ISSN: 0973-7510
E-ISSN: 2581-690X
The human oral environment allows the growth of various microorganisms like staphylococcus aureus; streptococcus; enterococcus; klebsiella; e.coli; pseudomonas; candida albicans. As the colonization progress the pathogens start affecting the soft tissues of oral cavity. The maintainance of dentures plays a key role in preventing the pathogens to affect the oral cavity. The aim of the study is to identify the most common pathogens that invades the surface of the denture and to eradicate the pathogens. A study was conducted among 30 geriatric patients attending the OPD of saveetha dental college, saveetha university. 20 denture blocks were prepared with a size of 5x5mm of which 10 were impregnated with an antibiotic agent amoxicillin 500mg and another 10 were impregnated with an antifungal agent fluconazole 150mg. The blocks were reevaluated for the degree of growth of microbial pathogens. The results reveals that the microbial growth medium inoculated with the swab samples collected from 30 different subjects shows ballooning of S.Aureus and C.Albicans. Fluconazole impregnated blocks show the decreased thickness of candidal growth whereas, Amoxicillin impregnated blocks shows the clear zone of inhibition of bacterial growth and the average clear zone is determined. As the candida infections are concerned, fluconazole is effective as expected in control of bacterial growth. The amoxicillin shows the marked effect against the bacteria. The study gave a scope for further investigations with incorporating newer agents or different agents at various concentrations.
Amoxycillin, Antimicrobial agents, Bacterial growth, Complete dentures, Fluconozole, Pathogens.
A Removable dental prosthesis which replaces the entire dentition and associated structures of the maxilla and mandible. The reasons for the loss of teeth are gum diseases; plaque accumulation and tooth decay. Complete dentures can help geriatric patients in maintaining masticatory function; aesthetics and speech. Thus the dentures also help the patients in maintaining health and nourishment. But the maintaince of the dentures is also a challenging task for the patients as the pathogens readily invade the denture surfaces. So there is also need to look into the method of eradication of such pathogens before they affect the oral tissues. Oral cavity harbours diverse microflora. It is a perfect ecological niche for the microorganisms, with maintained temperature (34-360C) and neutral pH. There always exists a dynamic equilibrium between the microbes and the host immunity1-3. When the microbial load overpowers the host immunity, it results in a wide array of oral diseases. In case of denture wearers, these organisms inhabitate the denture surface which serves as a reservoir2. Also, geriatric patients have low level of immunity against pathogens. Thus, an altered equilibrium in elderly patients favors the growth of oral pathogens, resulting in multitude of oral health diseases associated with denture insertion.
Angular chelitis, denture stomatitis, frictional keratosis, traumatic ulcers, denture hyperplasia etc are the routinely encountered issues in elderly patients3. Denture stomatitis refers to an erythematous lesion occurring underneath the denture, caused due to candidal infection, with greater frequency of occurence4. Several pathogenic and non-pathogenic organisms present in the oral cavity and on the dentures are responsible for altering the healthy alveolus and surrounding oral mucosa, also acting as a source of infections eg., bacterial endocarditis5,6. Streptococci and candidal sp. are frequently isolated from saliva and plaque. Streptococcus sp. is initial colonizer during plaque formation7. In 2005, Baena T. Monroy8 identified the predominant role of candidal sp.(86%) in ensuing denture stomatitis. Several studies have been published with the intent of understanding the pathogenic role of oral microorganisms involved in different dental conditions.
Also consensus data is available for fabrication of innovative dentures, such as antimicrobial monomer, silver-zinc zeolite incorporated acrylic resins, antimicrobial polymers etc9,10,11. These studies have incited an inquisitive approach towards the development of pathogen resistant dentures12. The significance of these researches have motivated us to develop a microbial resistant denture with the help of anti-microbial agent, targeted at the most dominant pathogenic species in the denture bearers. Therefore the aim of the study is to identify the most common pathogens that invades the surface of the denture and to eradicate the pathogens.
