobic bacteria (Steinkraus et al., 1997). The LAB counts increased by 2 logs after the fermentation and their inhibition activity reduced the aerobic bacteria counts by approximately 2 logs.
Table (2):
Total aerobic and anaerobic bacteria counts isolated from the stem and leaves of fresh and fermented Maman
| Samples | Aerobic Bacterial (log CFU/ml) | Anaerobic bacteria (Log CFU/ml) | ||||
|---|---|---|---|---|---|---|
| Stem | Leaves | Stem | Leaves | |||
| Fresh Maman | 5.10 ± 0.03a | 4.50 ± 0.38a | 3.80 ± 1.04a | 3.19 ± 0.52a | ||
| Fermented Maman | 3.48 ± 0.06b | 3.47 ± 0.15b | 5.56 ± 0.07b | 5.46 ± 0.08b | ||
Results are expressed in means ± standard deviation(n=3)
a,bLetters indicate significant difference at p < 0.05 between rows
Antioxidant activity
The antioxidant activity was determined by two direct methods namely DPPH and FRAP to evaluate the effects of fermentation on Maman leaves and stem. Moreover, the phenolic compounds and flavonoids content were determined (Table 3). The results demonstrated no significant effects of fermentation on the antioxidant activity of Maman and the fresh samples had slightly higher antioxidant activity. However, the phenolic and flavonoids contents were reduced more than 50% after the fermentation for the stem and leaves. The fresh leaves showed the highest total phenolic content, flavonoid content and FRAP, 83.51±2.32 mg/g, 5.40±0.71 mg/g and 20.11±0.27 mg GAE/mg, respectively. Meanwhile, highest DPPH value was observed for fresh stem that was 91.49 ± 0.02%. The antioxidants activity was slightly reduced after the fermentation for both stem and leaves. There is correlation between the phenolic and flavonoids content, and the antioxidant activity (Piluzza and Bullitta, 2011). The higher the phenolic and flavonoid content, the higher the antioxidant activity. The increased counts of anaerobic bacteria during fermentation require certain nutrient, phenolic and flavonoids are very good source for the anaerobic bacteria growth. The reduction of phenolic flavonoids compounds cause the slight reduction of the antioxidant activity of Maman leaves and stem (Adetuyi and Ibrahim, 2014).
Table (3):
Antioxidant activity, total phenolic compounds, and total flavonoid compounds of fresh and fermented Maman
| Stem | Leaves | ||||
|---|---|---|---|---|---|
| Fresh | Fermented | Fresh | Fermented | ||
| Total phenolic mg/g | 46.20 ± 2.91 | 22.93 ± 0.74 | 83.51 ± 2.32 | 38.66 ± 10.00 | |
| Total flavonoid mg/g | 0.91 ± 0.10 | 0.11 ± 0.05 | 5.40 ± 0.71 | 1.07 ± 0.19 | |
| FRAP (mM) | 7.17 ± 0.24 | 5.22 ± 0.06 | 20.11 ± 0.27 | 11.48 ± 0.07 | |
| DPPH (%) | 91.49 ± 0.02 | 88.49 ± 0.54 | 79.79 ± 2.29 | 76.61 ± 0.86 | |
Results are expressed in means ± standard deviation (n=3)
Antibacterial activity
The antibacterial activity of fresh and fermented Maman was evaluated to determine the effects of fermentation on the plant. The results showed very high antibacterial activity of fermented leaves and stem in comparison to the fresh samples (Table 4). The fermented leaves inhibited the growth of S. Typhimurium, E. coli, and S. aureus for more than 94%. The fermented stem inhibited the growth of S. Typhimurium, E. coli, and S. aureus for almost 88%, 80%, and 91%, respectively. On the other hand, the fresh samples of Maman stem and leaves showed very low antibacterial activity which is below 50%. Organic acids including lactic acid are the main compounds produced by LAB and they very strong antimicrobial agents associated with many fermented foods and can enhance the shelf life (Reis et al., 2012). Thus, it is observed by the pH value of fermented Maman that was significantly dropped after the fermentation. Moreover, LAB utilizes several phenolic compounds and flavonoids for their growth requirements and stimulating the production of volatile phenols with strong antibacterial activity (Silva et al., 2011). This is the reason of decreased phenolic content after fermentation and the occurrence of growth inhibition towards selected food-borne pathogens. The other factor that enhanced the antibacterial activity is the bioactive peptides that are generated by LAB proteolytic enzymes that hydrolyze complex proteins into biologically active peptides. In previous studies, the fermentation of several plant-based materials reported to increase the antibacterial activity due to the enzymatic activity of LAB (Borresen et al., 2012).
