ISSN: 0973-7510
E-ISSN: 2581-690X
Seaweed has been used in the field of aquaculture as a source of antioxidant, anti-pathogen and immunostimulant. The carrageenan content of seaweed is a potential immunostimulant that can act against bacterial attacks, such as Vibrio alginolyticus on white shrimp culture. This study aims to examine nutrition profile changes in red seaweed Kappaphycus alvarezii after fermentation using Saccharomyces cerevisiae. Fermentation process was done under aerobic conditions at 26-28 oC with an agitation of 125 rpm using. The treatments were varied based on inoculum size and comprised of 5% (v/v), 10% (v/v) and 15% (v/v) inoculum, where each treatment was analyzed in triplicates. Crude protein and fat increased in all treatments. The amino acid and fatty acid content rose to ±20% and ±48%, respectively as a result of biosynthesis by Saccharomyces cerevisiae. Fermented Kappaphycus alvarezii is nutritionally more beneficial compared to the unfermented type, supporting its potential as a feed supplement for white shrimp.
Kappaphycus alvarezii, Fermentation, Saccharomyces cerevisiae, Feed supplement
Seaweed is commonly used in the industry as a source of phycocolloids in the form of alginates, carrageenan and agars which are obtained through an extraction process (Gressler et al., 2010). The extracts can be incorporated as ingredients for food, cosmetics and fertilizers or be further developed into thickening agents and feed additives. Seaweed can also be used as feed supplements for fish and shrimp. Eclonia maxima, Laminaria japonica, Ulva rigida, Carpoblepharis flaccida, Glacilaria gracilis and Ulva lactura are examples of species that can potentially be used as a nutritional source for fish and shrimp.
Seaweed contains a complete source of nutrition in varying amounts. The content of seaweed can vary due to differences in species, location and seasonal temperature, condition of harvest, and age of harvest (Machado et al., 2003; Dawczynski et al., 2007). The fat content can vary from 1 – 6%, while the fiber and protein content range from 33 – 50% and 5.6 – 24%, respectively (Dawczynski, 2007; Gressler et al., 2010). The essential amino acid content of seaweed is considered high (45 – 49%) compared to the total amino acid content (Dawczynski et al., 2007). In the field of aquaculture, seaweed is known to have antioxidant, anti-pathogen and immunostimulant properties (Thanigaivel et al., 2016). The carrageenan content of seaweed is a potential immunostimulant that can act against bacterial attacks, such as Vibrio alginolyticus on white shrimp (Yeh and Chen, 2008).
A commonly cultivated red algae (Rodhophyta) that serves as a source of carrageenan is Kappaphycus alvarezii. Indonesia is a large producer of red algae Kappaphycus alvarezii, previously known as Eucheuma cottonii, where an estimated of 11 million tons was produced in 2016. Kappaphycus alvarezii is recognized to exhibit fast vegetative and generative growth, being able to double its biomass within 15-30 days after culturing.
The fermentation of seaweed using yeast can enhance its nutritional value by enriching the protein, vitamin, mineral, essential amino acid and fatty acid content, as well as improve its digestibility value (Uchida, 2003; Uchida and Murata, 2004; Felix and Pradeepa, 2011; Felix and Brindo, 2014). The employment of Saccharomyces cerevisiae is expected to to increase the nutritional value of the seaweed and to act as a potential immunostimulant from the presence of b-glucans in the cell walls of the yeast. Thus, the objective of this study is to explore the potential of fermented Kappaphycus alvarezii as a feed supplement for white shrimp (Litopenaeus vannamei) based on its nutritional profile.
Pretreatment of Seaweed
The seaweed Kappaphycus alvarezii (Eucheuma cottonii) originated from Bali in dried conditions and without pretreatment. The washing step was done to remove salt and dirt that remained on the surface of the seaweed. A sorting process was also done during the washing step. The drying step was done using a convection system oven at a temperature below 90 °C to prevent protein denaturation. The milling step was performed using a disc mill with a filter size of 5 mm, followed by a sieving step using a 250 µm metal sieve. The yield of the overall process was 25% of the total initial amount of dried seaweed used.