Objectives
The ojectives of the study are:
(i) To identify the pathogens that cohere to the denture surfaces
(ii) To diminish such pathogens in effecting the oral tissues
This study was conducted in two separate Phases. Initially, 31 geriatric patients were selected for collection of swab sample. The sample was then incubated, to observe the maximal colonizers on the denture surface. The next Phase was evaluation of antibiotic efficacy against the isolated microbes. It was assessed by the placement of 20 antibiotic infused denture blocks. Prior ethical consent was obtained and patients volunteered for the study. On the day of appointment patients were explained regarding the procedure. Then the dentures were taken out from the oral cavity and the swab was collected carefully from the tissue surface of the denture and on the surface of the oral cavity which is covered by the denture. The collected swab was send to the microbiological laboratory. The sample was inoculated onto Sabouraud’s dextrose agar plates, incubated at 37 degree Celsius for 24 hours and candidal colony forming units (CFU) were identified. Staphylococcal CFU’s were incubated on brain heart infusion agar (Figure 1,2).
 Fig. 1. Microbial Colonies Observed On Agar Dextrose Medium
Fig. 1. Microbial Colonies Observed On Agar Dextrose Medium
 Fig. 2. Microbial Colonies Observed On Brain Hearth Infusion Medium
Fig. 2. Microbial Colonies Observed On Brain Hearth Infusion MediumAfter determining the prominent microbes, antibiotic efficiency was evaluated. 20 acrylic denture blocks were impregnated with antibiotics. 10 blocks were embedded with fluconazole 150mg and the other 10 contained amoxicillin 500mg (Figure 3). The blocks were prepared with a modified polymer (powder) composition(DPI heat cure denture base resin), with a antimicrobial drug to polymer in the ratio of (1:3). Then polymer and monomer ratio for curing is followed as per the instructions given by the company of DPI21,22. The wax blocks of 5x5mm were prepared with 2mm thickness23. Those wax blocks were cured with the above mentioned parameters. The prepared blocks were manually observed for dimensional and colour stability. The changes in the colour and dimensions were not observed. The porosity is not observed. The surface is usual as seen in the normal conventional dentures.
Fig. 3. Denture Blocks Incorporated With Fluconazole And Amoxycillin
Later these blocks were placed in an artificial salivary medium, at pH 7. The salivary medium was replaced every day for 7 days. After 7 days, the acrylic blocks were embedded in agar medium inoculated with Candida species. The radius of inhibition (clear zone) is observed. The efficacy of the drug infused denture block is proportional to the area of inhibition (Figure 4).

Fig. 4. Observed Zone Of Inhibition
The organisms that were obtained from various samples shown as a pie chart with distribution of organisms accordingly (Figure 5). And the mean and standard deviation were analysed for all the organisms as shown in the Table I. Independent sample test were conducted and were shown in Table II for each organism. The P Value were evaluvated and S.aureus (P >0.070), candida albicans (P > 0.001) The mean difference, standard error difference and confidence interval were shown in Table II.
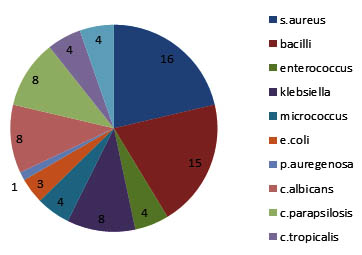
Fig. 5. Pie Chart Describing The Distribution Of Various Organisms Found On The Tissue Surfaces
Table (1):
