ISSN: 0973-7510
E-ISSN: 2581-690X
The present experiment was carried out with different floral preservatives to find out their efficacy on the POD and CAT enzyme activity on petals during vase life period of cut tuberose (Polianthes tuberosa L.) flower spikes in cv. “Single” and cv. “Double”. Among the floral preservatives tried the CAT activity was observed to be the minimum in 4% sucrose treatment whereas, the maximum in 25 ppm cobalt chloride treatment. GA3 100ppm and 25ppm cobalt chloride treated spikes recorded the highest POD activity whereas, 4% sucrose treatment recorded the lowest POD activity in the cut flower spikes. Lower activity of peroxide and catalase at senescence were associated with a longer vase life.
Floral preservatives, POD, CAT, Vase life, Quality.
Tuberose (Polianthes tuberosa Linn.) is essentially a florist’s flower and a leading commercial crop because of its multipurpose use. Due to its immense fragrance and highly valued natural flower oil, it is cultivated on a large scale in some parts of the world. The flower spikes last for a long duration and withstand long distance transportation. The use of floral preservatives is one of the most common techniques for prolonging the vase life and preserving the cut flowers. Different chemicals like sugars, organic acids, plant growth regulators and mineral salts are used in vase solution to improve the vase life and quality of cut tuberose. Different chemical preservatives in different formulations enhance the vase life of cut flowers (Nowak and Rundnicki, 1990). Keeping the above in view, the present investigation was conducted with an aim of improving the vase life of cut tuberose flower spikes.
The experiment was laid out in CRD factorial. The treatments were replicated thrice. All the flowers were displayed at 25 ± 5 °C and 60-70 % RH. Uniform spikes of two cultivars viz. “Mexican Single” and “Double” were harvested when the lower floret was fully open and placed immediately in water. The individual inflorescences were re-cut 40cm long and held in chemical preservative viz, T1– 2% CaCl2, T2– 400 ppm citric acid, T3– 25 ppm CoCl2, T4– 100 ppm GA3, T5– 50 ppm AgNO3, T6– 300 ppm Sodiumthio sulphate, T7– 4% sucrose and double distilled water served as control treatment. After recording the fresh weight each spike was placed in 200ml amber bottle containing 100ml of distilled water (control) or aqueous solutions of various treatments.
Peroxidase
Peroxidase activity was measured by the method as described by Putter (1974). 200 mg leaf samples were ground with 4 ml of 0.05 M Tris-HCI with 5.4 pH. The homogenate was centrifuged at 5000 rpm for 10 minutes. Supernatant was collected and the volume of this enzyme extract was made to 4 ml with Tris-buffer. All these steps were carried out at 4oC. For enzyme assay 2.6 ml of the working solution in cuvette and mixed well. Now zero absorbance was adjusted at 436 nm. Reaction was started by adding and mixing 0.2 ml of 1M H2O2 in the cuvette. Absorbance was taken at 30 seconds intervals upto 120 seconds. Enzyme activity was calculated as enzyme units. One enzyme unit is an increase in absorbance of 0.1 OD per minute per gram fresh weight of the samples. POD activity was measured on the 3rd and 5th day in vase and at senescence.
Catalase
The leaf tissues were ground with chilled 0.1M Tris-HCI (pH 7.5). The enzyme activity was estimated using slightly modified procedure of Sinha (1972). The reaction mixture consisted of 0.4 ml of 0.1 M phosphate buffer (pH 7.0), 0.4 ml of 0.1 M hydrogen peroxide and 0.2 ml of enzyme extract. After incubating at 37°C for 5 min, the enzyme activity was terminated by adding 2 ml of 5% dichromate and acetic acid (1:3 v/v) to the reaction mixture. The tubes were heated in a boiling water bath for 10 min. A control was run under similar conditions but the enzyme extract was added after stopping the reaction by dichromate-acetic acid. After cooling the tubes, absorbance of the test and control were measured at 570 nm. The enzyme activity was calculated by subtracting the absorbance of the test samples from that of control. One enzyme unit is defined as 1.0 mole of H2O2 utilized per min. CAT activity was measured on the 3rd and 5th day in vase and at senescence.
Vase life
The total vase life of both the cultivars i.e. “Single” and “Double” were recorded in days. Datas were analysed using ANOVA and the difference between means was compared by Fisher test (Fisher, 1967).
