ISSN: 0973-7510
E-ISSN: 2581-690X
The Current study revealed the natural occurrence of toxigenic fungi and mycotoxins production in grains in Saudi Arabia. Samples of yellow corn, white rice and red corn grains were collected from different local markets. Three fungal isolates were isolated from the examined corn grains using PDA media. The toxigenic Alternaria spp. was the most prominent fungi in yellow corn grains, white rice and red corn grains. Three Alternaria spp. isolated were identified using molecular characterization of ACTTS gene. DNA genome of the three Alternaria spp isolates (namely AWR; AYC and ARC which corresponds to isolates from white rice, yellow corn, red corn) was used as a template for PCR to amplify ACTTS gene. Partially sequenced ACTTS gene was amplified using a specific primer set to confirm its identity, phylogenetic relationships between the three isolates as well as determination of the corresponding antigenic determinants. The epitope prediction analysis demonstrated that there were 5, 6 and 5 epitopes whose score were above 0.90 in AWR, AYC and ARC, respectively. Interestingly, there were great variations in the epitope sequences among the three isolates except for the epitope, VYGASTATGTLAVQ. This work led to molecular identification of three Alternaria spp. using ACTTS gene and the unique antigenic determinants that could be used for design of a broad spectrum antibody for rapid detection of Alternaria spp. in foods.
Mycotoxins, Alternaria, ACTTS gene, PCR, antigenic determinants.
Several fungi attack the corn grains during harvest and storage. While more than 25 different fungi species known to invade stored grains (Duan et al., 2007), some species such as Aspergillus, Fusarium, Penicillum are responsible for most spoilage and germ damage during storage (Pitt, 2000a). They cause reduction in baking quality, nutritive values, produce undesirable odors, color and changes appearance of stored food grade seeds and decrease germination ability and total decay (Castillo et al., 2004). Besides their mycotoxins, they are considered as health hazard for man and animals, render products unacceptable for edible purposes or lower their market grade (Pitt, 2000b; Richard et al., 2007). Moreover, fungal infestation of seed coat decreases viability of seeds, or may cause abnormal seedlings (Selcuk et al., 2008). This has been demonstrated by isolation of fungi from seeds collected before seed set. Many of these fungi have no negative impact on seeds but there also many saprophytic and pathogenic fungi commonly isolated from seeds (Garcez et al., 2000) .The knowledge on frugal seed decay and its importance for plant demographic and community processes is quite limited (Blaney and kotanen, 2001). Fungal genera, such as Aspergillus spp; Fusarium spp; Penicillium spp; Alternaria spp; and Epicoccum spp. were isolated from same seeds of grains, beans, cowpea, peas, and cocoa (Rodrigues and Menezes, 2005; Sánchez-Hervás et al., 2008; Weder, 2002). In Saudi Arabia, very little information exists with respect to its natural contamination with toxigenic fungi and mycotoxins. Aflatoxin (s) were detected in some Aspergillus isolates while fumonisin was detected in some Fusarium isolates (Ibrahim et al., 1998) . Some pathotypes of Alternaria Alternata produces host selective ACT-toxin for which several open reading frames designated as ACTTS are implicated for its synthesis (Ajiro et al., 2010). Determination of ACTTS gene in Alternaria and unraveling its characteristics are important because it is involved in ACT-toxin production and pathogenicity.
The aim of the current study is the molecular identification of toxigenic Alternaria contaminating some grains and protein structural analysis depicted from the gene(s) responsible for toxin biosynthesis.
Grains samples
One hundred fifty grains corn (yellow and red corn grains) and rice (short and long white rice) were collected from different area of Saudi Arabia ( Riyadh, Hail, Qasim, Asir,Tabuk, Jizan, Jouf, Jeddah and Dammam), where collected from storage markets and houses . The collected grains were randomly and its weight between 0.5 – 1 kg of each grain in cleans and dries packaging.
Isolation of mycotoxigenic Alternaria spp.
Agar plate and blotter tests were used to isolate Alternaria spp. as described by Neergaard (1977). Grains were divided into two groups, the first group was disinfected with sodium hypochlorite 1% for 2 min and the second group was non-disinfected. All grains were washed several times by sterilized water, and then dried between sterilized filter papers. The half of each group was plated on potato dextrose agar (PDA). All dishes were incubated for 5 to 7 days at 25°C.