Inoculum Preparation
A loop of Saccharomyces cerevisiae culture was streaked on to PDA (Potato Dextrose Agar) medium and incubated at room temperature 28 – 30 °C for 24 – 48 h. Strain activation was later done by subculturing 1-2 loops of culture into 100 ml of activation medium, followed by incubation at room temperature 28 – 30 °C for 24 h with an agitation of 125 rpm. The activation step was then repeated using 10% (v/v) of inoculum from the previous culture. Following activation, the cells of Saccharomyces cerevisiae were counted until it reached a density of 106 cell/ml.
Subsequently, activated cultures of Saccharomyces cerevisiae were adapted onto the substrate that will be used during fermentation. The adaptation procedure was done three times using the following medium composition; (1) 75 % microbial growth medium + 25 % fermentation medium, (2) 50 % microbial growth medium + 50 % fermentation medium, and (3) 25 % microbial growth medium + 75 % fermentation medium.
Fermentation Process
The fermentation process was done by incorporating the different inoculums into a medium containing 20 g of seaweed flour, 1.5 g of maize flour, 1 g of glucose and 0.4 g of urea into an Erlenmeyer flask of 500 ml containing 200 ml of deionized water. The different treatment conditions comprised of 5 % (v/v) inoculum denoted as treatment I, 10 % (v/v) inoculum denoted as treatment II and 15 % (v/v) inoculum denoted as treatment III. The incubation was done at room temperature 28 – 30 °C with an agitation of 125 rpm for 72 hours. Parameters tested during fermentation are pH and microbial count (TPC and direct counting) which was done every six hours.
Post Fermentation
A drying step using an electrical conventional oven at 60 – 75 °C for 24 – 48 was performed after the fermentation process had finished. The temperature was important since too high of a temperature can affect the nutritional content and denaturate the proteins. Once the seaweed has dried (water content ³5%), it was milled through a disc mill using a filter size of 5 mm. Milled seaweed was then further sieved using sieve No. 60 (size 250 µm).
Nutritional Analysis
The nutritional content of the seaweed was analyzed using the following methods: proximate analysis consisting of dry matter content (oven method), crude protein content (Kjhedal titration), crude fat content (Soxhlet method) (Machado et al., 2004), energy (Bomb calorimetry), extract matter without nitrogen (calculated), fatty acid analysis (gas chromatography), crude fiber content as well as amino acid analysis (HPLC) (Ortiz et al., 2006).
Proximate Analysis of Seaweed Without Fermentation
Proximate analysis was done to examine nutrition value of pretreated seaweed Kappaphycus alvarezii. The result shows similar values as other studies by Istiani et al. (1986) and Liem (2013) (Table 1). Ash, fat, protein and crude fiber content of the seaweed was observed to be lower compared to other red seaweed species reported by Gressler et al. (2010), which are Gracilaria domingensis, Gracilaria birdiae, Laurencia filiformis and Laurencia intricate. This condition may occur as a result of differences in species, geographical condition and season (Kaehler and Kennish, 1996). However, these values are still in accordance with the acceptable ranges published by Dawczynski et al. (2007) and Gressler et al. (2008).
Table (1):
Proximate Analysis of the red algae Kappaphycus alvarezii.
| Nutrition Profile | Value | ||
|---|---|---|---|
| Measured | Reported by Istiani et al.(1986) | Reported by Liem (2013) | |
| Total Energy (kal) | 2883.8 | – | – |
| Dry matter (%) | 91.1 | 86.1 | 90.05 |
| Water content (%) | 8.9 | 13.9 | 9.95 |
| Ash (%) | 18.68 | 17.09 | 17.69 |
| Crude fat (%) | 0.22 | 0.37 | 0.53 |
| Crude protein (%) | 3.26 | 2.69 | 3.82 |
| Non protein nitrogen (%) | 0.56 | – | – |
| Extract matter without N (%) | 64.34 | 65 | 73.81 |
| Crude Fiber (%) | 4.6 | 0.95 | 4.15 |
| Carrageenan (%) | 67.3 | 61.52 | – |
Amino Acid& Fatty Acid Analysis of Seaweed Without Fermentation
The amino acid content of the measured seaweed was found to be comprehensive, although it still has lower values compared to other species (Table 2). Based on the analysis, the most abundant amino acid was found to be aspartate and glutamate at 0.29% and 0.35%, respectively. The two amino acids were also reported to be highest in the comparing species.