Table (4):
Antibacterial activity of fresh and fermented Maman leaves and steams against food-borne pathogens after 24 h incubation at 37 °C in 96 well micro-titer plates
| Pathogens | Fresh sample | Fermented sample | ||
|---|---|---|---|---|
| Leaves | Steam | Leaves | Steam | |
| S. Typhimurium | 25.6 | 31.1 | 97.2 | 88.8 |
| E. coli | 38.5 | 38.8 | 94.7 | 80.5 |
| S. aureus | 47.3 | 36.6 | 97.7 | 91.1 |
The natural fermentation process significantly influenced Maman leaves and stem. The color of the fermented Maman was darker than fresh samples because of the incubation temperature and the production of organic acids. The protein, fiber, and carbohydrates contents were decreased after fermentation while ash content was increased. The pH value of fermented Maman was low due to the production of organic acids by LAB during fermentation. Fresh and fermented Maman demonstrated strong antioxidant activity while the phenolic and flavonoids content was 50% reduced during fermentation. The fermentation enhanced the antibacterial activity by two folds. The results indicate that natural fermentation improved the nutritional qualities and biological activity of Maman. It is recommended that future work should be carried out to identify the nutrients contents and the antibacterial related compounds to further explore the health benefits of fermented Maman.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the support and generosity of Universiti Putra Malaysia without which the present study could not have been completed.
- Adetuyi, F.O. and Ibrahim, T.A., Effect of fermentation time on the phenolic, flavonoid and vitamin C contents and antioxidant activities of okra (Abelmoschus esculentus) seeds. Nigerian Food Journal, 2014; 32(2), pp.128-137.
- AOAC International. Official Methods of Analysis of AOAC International, 19th ed. Arlington, USA 2012.
- Balagopalan, C. Nutritional improvement of cassava products using microbial techniques for animal feeding. Monograph of the Central Tuber Crops Research Institute, Kerala, India, pp: 44 1996.
- Bolling, B.W., Dolnikowski, G., Blumberg, J.B. & Chen, C.Y. Polyphenol content and antioxidant activity of California almonds depend on cultivar and harvest year. Food Chemistry, 2010; 122(3): 819-825.
- C Borresen, E., J Henderson, A., Kumar, A., L Weir, T. and P Ryan, E., Fermented foods: patented approaches and formulations for nutritional supplementation and health promotion. Recent patents on food, nutrition & agriculture, 2012; 4(2), pp.134-140.
- Calo-Mata, P., Arlindo, S., Karola, B., de Miguel, T. , Pascoal, A. & Barros-Velazquez J. Current applications and future trends of lactic acid bacteria and their bacteriocins for the biopreservation of aquatic food products. Food and Bioprocess Technology, 2008; 1(1): 43-63.
- Chan, E.W.C., Lim, Y.Y. & Chew, Y.L. Antioxidant activity of Camellia sinensis leaves and tea from a lowland plantation in Malaysia. Food Chemistry, 2007; 102(4): 1214-1222.
- Chen, H. Moden Solid State Fermentation: Theory and Practices. Netherlands: Springer 2013.
- Chweya, J.A. Identification and nutritional importance of indigenous green leaf vegetables in Kenya. Paper presented at the IX African Symposium on Horticultural Crops, 1983; 153.
- Claussen, I.C., Strommen, I., Egelandsdal, B. & Stratkvern, K.O. Effects of drying methods on functionality of a native potato protein concentrate. Drying Technology, 2007; 25: 1091-1098.
- Daeschel, M.A., Andersson, R.E., Fleming, H.P. Microbial ecology of fermented plant materials. FEMS Microbiology Letters, 1987; 46(3): 357-367.
- Daliri, E. B.-M., Oh, D. H., & Lee, B. H. Bioactive Peptides. Foods, 2017; 6(5), 32. http://doi.org/10.3390/foods6050032
- FAO, 1998. Feeding the Cities (excerpt from: The State of Food and Agriculture, 1998). “Food into Cities” Collection, DT/39-98E. Rome.
- Fernández, M., Hudson, J.A., Korpela, R. and de los Reyes-Gavilán, C.G., Impact on human health of microorganisms present in fermented dairy products: an overview. BioMed research international Article ID 412714; 2015.
- Hibbing, M.E., Fuqua, C., Parsek, M.R. and Peterson, S.B., Bacterial competition: surviving and thriving in the microbial jungle. Nature Reviews Microbiology, 2010; 8(1): p.15.
- Hwei-Ming, B., Villaume, C., Ching-Fwu, L., Evrard, J., Quemener, B., Jean-Pierre, N. & Mejean, L. Effect of a solid state fermentation using Rhizopus oligosporus sp. t- 3 on elimination of anti-nutritional substances and modification of biochemical constituents of defatted rapeseed meal. Journal of the Science of Food Agriculture, 1994; 65: 315-322.