Mean And Standard Deviation Of Organisms For The Drugs Amoxycilin Fluconozole (Group Statistics)
| Groups | N | Mean | Std. Deviation | Std. Error Mean | |
|---|---|---|---|---|---|
| Saureus | Amoxycilin | 3 | 5.0000 | .50000 | .28868 |
| fluconozole | 3 | 4.0000 | .50000 | .28868 | |
| bacilli | Amoxycilin | 3 | 8.0000 | .50000 | .28868 |
| fluconozole | 3 | 5.0000 | .00000 | .00000 | |
| enterococcus | Amoxycilin | 3 | 6.0000 | .00000 | .00000 |
| fluconozole | 3 | 2.9333 | .11547 | .06667 | |
| klebsiella | Amoxycilin | 3 | 6.5000 | .00000a | .00000 |
| fluconozole | 3 | 5.0000 | .00000a | .00000 | |
| micrococcus | Amoxycilin | 3 | 7.3333 | 1.04083 | .60093 |
| fluconozole | 3 | 6.0000 | 1.00000 | .57735 | |
| e.coli | Amoxycilin | 3 | 5.6000 | .00000 | .00000 |
| fluconozole | 3 | 4.0000 | .50000 | .28868 | |
| Aurgenosa | Amoxycilin | 3 | 8.0000 | .00000 | .00000 |
| fluconozole | 3 | 6.0000 | 1.00000 | .57735 | |
| c.albicans | Amoxycilin | 3 | 6.5000 | .00000 | .00000 |
| fluconozole | 3 | 4.6667 | .57735 | .33333 | |
| c.parapsilosis | Amoxycilin | 3 | 7.5000 | .50000 | .28868 |
| fluconozole | 3 | 4.8333 | .28868 | .16667 | |
| c.tropicalis | Amoxycilin | 3 | 7.0000 | .50000 | .28868 |
| fluconozole | 3 | 5.9333 | .11547 | .06667 |
Table (2):
Independent Samples Test
| Levene’s Test for Equality of Variances | t-test for Equality of Means | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| F | Sig. | t | df | Sig. (2-tailed) | Mean Difference | Std. Error Difference | 95% Confidence Interval of the Difference | ||
| Lower | Upper | ||||||||
| Saureus | .000 | 1.000 | 2.449 | 4 | .070 | 1.00000 | .40825 | -.13348 | 2.13348 |
| 2.449 | 4.000 | .070 | 1.00000 | .40825 | -.13348 | 2.13348 | |||
| bacilli | 4.000 | .116 | 10.392 | 4 | .000 | 3.00000 | .28868 | 2.19851 | 3.80149 |
| 10.392 | 2.000 | .009 | 3.00000 | .28868 | 1.75793 | 4.24207 | |||
| enterococcus | 16.000 | .016 | 46.000 | 4 | .000 | 3.06667 | .06667 | 2.88157 | 3.25176 |
| 46.000 | 2.000 | .000 | 3.06667 | .06667 | 2.77982 | 3.35351 | |||
| micrococcus | .073 | .801 | 1.600 | 4 | .185 | 1.33333 | .83333 | -.98037 | 3.64704 |
| 1.600 | 3.994 | .185 | 1.33333 | .83333 | -.98183 | 3.64850 | |||
| e.coli | 4.000 | .116 | 5.543 | 4 | .005 | 1.60000 | .28868 | .79851 | 2.40149 |
| 5.543 | 2.000 | .031 | 1.60000 | .28868 | .35793 | 2.84207 | |||
| Aurgenosa | 4.000 | .116 | 3.464 | 4 | .026 | 2.00000 | .57735 | .39702 | 3.60298 |
| 3.464 | 2.000 | .074 | 2.00000 | .57735 | -.48414 | 4.48414 | |||
| c.albicans | 16.000 | .016 | 5.500 | 4 | .005 | 1.83333 | .33333 | .90785 | 2.75882 |
| 5.500 | 2.000 | .032 | 1.83333 | .33333 | .39912 | 3.26755 | |||
| c.parapsilosis | .400 | .561 | 8.000 | 4 | .001 | 2.66667 | .33333 | 1.74118 | 3.59215 |
| 8.000 | 3.200 | .003 | 2.66667 | .33333 | 1.64239 | 3.69094 | |||
| c.tropicalis | 2.114 | .220 | 3.600 | 4 | .023 | 1.06667 | .29627 | .24408 | 1.88925 |
| 3.600 | 2.213 | .059 | 1.06667 | .29627 | -.09756 | 2.23090 | |||
The zone of inhibition for bacteria and fungi were shown in the graphical representation which were found to be significant. The results reveals that the microbial growth medium inoculated with the swab samples collected from 30 different subjects shows ballooning of S.Aureus and C.Albicans. Where as in the subsequent study the fluconazole impregnated blocks show the decreased thickness of candidal growth. The amoxicillin impregnated blocks shows the clear zone of inhibition of bacterial growth and the average clear zone is determined (Figure 6 and Figure 7).