Table (2):
The amino acid content of red algae.
| Amino acid (%) | Kappaphycus alvarezii | Species reported by Gressler et al. (2010) | |||
|---|---|---|---|---|---|
| Gracilaria domingensis | Gracilaria birdiae | Laurencia filiformis | Laurencia intricate | ||
| Essential | |||||
| Cysteine | nd. | 0.03 | 0.04 | 0.1 | 0.04 |
| Isoleucine | 0.12 | 0.4 | 0.4 | 0.5 | 0.3 |
| L-Arginine | 0.15 | 0.4 | 0.6 | 0.6 | 0.2 |
| Leucine | 0.22 | 0.7 | 0.7 | 0.8 | 0.5 |
| L-Tyrosine | 0.07 | 0.2 | 0.2 | 0.6 | 0.3 |
| Lysine | 0.14 | 0.4 | 0.6 | 1 | 0.5 |
| Methionine | 0.05 | 0.2 | 0.2 | 0.3 | 0.1 |
| Phenylalanine | 0.13 | 0.4 | 0.5 | 0.5 | 0.3 |
| Threonine | 0.15 | 0.4 | 0.5 | 0.6 | 0.4 |
| Valine | 0.16 | 0.4 | 0.5 | 0.5 | 0.3 |
| Non-Essential | |||||
| Glycine | 0.16 | 0.5 | 0.6 | 0.7 | 0.5 |
| Histidine | 0.02 | 0.1 | 0.2 | 0.2 | 0.1 |
| L-Alanine | 0.18 | 0.6 | 0.7 | 0.7 | 0.5 |
| L-Aspartate | 0.29 | 1 | 1.2 | 1.5 | 1 |
| L-Glutamate | 0.35 | 0.9 | 1 | 1.4 | 0.9 |
| L-Proline | 0.13 | 0.4 | 0.5 | 0.5 | 0.3 |
| L-Serine | 0.15 | 0.4 | 0.5 | 0.6 | 0.4 |
| Tryptophan | nd. | 0.2 | 0.2 | 0.1 | 0.1 |
| Total AA | 2.47 | 7.63 | 9.14 | 11.2 | 6.74 |
| EAA | 1.19 | 3.53 | 4.24 | 5.5 | 2.94 |
| NAA | 1.28 | 4.1 | 4.9 | 5.7 | 3.8 |
| EAA/NAA | 0.93 | 0.86 | 0.87 | 0.96 | 0.77 |
| EAA/Total AA | 0.48 | 0.46 | 0.46 | 0.49 | 0.44 |
The amino acid content is linearly dependent on the protein content of the seaweed where a low protein content would translate to a low amino acid content. Thus, similar to the factors affecting protein, the amino acid content is also affected by the difference in species, environment and age of harvest.
The fatty acid content of the measured seaweed was found to be lower compared to other species. The highest amount of fatty acid observed was palmitate (C16:0) at 0.15%. However, this value was considerably lower than other species reported by Gressler et al.(2010). Factors affecting fatty acid content can include type of seaweed, temperature of environment, characteristic of seaweed, intensity of light, mineral content, nitrogen content and period of life cycle the seaweed is in (Takagi et al., 1985; Dawczynski et al., 2007).
Growth Curve of Saccharomyces cerevisiae
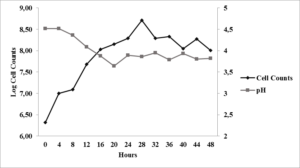
Growth curve of Saccharomyces cerevisiae on PDB (Potato Dextrose Broth) can be divided into two phases, a logarithmic phase between 0 – 28 hours and a stationary phase between 28 – 48 hours (Figure 1). The absence of a lag phase indicate that the culture was in an active state and had been well-adapted to the PDB medium.
The growth of Saccharomyces cerevisiae used in this study was relatively fast. A significant change in cell density was observed between t0 andt4 where it rose from 2.1 x 106 cells/ml to 1.07 x 107 cells/ml. The significant increase of cell density indicate that the growth is at the logarithmic phase where the yeast is actively undergoing cell division. The highest growth rate on PDB medium was observed at t2 where µ was equivalent to 0.39 h-1.