- Irkin, R. & Songun, G.E. Applications of Probiotic Bacteria to the Vegetable Pickle Products. Scientific Revision and Chemical Communication, 2012; 2(4): 562-567.
- Ismail, A., Marjan, Z.M. & Chin ,W.F. Total antioxidant activity and phenolic content in selected vegetables. Food Chemistry, 2004; 87: 581–586.
- Khetarpaul, N. & Chauhan, B.M. Effect of germination and pure culture fermentation on HCl-extractability of minerals of pearl millet (Pennisetum typhoideum). International Journal of Food Science & Technology, 1989; 24: 327–331.
- Kori, M.L., Gaur, K. & Dixit, V.K. Andra investigation of immunomodulatory potential of Cleome gynandra. Asian Journal of Pharmaceutical & Clinical Research. 2009; 2(1): 35-39.
- Kusznierewicz, B., S´miechowska, A., Bartoszek, A. & Namies´nik, J. The effect of heating and fermenting on antioxidant properties of white cabbage. Food chemistry, 2008; 108: 853–861.
- Lee, C-H. Lactic acid fermented foods and their benefits in Asia. Food Control, 1997; 8(5): 259-269.
- Maki, M. 2004. Lactic acid bacteriain vegetables fermentation. In Lactic Acid Bacteria, Microbiological and Functional Aspects. Third ed. Edited by Salminen S., von Wright A, and Ouwehand A. New York: Marcel Dekker.
- Matthews, A., Grimaldi, A., Walker, M., Bartowsky, E., Grbin, P. and Jiranek, V., Lactic acid bacteria as a potential source of enzymes for use in vinification. Applied and environmental microbiology, 2004; 70(10), pp.5715-5731.
- Oke, O.L. Leaf protein research in Nigeria: a review. Tropical Science, 1973; 15(2):139-155.
- Oulaï, P.D., Zoue, L.Y.T. and Niamké, S.L., Nutritive and Antioxidant properties of Shade Dried Leafy Vegetables Consumed in Northern Côte d’Ivoire. Turkish Journal of Agriculture-Food Science and Technology, 2016; 4(2), pp.84-91.
- Patil, P.J., & Ghosh, J.S. Antimicrobial Activity of Catharanthus roseus-A Detailed Study. British Journal of Pharmacology and Toxicology, 2010; 1(1): 40-44.
- Piluzza, G. and Bullitta, S., Correlations between phenolic content and antioxidant properties in twenty-four plant species of traditional ethnoveterinary use in the Mediterranean area. Pharmaceutical biology, 2011; 49(3), pp.240-247.
- Reis, J.A., Paula, A.T., Casarotti, S.N. and Penna, A.L.B., Lactic acid bacteria antimicrobial compounds: characteristics and applications. Food Engineering Reviews, 2012; 4(2), pp.124-140.
- Rhee, S.J., Lee, J.E. and Lee, C.H., Importance of lactic acid bacteria in Asian fermented foods. Microbial Cell Factories, 2011; 10(1): S5.
- Salah, N., N.J. Miller, G. Paganga, L. Tijburg, G.P. Bolwell & C. Rice-Evans, Polyphenolic flavonols as scavenger of aqueous phase radicals and as chain-breaking antioxidants. Archives of Biochemistry and Biophysics, 1995; 2: 339-346.
- Silva, I., Campos, F.M., Hogg, T. and Couto, J.A., Wine phenolic compounds influence the production of volatile phenols by wine related lactic acid bacteria. Journal of applied microbiology, 2011; 111(2), pp.360-370.
- Siow, L.F. & Hui, Y.W. 2013. Comparison on the antioxidant properties of fresh and convection oven-dried guava (Psidium guajava L.). International Food Research Journal 20(2): 639-644.
- Steinkraus, K.H. Classification of fermented foods: worldwide review of household fermentation techniques. Food Control, 1997; 8(5-6): 311-317.
- Sulaiman, C.T. & Balachandran, I. Total phenolics and total flavonoids in selected Indian medicinal plants. Indian Journal of Pharmaceutical Science, 2012; 74: 258-60.
- Tortora, G.J., Funke, B.R., & Case, C.L. Microbiology: An Introduction, including Microbiology Place (TM) Website, Student Tutorial CD-ROM, and Bacteria ID CD-ROM . Edition: Benjamin Cummings Publishing SF, USA 2001.
- Wong, S.P., Leong, L.P. & Koh, J.H.W. Antioxidant activities of aqueous extracts of selected plants. Food Chemistry, 2006; 99: 775-783.
- Woodford, M.M. 1985. The silage fermentation. In: The microbiology of fermented food.Volume 2 (Wood, B.J.B. Ed) Pg 85-112. New York: Elsevier.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