Purification and identification of Alternaria spp.
Single colony was transferred and purified by hypha tip technique onto PDA medium in the presence of streptomycin (50 mg /ml). The developing fungi were prepared for molecular identification using primers specific for the ACTTS gene.
Molecular identification of ACTTS gene
The molecular identification of the ACTTS gene was carried out by PCR and sequencing of amplicons.
Isolation of DNA genome
The mycelium mass of Alternaria spp isolates grown on PDA broth medium was harvested by centrifugation at 6000rpm for 10 min. The pellets were washed twice by PBS buffer and stored at 200C. Total DNA of the three isolates was isolated using lysozyme – dodecyl sulfate lysis method as described by Leach, et al (1990).
Amplification and purification of ACTTS gene
Specific PCR reactions were conducted to assess the presence of ACTTS gene. The primers used in this study are provided in a supplementary table. The PCR amplification conditions included initial denaturation at 94°C for 5min then 35 cycles at 94°C for 30s, 55°C for 60s followed by extension step at 72°C for 90s. and a final extension at 72°C for 7 min. The amplification reaction was performed by thermal cycler (COT Thermocycler model 1105). Purification of PCR product was detected by electrophoresis using agarose 1.5% in 1x TAE buffer and staining with ethidium bromide (Sambrook et al., 1989). The resultant fragment of ACTTS gene was excised from the gel and purified using a QIA quick gel extraction kit (Qiagen, Berlin, Germany).
DNA sequencing
The purified PCR products were prepared for Sanger sequencing technology using DNA sequencer technique (Sigma, central lab, PNU, KSA). DNA sequences of Alternaria isolates were aligned using Bio Edit software version 7(www. mbio-ncus. edu/bio. edit) and were compared of the often accessions of Alternaria spp. available in the NCBI data base using BLAST algorithm to identify closely related sequences (http/www.ncbi.nih.gov). Dendrogram were constructed by using un-weighed pair Group method with Arithmetic (UPGMA) on Gen bank.
Epitope prediction and antigenicity
The primary amino acids sequence of the ACTTS protein was evaluated from the corresponding nucleotide sequence using MEGA 6.0 software. The linear B-cell epitopes in the primary amino acid sequence of the coat protein was performed using BCPREDS server with default parameters (http://ailab.cs.iastate.edu/bcpreds/) which implements a support vector machine (SVM) and the subsequence kernel method (El-Manzalawy et al., 2008a). Flexible length linear B-cell epitopes were predicted using FBCPred (El-Manzalawy et al., 2008b) method with a specificity cut-off; 75%.
The antigenicity of each amino acid residue in the primary protein sequence was determined using a semi-empirical method (Kolaskar and Tongaonkar, 1990) which makes use of physicochemical properties of each amino acid and their frequencies of occurrence in experimentally known segmental epitopes.
Three Alternaria isolated from tested grains by PDA method was purified by single spore and hypha tip on PDA slant medium. The Alternaria isolates were selected for molecular identification using ACTTS gene sequencing. Three Alternaria isolates represented grains from yellow corn, white rice and red corn and designated as Alternaria AYC, AWR and ARC, respectively.