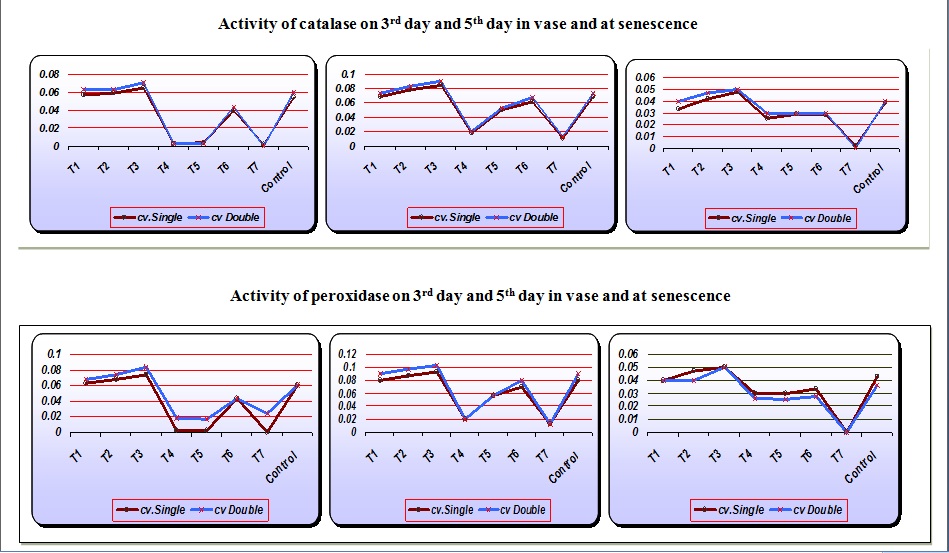
The aim of the present study was to improve the vase life of cut tuberose by delaying senescence. Senescence is defined as the final phase of ontogeny of the organ in which series of irreversible changes are initiated in the plant tissues leading to cellular breakdown and death of the organ. In the present study it was observed that the peroxidase (POD) activity in tuberose spikes was the maximum in the spikes treated with cobalt chloride (0.078) whereas the minimum in gibberellic acid (0.003) treated spikes on the 3rd day in vase (Table 1). The peroxidase (POD) activity increased from the 3rd day to 5th day. On day 5th it was observed that the maximum POD activity was recorded in cobalt chloride (0.098) treated spikes whereas sucrose treated spikes recorded the minimum POD (0.013) activity. At senescence the peroxidase activity stopped completely in the sucrose treated spikes whereas it was the maximum in cobalt chloride (0.050) treated spikes. The early marked increase and then a subsequent decrease in the peroxidase (POD) activity explains the onset of senescence in rose petals (Bhaskar et al., 2006). Shew felt and del Rosario (2000) reported that the lipid peroxidation is generally relegated to the status of a secondary effect of a primary event responsible for the degradation process, could actually be a critical, controllable event common to the mechanism of many of the post harvest disorders. Bhaskar et al. (2006) reported that control recorded the highest value of (POD) activity in flower spikes.
Table (1):
Effect of floral preservatives on POD activity.
| Treatment | Activity of POD (units g tissues-1 min-1) | |||||
|---|---|---|---|---|---|---|
| 3rd day in vase | 5th day in vase | Senescence | ||||
| V1 | V2 | V1 | V2 | V1 | V2 | |
| T1 | 0.063 | 0.067 | 0.080 | 0.090 | 0.040 | 0.040 |
| T2 | 0.067 | 0.073 | 0.087 | 0.097 | 0.047 | 0.047 |
| T3 | 0.073 | 0.083 | 0.093 | 0.103 | 0.050 | 0.050 |
| T4 | 0.003 | 0.003 | 0.020 | 0.020 | 0.030 | 0.030 |
| T5 | 0.003 | 0.003 | 0.057 | 0.057 | 0.030 | 0.030 |
| T6 | 0.043 | 0.043 | 0.070 | 0.080 | 0.033 | 0.033 |
| T7 | 0.000 | 0.000 | 0.013 | 0.013 | 0.000 | 0.000 |
| Control | 0.060 | 0.060 | 0.080 | 0.090 | 0.043 | 0.043 |
| Mean | 0.039 | 0.042 | 0.063 | 0.069 | 0.034 | 0.034 |
| Factor | (V) | (T) | (V) | (T) | (V) | (T) |
| S. Em | 0.0013 | 0.0025 | 0.0008 | 0.0017 | 0.0010 | 0.0020 |
| C.D. 0.05% | N.S. | 0.0072 | 0.0024 | 0.0048 | N.S. | 0.0059 |
It was observed (Table 2) that catalase (CAT) activity was the minimum in sucrose treated flowers (0.001) whereas the maximum in cobalt chloride treated flowers (0.067) on 3rd day, 5th day and at senescence. The catalase (CAT) activity increased from the 3rd to 5th day in vase and gradually decreased at senescence. The catalase (CAT) activity was higher in cv. Double. However, Bhaskar et al. (2006) reported that the catalase (CAT) activity remains constant in all the treatments up to day 7th and increases on the onset of senescence. The action of CAT activity in plants leads to the lipid degradation and the degradation of membrane permeability, which is responsible for early marked senescence (Sankar et al., 2008).