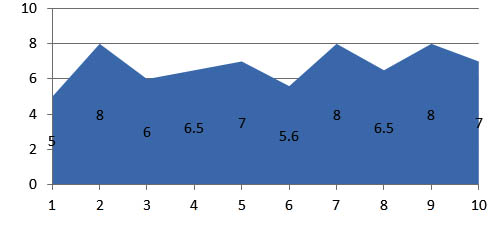
Fig. 6. Zone of inhibition observed in the sample blocks incorparated with amoxycillin
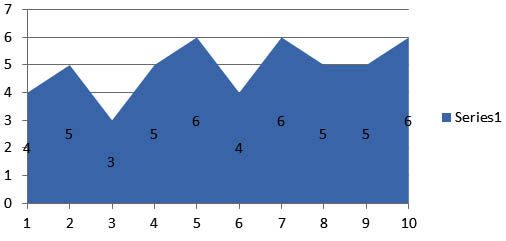
Fig. 7. Zone of inhibition observed in the sample blocks incorparated with fluconozole
As oral cavity is the common passage for all the microorganisms to enter the human body. In old age geriatric patients may loose their teeth due to various reasons17. As the disinfection of removable partial dentures plays an important role in the maintainance of oral hygiene. So, in this study it is evident that the tissue surfaces of the complete dentures and oral tissue surfaces in the geriatric patients were predominant with staphylococcus aureus and candida albicans18.
S.aureus was the secondary colonizer present in the plaque and staph component is the natural inhabitant. Staphylococcus aureus is a gram positive bacteria, which is round shaped. This is a member of Firmicutes. This is a normal flora of the body which can be found in nose, skin and respiratory tract of the body. It can grow without the need of oxygen. It is the regular cause of skin infections like sinusitis, food poisioning and abscess. This organism can cause a range of infections from minor skin disorders to life threatening diseases, like it causes pimples, boils, abscess to major diseases like toxic shock syndrome, osteomyelitis. Each year 50000 deaths were reported in united states of America with staph aureus infections19.
Candida albicans, it is an Oppurtunistic pathogen, belongs to yeast. This is commonly found in human gut flora. Usually detected in GIT (Gastro intestinal tract) and mouth. As it is a commensal, it will become pathogen in immunocomprimised individuals. Where as the candida species are not present usually in the oral cavity except in the candida infections. It is a dimorphic fungus, so it appears both as yeast and filamentous cells. Annually 2800 to 11200 deaths occur in united states of America due to candida albicans20. Ususally it will found together with staphylococcus aureus. The preventive measures from this includes maintaining proper oral hygiene, good and proper nutrition which keeps healthy life style.
Here in this study the complete denture cases which are six months old from the date of delivery were chosen. Biofilm adhered to the denture base is an ideal sample for swab collection. Because biofilm formation due to absence of ionic charge in acrylic denture base, irregular denture surface, poor dental hygiene, immune deficiency, dry mouth and hydrophobic interaction of bacteria, favors microbial colonization13,14,15,16. There are different organisms that are grown on the bed in which staph aureus and candida albicans were found to be predominant.
Given the complexity of the oral environment, comprehending the intricate interactions of this ecosystem is a difficult task, and the addition of a denture further adds to the complexity. However, this study has taken a significant step forward towards understanding this environment. With a detailed knowledge of the microbial composition, research in this area can progress further, with a more in depth focus on how these organisms interact with one another as well as the host, allowing us to develop meaningful biofilm models of denture plaque.
Limitations
(i) Increasing in the sample size might vary the results
(ii) Side effects of the drugs were not evaluated
(iii) Leaching capacity of the drug was not evaluated
Future Prospectives
As the invitro study seems to be successful in eliminating the colonization of microbial flora around the acrylic denture blocks, we are planning to continue the study invivo by preparing the complete dentures with various drugs by various concentrations to evaluate the better drug for the desired criteria
As the staph. aureus and candida albicans were found to be predominant pathogens in the oral cavity. As their effects on the oral cavity varies in different ways in oral health. The trial in eliminating the pathogens in this study was found to be successful with a clear zone of inhibition around the customized acrylic blocks. This gives us hope for the further studies
- Lillard JW, Boyaka PN, Chertov O, Oppenheim JJ, McGhee JR. Mechanisms for induction of acquired host immunity by neutrophil peptide defensins. Proceedings of the National Academy of Sciences. 1999; 96(2):651-6.
- Gendron R, Grenier D, Maheu-Robert LF. The oral cavity as a reservoir of bacterial pathogens for focal infections. Microbes and infection. 2000 ; 2(8):897-906.
- Coelho CM, Sousa YT, Dare AM. Denture related oral mucosal lesions in a Brazilian school of dentistry. Journal of oral rehabilitation. 2004 ; 31(2):135-9.