The pH value of the medium changed during the growth of S. cerevisiae where it dropped from an initial value of 4.52 to 3.82 (Figure 1). The decrease of pH suggests that there is a production of acid from the metabolism of carbohydrate in the seaweed sample. The decrease in pH corresponded well to the increase of cell mass. This decrease of pH was observed until t12, while at t14the pH increased signifying the end of growth. During cell lysis, ammonia can be released into the medium causing the pH to rise (Boulton and Quain, 2001).
Fig. 1. Growth curve of Saccharomyces cerevisiae on PDB medium at 26 – 28 °C and agitation of 125 rpm
The growth curve of Saccharomyces cerevisiae on fermentation medium (Figure 2) has similar trend with its growth curve on PDB medium, as it displayed a logarithmic phase followed by a stationary phase. The highest growth rate observed for each treatments;10% (v/v), 15% (v/v) and 5% (v/v); were 0.38 ± 0.05h-1, 0.37 ± 0.06 h-1 and 0.23 ± 0.03 h-1, respectively.
All the fermentation medium contained the same nutritional content but a different initial cell density. Therefore, the ratio of nutrition compared to the initial cell density of S. cerevisiae is different. The treatment with 5% (v/v) inoculum has the highest availability ratio of nutrient over cells, as compared to 10% (v/v) and 15% (v/v) inoculum, respectively.

Fig. 2. Growth curve of Saccharomyces cerevisiae on fermentation medium at 26 – 28 oC and agitation of 125 rpm
With time, the available nutrition will become limited where the cells will then lose energy and the ability to grow, resulting in autolysis. Environmental factors can also affect growth, for example the increasing concentration of alcohol that can inhibit enzymes and cause cell death (Boulton and Quain, 2001). The autolysis that occurred during cell death is due to the work of hydrolytic enzymes such as proteinases and glucanases that are present in the intracellular matrix.
The fermentation medium consists of several different carbohydrate sources; carrageenan and other polysaccharides from Kappaphycus alvarezii, glucose which was added to the medium, and starch from maize flour. During the cultivation of S. cerevisiae in the fermentation medium, glucose will be the first resources to be utilized as it is the simplest form of carbon source to assimilate. Saccharomyces cerevisiae will also prefer glucose since it will suppress the use of the other available carbon sources (Boulton and Quain, 2001). The yeast can utilize dextrose, galactose, sucrose, maltose, raffinose, trehalose but not lactose as their carbon source.
The pH of all the treatments during fermentation decreased from an average of 6.48 to 4.6. The pH of the medium will greatly affect the growth of the yeast, where the optimum range is 4 – 5. A pH value of 3 or lower will inhibit the growth of yeast and the fermentation will run at a much slower pace (Adam et al., 1985). Saccharomyces cerevisiae produces organic acids from non-acidic compounds such as carbohydrates. These acids can take the form of pyruvic acid, citric acid and succinic acid that are formed by the metabolism of sugars in the medium. These organic acids can accumulate and decrease the pH of the medium. A drop of pH may also caused by the use of nitrogen where the yeast will use a cation resulting in a free nitrate ion that will react with water to form nitrate acid. As the growth enters the death phase, the pH of the medium will become more basic from the release of ammonia during autolysis.
Nutrient Content of Fermented Seaweed
The fermentation process led to a change in the nutritional content of the seaweed (Table 3).S. cerevisiae utilize carbon (C), nitrogen (N), phosphate (P), sulfur (S) and other compounds for bioenergy and biosynthesis. The main carbon source utilized by the yeast is carbohydrate. The carbohydrate content of seaweed differs to that of plants which are mainly composed of cellulose and hemicellulose (Sun and Cheng, 2002). In seaweed, the carbohydrate composition is highly dependent on the species (Park et al., 2012). The seaweed Kappaphycus alvarezii is classified as a carrageenophyte, as its most dominant carbohydrate take the form of carrageenan (Necas and Bartosikova, 2013). Carrageenan are sulfated galactans comprised of the monomers D-galactose and 3,6-anhydro-D-galactose linked together by -1,4 and -1,3 bonds. Red seaweed can also contain cellulose which are composed of -1,4-D-glucan chains (Park et al., 2012).