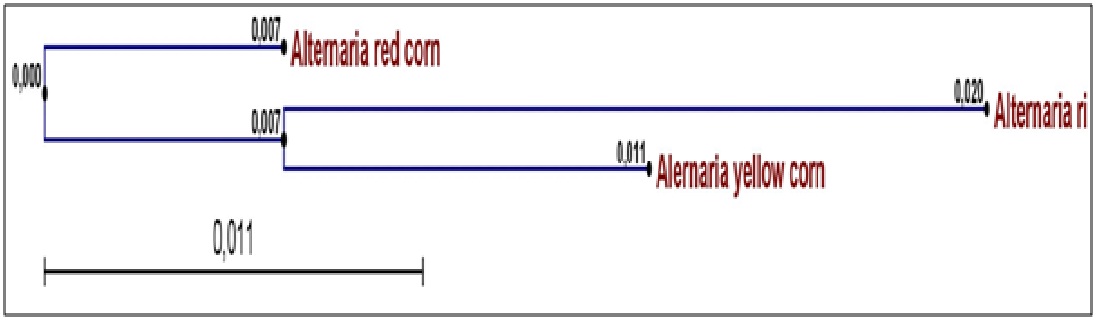
Fig. 1. Phylogeny of the three studied Alternaria isolates (A. Alternata AYC, A. alternata AWR and A. alternata ARC ) isolated from yellow corn, white rice and red corn
Molecular characterization of ACTTS gene
Total DNA was extracted from Alternaria spp. AWR; AYC and ARC infected grains. ACTTS gene of three Alternaria spp isolate AWR; AYC and ARC was amplified from isolated DNA of mycelium using PCR reaction mixture and specific primer sets. PCR amplicons were allowed for sequencing reaction through cycle sequencing method. The DNA Amplicons returned as electropherogram Files. Electropherogram showed distinct peaks for each base cell as well as high Q values for each Cell. Sequences obtained for each primer for each isolate had sufficient overlap between them and used to form one continuous sequence (Coting). The nucleotide partial sequence of ACTTS gene in the three isolates was compared with published isolates on GenBank. The sequence homology revealed that the gene of interest was ACTTS gene (coding for enoyl reductase) and the test fungal isolates were A. alternata isolates. A multiple sequence alignment was constructed using Clustal W software between the three studied isolates. The alignment showed many conserved regions in all sequences as well as distinguished the heterogeneity positions among the aligned sequences. Phylogenetic analysis was performed by construction of phylogenetic tree using a neighbor joining method to unravel the relationships among all Alternaria isolates (Fig. 2). The phylogenetic tree resulted in two clades in which AYC (yellow corn isolate) and AWR (white rice isolate) were in the same cluster whilst ARC (red corn isolate) was separate in a different cluster. Thus, the molecular identification based on sequence homology of the ACTTS gene confirmed the identity and phylogeny of the studied three Alternaria isolates.
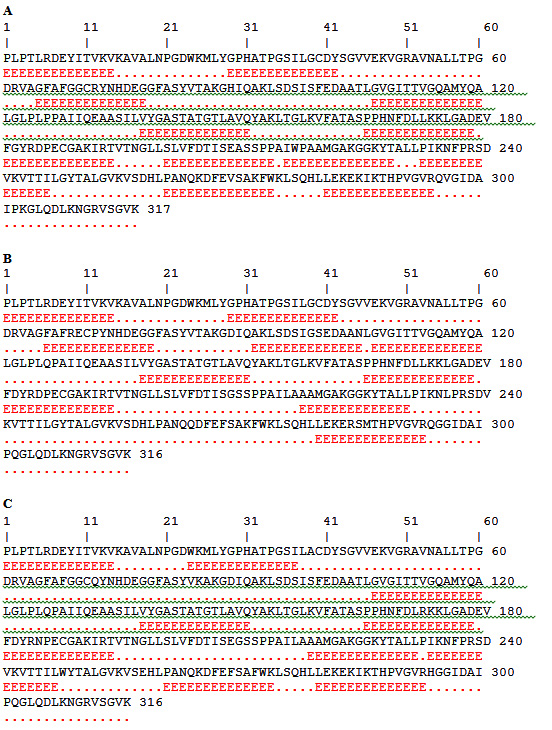
Fig. 2. Amino acid residues of ACTTS protein in ARC (A), AWR (B) and ARC (C) showing predicted as epitopes (Red) that are highlighted
Detection of epitope sequences of the Alternaria ACTTS gene
For prediction of the linear B-cell epitopes in the ACTTS protein primary amino acids sequence, BCPREDS server was used. Five to six epitopes, whose sequence length was 14 residues, were retrieved in the amino acid sequences of the three Alternaria isolates (Table 1). The highest epitope according to specificity was AMGAKGGKYTALLP, LEKEKIKTHPVGVR and LEKEKIKTHPVGVR in the ACTTS in AWR, AYC and ARC, respectively. The epitope prediction analysis demonstrated that there were 5, 6 and 5 epitopes whose score were above 0.90 in AWR, AYC and ARC, respectively. Interestingly, there were great variations in the epitope sequences among the three isolates except for the epitope, VYGASTATGTLAVQ which was found to be conserved among all. The predicted epitopes were displayed and highlighted (in red) with their positions on the amino acids sequence as shown in Figure (2).
Table (1):
Flexible length predictions of epitopes in the amino acids sequence of ACTTS protein of the three studied Alternaria isolates.