- Pfaller MA, Diekema DJ, Jones RN, Messer SA, Hollis RJ, SENTRY Participants Group. Trends in antifungal susceptibility of Candida spp. isolated from pediatric and adult patients with bloodstream infections: SENTRY Antimicrobial Surveillance Program, 1997 to 2000. Journal of Clinical Microbiology. 2002; 40(3):852-6.
- Berbari EF, Cockerill FR, Steckelberg JM. Infective endocarditis due to unusual or fastidious microorganisms. InMayo Clinic Proceedings 1997 Jun 30 (Vol. 72, No. 6, pp. 532-542). Elsevier.
- Dismukes WE, Karchmer AW, Buckley MJ, Austen WG, Swartz MN. Prosthetic valve endocarditis. Circulation. 1973; 48(2):365-77.
- Rosan B, Lamont RJ. Dental plaque formation. Microbes and infection. 2000; 2(13):1599-607.
- Baena-Monroy T, Moreno-Maldonado V, Franco-Martinez F, Aldape-Barrios B, Quindos G, Sánchez-Vargas LO. Candida albicans, Staphylococcus aureus and Streptococcus mutans colonization in patients wearing dental prosthesis. Medicina oral, patologia oral y cirugia bucal. 2005; 10:E27-39.
- DeBoer J, Vermilyea SG, Brady RE. The effect of carbon fiber orientation on the fatigue resistance and bending properties of two denture resins. The Journal of prosthetic dentistry. 1984; 51(1):119-21.
- Takahashi Y, Chai J, Takahashi T, Habu T. Bond strength of denture teeth to denture base resins. International journal of prosthodontics. 2000 ; 13(1).
- Larson WR, Dixon DL, Aquilino SA, Clancy JM. The effect of carbon graphite fiber reinforcentent on the strength of provisional crown and fixed partial denture resins. The Journal of prosthetic dentistry. 1991; 66(6):816-20.
- Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. Biofilm formation by the fungal pathogenCandida albicans: development, architecture, and drug resistance. Journal of bacteriology. 2001; 183(18):5385-94.
- Zomorodian K, Haghighi NN, Rajaee N, Pakshir K, Tarazooie B, Vojdani M, Sedaghat F, Vosoghi M. Assessment of Candida species colonization and denture-related stomatitis in complete denture wearers. Medical mycology. 2011; 49(2):208-11.
- Gendreau L, Loewy ZG. Epidemiology and etiology of denture stomatitis. Journal of Prosthodontics. 2011; 20(4):251-60.
- Sachdeo A, Haffajee AD, Socransky SS. Biofilms in the edentulous oral cavity. Journal of Prosthodontics. 2008; 17(5):348-56.
- Okita N, Ørstavik D, Ørstavik J, Østby K. In vivo and in vitro studies on soft denture materials: microbial adhesion and tests for antibacterial activity. Dental Materials. 1991; 7(3):155-60.
- Müller F, Naharro M, Carlsson GE. What are the prevalence and incidence of tooth loss in the adult and elderly population in Europe?. Clinical oral implants research. 2007; 18(s3):2-14.
- Minagi S, Miyake Y, Inagaki K, Tsuru H, Suginaka H. Hydrophobic interaction in Candida albicans and Candida tropicalis adherence to various denture base resin materials. Infection and immunity. 1985; 47(1):11-4.
- Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clinical infectious diseases. 2003; 36(1):53-9.
- Beck-Sagué CM, Jarvis WR, National Nosocomial Infections Surveillance System. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980-1990. The Journal of infectious diseases. 1993:1247-51.
- Arundati R, Patil NP. An investigation into the transverse and impact strength of a new indigenous high-impact denture base resin, DPI-TUFF and its comparison with most commonly used two denture base resins. The Journal of Indian Prosthodontic Society. 2006; 6(3):133.
- Singh SV, Aggarwal P. Effect of tea, coffee and turmeric solutions on the colour of denture base acrylic resin: An in vitro study. The Journal of Indian Prosthodontic Society. 2012; 12(3):149-53.
- Barpal D, Curtis DA, Finzen F, Perry J, Gansky SA. Failure load of acrylic resin denture teeth bonded to high impact acrylic resins. The Journal of prosthetic dentistry. 1998; 80(6):666-71.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