Table (3):
Nutritional content of seaweed before and after fermentation.
| Nutritional Content | Treatments | |||
|---|---|---|---|---|
| Before Fermentation | After Fermentation | |||
| 5% Inoculum | 10% Inoculum | 15% Inoculum | ||
| Total Energy (kal) | 288.38 | 298.6 | 308.81 | 308.28 |
| Dry matter (%) | 91.1 | 95.11 | 97.33 | 96.12 |
| Water content (%) | 8.78 | 4.89 | 2.67 | 3.88 |
| Ash (%) | 16.84 | 20.13 | 20.2 | 22.11 |
| Crude fat (%) | 0.27 | 0.26 | 0.29 | 0.3 |
| Crude protein (%) | 8.24 | 8.82 | 9.13 | 9.11 |
| Non-Protein Nitrogen (%) | 5.54 | 4.82 | 4.27 | 4.21 |
| Extract matter without N (%) | 68.98 | 62.1 | 61.42 | 58.16 |
| Crude Fiber (%) | 4.06 | 5.8 | 6.28 | 6.44 |
| Carrageenan | 67.3 | 63.1 | 61.52 | 61.33 |
During fermentation, S. cerevisiae utilize the galactose and glucose present in the seaweed. The yeast may utilize the glucose present in the carrageenan after overcoming metabolite repression or GAL repression from the usage of glucose. The presence of glucose is needed to support initial growth of the yeast. Once the glucose has become limited, galactose can be utilized as the subsequent carbon source. The decrease in carrageenan after fermentation was found not to be significant and this can be due to the fact that the yeast utilized glucose prior to the galactose in the carrageenan. The accessibility of using carrageenan as a carbon source could have also been limited. To optimize the utilization of seaweed as a carbon source, several approaches such as microwave extraction and milling (Sambusisti et al., 2015), heating above 85 °C (Ruiz et al., 2013) and using alkaline (Wi et al., 2009) can be used.
The fermentation process also altered the fat content of the seaweed where a slight increase in amount was observed. The increase was however not significant from 0.27% prior to fermentation to 0.26%, (5% inoculum), 0.29% (10% inoculum) and 0.3% (15% inoculum). In parallel to the increase in fat content, the fatty acid content also experienced an increase (Table 4). Only fatty acids with long chains were detected in both the unfermented and fermented samples. The change in the fatty acid content is a result of biosynthesis by S. cerevisiae.
Table (4):
Fatty acid content seaweed before and after fermentation.
| Fatty acid (%) | Treatments | |||
|---|---|---|---|---|
| Before fermentation | After Fermentation | |||
| 5% Inoculum | 10% Inoculum | 15% Inoculum | ||
| Saturated | ||||
| C 14:0 | 0.127 | n.d.* | n.d.* | n.d.* |
| C 16:0 | 0.151 | 0.177 | 0.193 | 0.202 |
| C 18:0 | 0.012 | 0.018 | 0.023 | 0.024 |
| Monosaturated | ||||
| C 16:1 | 0.005 | n.d.* | 0.014 | n.d.* |
| C 18:1 ω9C | 0.025 | 0.036 | 0.044 | 0.046 |
| Polysaturated | ||||
| C 18:2 ω6C | n.d.* | 0.013 | 0.021 | 0.024 |
| C 18:3 ω3 | 0.010 | n.d.* | n.d.* | n.d.* |
| C 20:4 ω6 | 0.004 | n.d.* | n.d.* | n.d.* |
| Mono-unsaturated fatty acid (MUFA) | 0.029 | 0.048 | 0.058 | 0.065 |
| Poly-unsaturated fatty acid (PUFA) | 0.015 | 0.018 | 0.021 | 0.022 |
| Unsaturated lipids | 0.044 | 0.067 | 0.079 | 0.085 |
| Saturated lipids | 0.176 | 0.200 | 0.216 | 0.222 |
| Arachidonic acid (AA) | 0.004 | n.d.* | n.d.* | n.d.* |
| Omega 6 total | 0.015 | 0.018 | 0.021 | 0.022 |
| Omega 9 total | 0.025 | 0.037 | 0.044 | 0.047 |
| Linoleic Acid | 0.010 | 0.017 | 0.021 | 0.022 |
| Oleic Acid | 0.025 | 0.037 | 0.044 | 0.048 |
Fatty acids are a vital component of eukaryotic cells, including S. cerevisiae. Yeast cells are capable of synthesizing long fatty acid chains which are later used in the formation of membranes, energy storage and protein modification (Feldmann, 2012). The most common fatty acids produced by yeasts are palmitoleic acid (C16) and oleic acid (C18). This is in good agreement with the observed results showing that fatty acids with longer chains were significantly produced.