Number |
Epitope/AWR |
Score/AWR |
Epitope/AYC |
Score/AYC |
Epitope/ ARC |
Score/ ARC |
|---|---|---|---|---|---|---|
1 |
AMGAKGGKYTALLP |
0.996 |
LEKEKIKTHPVGVR |
1 |
LEKEKIKTHPVGVR |
1 |
2 |
GPHATPGSILGCDY |
0.992 |
AMGAKGGKYTALLP |
0.996 |
WPAAMGAKGGKYTA |
0.999 |
3 |
LEKERSMTHPVGVR |
0.991 |
WKMLYGPHATPGSI |
0.977 |
GPHATPGSILGCDY |
0.992 |
4 |
VYGASTATGTLAVQ |
0.973 |
VYGASTATGTLAVQ |
0.973 |
VYGASTATGTLAVQ |
0.973 |
5 |
FAFRECPYNHDEGG |
0.958 |
FDYRNPECGAKIRT |
0.938 |
IKNFPRSDVKVTTI |
0.911 |
6 |
– |
– |
PANQKDFEFSAFWK |
0.901 |
– |
– |
The antigenicity profile of the amino acid residues of the ACTTS protein demonstrated the residue of high frequency to be part in the genetic determinant (fig. 4). The highest residues above the threshold value were numerous such as Valine (V), Leucine (L), Isoleucine (I), serine (S), Cytosine (C), Aspartic acid (D), glutamine (Q) and glutamic acid (E) with antigenicity score and spanning the region, 281- 293, 218-230, 280-290 in the primary protein sequence of ARC, AWR and AYC, respectively. These residues with high frequencies of occurrences in antigenic determinants were highlighted (yellow) in the antigenicity profile (fig. 3). Figure 3 also show the variability in the positions and types of amino acid residues with high antigenic frequency.
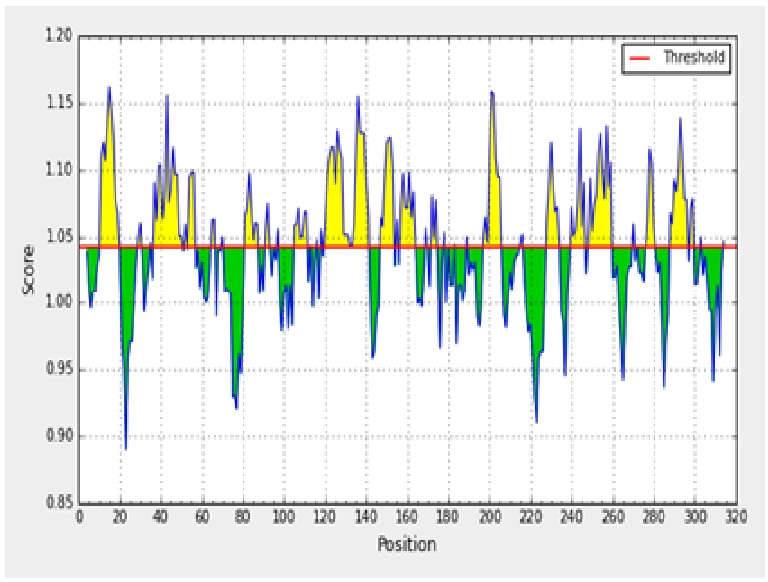
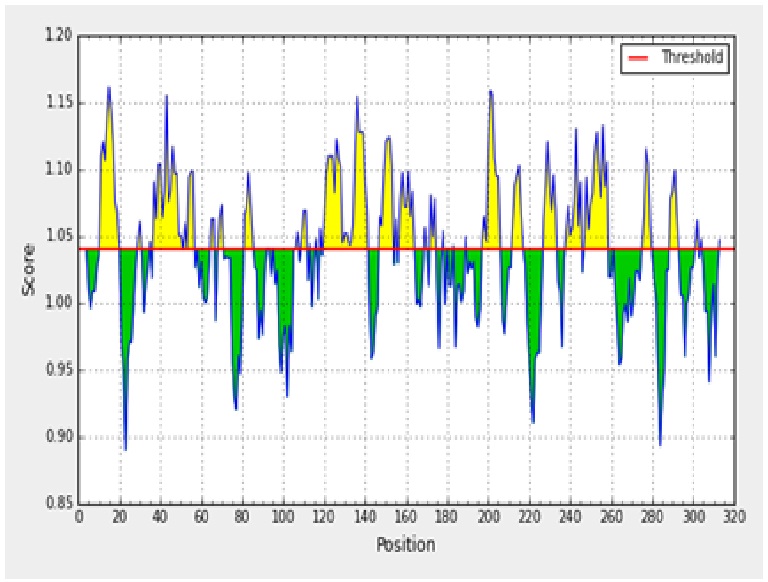
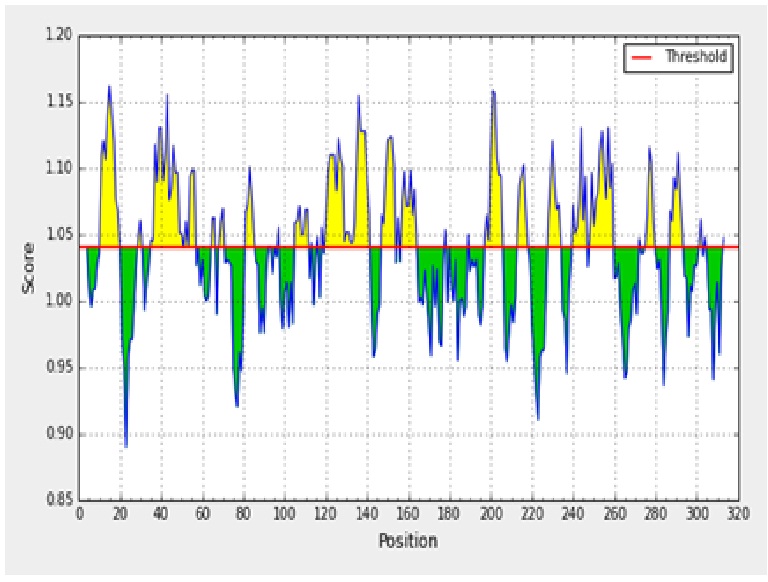
Fig. 3. Kolaskar and Tongaonkar antigenicity scale for prediction of antigenic determinants in ACTTS protein in ARC (A), AWR (B) and AYC (C). Amino acid residues of high frequencies in epitopes are distinguished (yellow)
The emergence of toxigenic fungi on small grains has a negative impact on the safety and quality of feed and food. The genus Alternaria includes cosmopolitan and ubiquitous mold fungi in which saprobes and plant pathogens are many (Thomma, 2003). Alternaria species causes yield losses in processing and production (Vukovi, 2012). Being able to grow at low temperature, Alternaria spp. are responsible for spoilage of food commodities during transport and storage (Barkai-Golan, 2008; Dall’Asta et al., 2014). In addition, reduction in nutritive value, insipidness and discoloration are other problems resulted from contamination of grains by Alternaria (Kosiak et al., 2004). In