Table (2):
Effect of floral preservatives on catalase activity and vase life of cut tuberose spikes.
| Treatment | Activity of CAT (units g tissues-1 min-1) | |||||||
|---|---|---|---|---|---|---|---|---|
| 3rd day in vase | 5th day in vase | senescence | Vase life | |||||
| V1 | V2 | V1 | V2 | V1 | V2 | V1 | V2 | |
| T1 | 0.057 | 0.063 | 0.069 | 0.073 | 0.033 | 0.040 | 12.00 | 10.00 |
| T2 | 0.059 | 0.063 | 0.078 | 0.083 | 0.042 | 0.047 | 13.67 | 16.00 |
| T3 | 0.065 | 0.070 | 0.084 | 0.090 | 0.048 | 0.050 | 14.67 | 14.00 |
| T4 | 0.003 | 0.003 | 0.018 | 0.020 | 0.025 | 0.030 | 11.33 | 16.00 |
| T5 | 0.004 | 0.003 | 0.051 | 0.053 | 0.029 | 0.030 | 13.67 | 16.50 |
| T6 | 0.040 | 0.043 | 0.062 | 0.067 | 0.028 | 0.030 | 15.33 | 14.50 |
| T7 | 0.002 | 0.000 | 0.011 | 0.013 | 0.002 | 0.000 | 12.00 | 19.00 |
| Control | 0.054 | 0.060 | 0.069 | 0.073 | 0.038 | 0.040 | 12.77 | 16.00 |
| Mean | 0.036 | 0.038 | 0.055 | 0.059 | 0.031 | 0.033 | 13.18 | 15.25 |
| Factor | (V) | (T) | (V) | (T) | (V) | (T) | (V) | (T) |
| S. Em | 0.0008 | 0.0016 | 0.001 | 0.002 | 0.0008 | 0.0017 | 0.59 | 1.18 |
| C.D. 0.05% | 0.0023 | 0.0046 | 0.003 | 0.006 | N.S. | 0.0048 | 1.71 | N.S |
Sucrose was the most effective treatment for increasing the vase life (Table 2) of cut flower spikes. The flowers treated with calcium chloride recorded the lowest vase life. The spikes of cv. Double had higher vase life than cv. Single. Sucrose increases the vase life of the flowers by acting as the main source of food and respirable substrate in flower petals. Naidu et al. (1989) observed that the rapid respiration and carbohydrate supply through sucrose increases the vase life of several flowers. The positive water relation helps in the utilization of sugars, proteins and lowers POD activities thereby leading to the longest vase life.
Summary
The CAT activity was observed to be the minimum in 4% sucrose treatment whereas, the maximum in cobalt chloride treatment. Gibberellic acid and cobalt chloride treated spikes recorded the highest POD activity whereas, sucrose treated spikes recorded the lowest POD activity in the cut flower spikes.
- Bhaskar, V. V., Rao, P. V. and Babu, J.D. Effect of post harvest application of antioxidants on physiological and biochemical changes of cut rose (Rosa hybrida L.) petals during vase life period. Journal of Ornamental Horticulture, 2006; 9(2):75-79.
- Fisher, R.A. Hand Book of Agriculture Statistics by C.S. Chandel. Achal Prakash Mandir Kanpur, 1967; 149-191.
- Naidu, S.N. and Reid, M.S. (1989). Post harvest handling of tuberose (Polianthes tuberosa L.) Acta Hort. 261- 313- 7.
- Nowak, J. and Rudnicki, R.M. Post harvest handling and storage of cut flowers, Florist Greens and Potted plants. Chapman and Hall, 1990; London.
- Putter, J. Method of Enzymatic method analysis 2, (Ed.): Bergmeyer. Academic Press, New York., 1974; pp. 685.
- Sankar, V. M., Bhattacharjee, S.K. and Chatterjee, S.R. Enzyme activity in petals of cut rose cv. Raktagandha as influenced by chemical treatments and low temperature storage. In: National Symposium on Recent Advances in Floriculture, 05-1, 4-6 March, Navsari Agriculture University, 2008; Gujarat.
- Shewfelt, R. L. and del Rosario, B. A. The role of lipid peroxidation in storage disorders of fresh fruits and vegetables. Hort. Sci., 2000; 35: 575-579.
- Sinha, A.K. Calorimetric assay of catalase. Anal. Biochem., 1972; 47: 389-95.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