Crustaceans require dietary lipids as a source of essential fatty acids and other lipid classes like phospholipids (PL), sterols and carotenoids. In the case of marine organisms, polyunsaturated and especially highly unsaturated fatty acids (PUFA and HUFA) are important and essential because of their limited ability to synthesize them (Gonzalez-Felix et al., 2002). Providing energy is one of major function of dietary lipids, besides providing membrane components and affect the growth and immune system of L. vannamei (Zhang et al., 2014). When lipid supply is insufficient, animals may use protein as an energy source. This condition can make protein deficiency. Shrimp are lack to adaptive immunity and completely depend on their innate immunity, which includes both cellular and humoral components. High dietary lipid level could enhance the innate immune response capacity of shrimp system, which mainly consists of pPO activating system, clotting system, phagocytosis, encapsulation and nodule formation, antimicrobial peptides formation and cell agglutination (Zhang et al.,2013).
Increase of amino acids in the fermented seaweed was observed and indicates conversion of NPN(non-protein nitrogen) that was added into protein nitrogen (Table 5). Increase of amino acids was observed on all the fermented treatments, where an increase in aspartate and glutamate was found to be highest. The fermentation process also changed the ratio between the essential amino acids and non-essential amino acids, however the change was not significant. During fermentation, the protein nitrogen content may increase as an implication of microbial protein presence due to ammonium utilization by Saccharomyces cerevisiae for biosynthesis (Feldmann, 2012).
Table (5):
Amino Acid content of seaweed before and after fermentation.
| Amino acid (%) | Treatments | |||
|---|---|---|---|---|
| Before Fermentation | After Fermentation | |||
| 5% Inoculum | 10% Inoculum | 15% Inoculum | ||
| Essential | ||||
| Isoleucine | 0.119 | 0.135 | 0.146 | 0.15 |
| L-Arginine | 0.151 | 0.155 | 0.158 | 0.158 |
| Leucine | 0.22 | 0.244 | 0.266 | 0.267 |
| L-Tyrosine | 0.069 | 0.069 | 0.068 | 0.068 |
| Lysine | 0.141 | 0.135 | 0.128 | 0.129 |
| Methionine | 0.05 | 0.053 | 0.054 | 0.055 |
| Phenylalanine | 0.13 | 0.146 | 0.161 | 0.161 |
| Threonine | 0.153 | 0.17 | 0.183 | 0.186 |
| Valine | 0.156 | 0.181 | 0.2 | 0.206 |
| Non Essential | ||||
| Glycine | 0.157 | 0.184 | 0.201 | 0.211 |
| Histidine | 0.021 | 0.028 | 0.036 | 0.035 |
| L-Alanine | 0.184 | 0.219 | 0.243 | 0.252 |
| L-Aspartate | 0.294 | 0.339 | 0.378 | 0.381 |
| L-Glutamate | 0.351 | 0.447 | 0.509 | 0.538 |
| L-Proline | 0.128 | 0.147 | 0.16 | 0.164 |
| L-Serine | 0.149 | 0.162 | 0.177 | 0.174 |
| Total AA | 2.474 | 2.813 | 3.068 | 3.134 |
| EAA | 1.189 | 1.288 | 1.364 | 1.38 |
| NAA | 1.284 | 1.526 | 1.704 | 1.755 |
| EAA/NAA | 0.926 | 0.844 | 0.800 | 0.786 |
| EAA/Total AA | 0.481 | 0.458 | 0.445 | 0.440 |
The quality of protein sources is expressed as the amount of essential amino acids in the crude protein. Bioavailability of proteins and amino acids in feedstuffs is an important factor to consider, in part because it is related to the quantity of nitrogen absorbed by shrimp. Amino acids were classified as nutritionally essential or nonessential based on nitrogen balance on growth. Amino acids (AA) are not only building blocks for tissue proteins, but also essential substrates for the synthesis of many biologically active substances (e.g. polyamines, glutathione, creatine, carnitine, hormones, neurotransmitters) with crucial role in maintaining normal physiological and nutritional status of the body (Xie et al., 2015).Therefore, increasing amino acid content may significantly enhance immunity in shrimp. Hydroxyproline, arginine and glycine are three functional amino acid, which play important role in nutrient utilization and immune response (Xie et al., 2014).