addition to economic losses, many species are well known mycotoxins producers with various toxicological properties and high risks for human and animal health (Logrieco et al., 2009). Rapid and accurate identification of Alternaria and / or their metabolites are mandatory for the implementation of preventive measures in the whole food production system. The molecular characterization of the three Alternaria spp. isolated from small grains (yellow corn, white rice and red corn) using the mycotoxins gene was performed and the ACTTS gene sequence analysis allowed for coupled identification and mycotoxins screening in the three Alternaria isolates. Mycotoxins-producing fungi were isolated from sorghum grains from Saudia Arabia before (Mahmoud et al., 2013; Yassin et al., 2010). Following the molecular identification of Alternaria spp., B-cell epitopes in the ACTTS gene were predicted. The characterization of B-cell epitopes using computational tools is highly advantageous for the synthesis of specific antibodies for rapid detection of microbial pathogens in their environments. The epitopes prediction saves labor and time for validation experiments. The identification of epitopes plays a crucial role in the vaccine design, immunodiagnostic testing and antibody production (Sette and Fikes, 2003). In this study, BCPREDS server was used to predict epitopes found in the primary amino acids sequence of ACTTS protein. BCPREDS proved high efficiency to predict linear B-cell epitopes in SARS-CoV S protein (El-Manzalawy et al., 2008). There was variability in the sequence and numbers of epitopes among the three toxin proteins analyzed. Here, a fixed length of epitopes (14 residues) was observed. The epitope, VYGASTATGTLAVQ, was found to be common between all isolates suggesting its exploitation for design of a specific antibody to be used for rapid detection of different Alternaria species in small grains. The highly frequent residues with high antigenicity profiles such as Valine, Leucine, Isoleucine, Aspartic acid, Glutamine and Glutamic acid are mostly hydrophobic. The occurrence of hydrophobic residues in epitopes is frequent and do have a hierarchy signature (Aftabuddin and Kundu, 2007; Mine and Zhang, 2002). Epitope prediction has many implications in pathogen detection and differentiation applications.