Seaweed has been used in the field of aquaculture as a source of antioxidant, anti-pathogen and immunostimulant. The carrageenan content of seaweed is a potential immunostimulant that can act against bacterial attacks, such as Vibrio alginolyticus on white shrimp. In this study, the potential of fermented Kappaphycus alvarezii as a feed supplement for white shrimp (Litopenaeus vannamei) was observed based on its nutritional profile.
Seaweed contains a complete source of nutrition in varying amounts. The content of seaweed can vary due to differences in species, location, seasonal temperature condition of harvest and age of harvest. The proximate analysis showed that the seaweed used in the study had comparable nutritional content to that of literature with lower amounts of crude protein and fat content.
The fermentation process is expected to reduce the carbohydrate and carrageenan content of seaweed where both glucose and galactose will be used as carbon sources. The carbon along with other nutritional elements in the medium will be used to generate bioenergy and biosynthesis of cellular building blocks, such as phosphate sugars, organic acids, fatty acids and amino acids. Fermentation can also convert non protein nitrogen into microbial protein.
The decrease in the carrageenan content through the fermentation process was not significant, therefore retaining its role as an immunostimulant. Aside from the carrageenan, the increase of several amino acids such as glycine, alanine, proline and serine in the fermented seaweed can also act as an immunostimulant in towards white shrimps. Hence the result of the study suggests that the fermented seaweed use 10% inoculum for the application of a feed supplement for white shrimp, because this treatment give the highest microbial growth rate about 0,38 h-1 in 6 hours and this treatment was more nutritionally beneficial as compared to the other seaweed (treated and untreated).
- Adams, M. R., Cook, R. D. Rattagool, P. Fermented fish products of Southeast Asia. Tropical Science, 1985; 25:61–73
- Boulton C. Quain, D. Brewing yeast and fermentation. London: Blackwell Science Ltd., 2001
- Dawczynski, C., Schubert, R. Jahreis, G. Amino acid, fatty acid, and dietary fiber in edible seaweed products. Food chemistry, 2007; 103:891 – 899
- Feldmann, H. Yeast Molecular and Cell Biology 2nd: Completely Revised and Greatly Enlarged Edition. Weinheim: Wiley-VCH Verlag & Co., 2012
- Felix, N., Brindo, R. A. Substituting fish meal with fermented seaweed, Kappaphycus alvarezii in diets of juvenile freshwater prawn Macrobrachium rosenbergii. International Journal of Fisheries and Aquatic Studies, 2014; 1(5):261-265
- Felix, N., Pradeepa, P. Seaweed (Ulva reticulata) Based Fermented Marine Silage Feed Preparation under Controlled Conditions for Penaeus monodon Larval Development. Journal of Marine Science: Resources & Development, 1010; 1:103
- González-Félix, M. L., Lawrence, A. L., Gatlin III, D. M. Perez-Velazquez, M. Growth, survival and fatty acid composition of juvenile Litopenaeus vannamei fed different oils in the presence and absence of phospholipids. Aquaculture, 2002; 205(3-4):325-242
- Gressler, V., Yokoya, N. R., Fujii, M. T., Colepicolo, P., Filho, J. M., Torres, R. P. Pinto, E. Lipid, fatty acid, protein, amino acid and ash contents in four Brazilian red algae species. Food chemistry, 2010; 120: 585 – 590
- Istiani, S., Zatnika, A., Suhaimi, Anggadiredja, J. Manfaat dan Pengolahan Rumput Laut [Benefits and Processing of Seaweed]. Jakarta: BPPT, 1986
- Kaehler, S. Kennish, R. Summer and winter comparisons in the nutritional value of marine macroalgae from Hong Kong. Botanica Marina, 1996; 39: 11–17
- Liem, Z. A. Kandungan proksimat dan aktivitas antioksidan rumput laut merah di perairan Kupang Barat [Proximate Content and Antioxidant Activity of Red Seaweed (Eucheuma cottonii) in Kupang Barat waters. M.Sc Thesis, 2013. Satya Wacana Christian University, Indonesia.
- Machado, D. I. S., Cervantes, J. L., Hernandez, J. L., Losada, P.P. Fatty acids, total lipid, protein and ash contents of processed edible seaweed. Food chemistry, 2004; 85: 439 – 444.
- Necas, J. Bartosikova, L. Carrageenan: a review. Veterinarni Medicina, 2013; 58(4):187–205
- Ortiz, j., Romero, N., Robert, P., Araya, J., Hernandez, J. L., Bozzo, C., Navarrete, E., Osario, A., Rio, A. Dietary fiber, amino acid, fatty acid and tocopherol contents of the edible seaweeds Ulva lactuca and Durvillaea antartica. Food chemistry, 2006; 99: 98 – 104.
- Park, J.H., Hong, J.Y., Jang, H.C., Oh, S.G., Kim, S.H., Yoon, J.J. Kim, Y.J. Use of Gelidium amansii as a promising resource for bioethanol: a practical approach for continuous dilute-acid hydrolysis and fermentation. Bioresource Technology, 2012; 108: 83–88.
- Ruiz, H. A., Rodríguez-Jasso, R. M., Fernandes, B. D., Vicente, A. A. Teixeira, J. A. Hydrothermal processing, as an alternative for upgrading agriculture residues and marine biomass according to the biorefinery concept: a review. Renewable Sustainable Energy Reviews, 2013; 21:35–51.
- Sambusiti, C., Bellucci, M., Zabaniotou, A., Beneduce, L. Monlau, F. Algae as promising feedstocks for fermentative biohydrogen production according to a biorefinery approach: a Comprehensive review. Renewable Sustainable Energy Reviews, 2015; 44:20– 36.
- Sun, Y. Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresource Technology, 2002; 83(1):1–11.
- Takagi, T., Asahi, M. Itabashi, Y. Fatty acid composition of twelve algae from japanese waters. Journal of Japan Oil Chemists’ Society, 1985; 34(12):1008-1012.
- Thanigaivel, S., Chandrasekaran, N., Mukherjee, A. Thomas, J. Seaweed as an alternative therapeutic for aquatic disease management. Aquaculture, 2016; 464:429 – 536.
- Uchida, M. Use of fermented seaweed as a hatchery diet. Aqua Feed International, 2013; 2:15-17.
- Uchida, M., Murata, M. Isolation of a lactic acid bacterium and yeast consortium from a fermented material of Ulva spp. (Chlorophyta). Journal of Applied Microbiology, 2004; 97: 1297-1310.
- Wi, S. G., Kim, H. J., Mahadevan, S. A., Yang, D. J. Bae, H. J. The potential value of the seaweed Ceylon moss (Gelidium amansii) as an alternative bioenergy resource. Bioresource Technology, 2009; 100: 6658–6660.
- Xie, S., Tian, L., Jin, Y., Yang, H., Liang, G. Liu, Y. Effect of glycine supplementation on growth performance, body composition and salinity stress of juvenile Pacific white shrimp, Litopenaeus vannamei fed low fishmeal diet. Aquaculture, 2014; 418-419:159-164.
- Xie, S., Tian, L., Li, Y., Zhou, W., Zeng, S., Yang, H. Liu, Y. Effect of proline supplementation on anti-oxidative capacity, immune response and stress tolerance of juvenile Pacific white shrimp, Litopenaeus vannamei. Aquaculture, 2015; 448:105-11.
- Yeh, S. T., Chen, J. C. Immunomodulation by carrageenans in the white shrimp Litopenaeus vannamei and its resistance against Vibrio alginolyticus. Aquaculture, 2008; 276:22-28.
- Zhang, M., Sun, Y., Chen, K., Yu, N., Zhou, Z., Chen, L., Du, Z. Li, E. Characterization of the intestinal microbiota in Pacific white shrimp, Litopenaeus vannamei, fed diets with different lipid sources. Aquaculture, 2014; 434:449-455
- Zhang, S., Li, J., Wu, X., Zhong, W., Xian, J., Liao, S., Miao, Y. Wang, A. Effects of different dietary lipid level on the growth, survival and immune-relating genes expression in Pacific white shrimp, Litopenaeus vannamei. Fish & Shellfish Immunology, 2013; 34(5):1131-1138.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.