The consideration of occurrence of Alternaria spp. on small grains is important in risk assessment of mycotoxins and setting up preventive measures proactively.
ACKNOWLEDGMENTS
The authors are grateful to Deanship of Scientific Research; PNU for financial support. And thanks for all technicians in Research Labe, Biology department, PNU.
- Aftabuddin, M., Kundu, S., Hydrophobic, hydrophilic, and charged amino acid networks within protein. Biophys. J. 2007; doi:10.1529/biophysj.106.098004.
- Ajiro, N., Miyamoto, Y., Masunaka, A., Tsuge, T., Yamamoto, M., Ohtani, K., Fukumoto, T., Gomi, K., Peever, T.L., Izumi, Y., Tada, Y., Akimitsu, K., Role of the host-selective ACT-toxin synthesis gene ACTTS2 encoding an enoyl-reductase in pathogenicity of the tangerine pathotype of Alternaria alternata. Phytopathology, 2010; 100: 120–126. doi:10.1094/PHYTO-100-2-0120
- Barkai-Golan, R., Alternaria Mycotoxins, in: Mycotoxins in Fruits and Vegetables., 2008; pp. 185–203. doi:10.1016/B978-0-12-374126-4.00008-5
- Blaney, C.S., Kotanen, P.M., Effects of fungal pathogens on seeds of native and exotic plants: A test using congeneric pairs. J. Appl. Ecol, 2001; 38, 1104–1113. doi:10.1046/j.1365-2664.2001.00663.x
- Castillo, M.D., González, H.H.L., Martínez, E.J., Pacin, A.M., Resnik, S.L., Mycoflora and potential for mycotoxin production of freshly harvested black bean from the Argentinean main production area. Mycopathologia., 2004; doi:10.1023/B:MYCO.0000038426.05215.89
- Dall’Asta, C., Cirlini, M., Falavigna, C., Mycotoxins from Alternaria: Toxicological implications. Adv. Mol. Toxicol., 2014; 8: 107–121. doi:10.1016/B978-0-444-63406-1.00003-9
- DUAN, C. xing, WANG, X. ming, ZHU, Z. dong, WU, X. fei, Testing of Seedborne Fungi in Wheat Germplasm Conserved in the National Crop Genebank of China. Agric. Sci. China, 2007; 6: 682–687. doi:10.1016/S1671-2927(07)60100-X
- El-Manzalawy, Y., Dobbs, D., Honavar, V., Predicting linear B-cell epitopes using string kernels. J. Mol. Recognit., 2008a; 21: 243–255. doi:10.1002/jmr.893
- El-Manzalawy, Y., Dobbs, D., Honavar, V., Predicting flexible length linear B-cell epitopes. Comput. Syst. Bioinformatics Conf., 2008b; 7: 121–32.
- Garcez, W.S., Martins, D., Garcez, F.R., Marques, M.R., Pereira, A.A., Oliveira, L.A., Rondon, J.N., Peruca, A.D., Effect of sppores of saprophytic fungi on phytoalexin accumulation in seeds of frog-eye leaf sppot and stem canker-resistant and -susceptible soybean (Glycine max L.) cultivars. J. Agric. Food Chem., 2000; 48: 3662–3665. doi:10.1021/jf991146o
- Ibrahim, T.F., El-Abedeen, A.Z., El-Morsy, G.A., El-Azhary, T.M., Aflatoxins in Egyptian sorghum grains: detection and estimation. Egypt. J. Agric. Res., 1998; 76: 923–931.
- Kolaskar, A.S., Tongaonkar, P.C., A semi-empirical method for prediction of antigenic determinants on protein antigens. FEBS Lett., 1990; 276: 172–174.
- Kosiak, B., Torp, M., Skjerve, E., Andersen, B., Alternaria and Fusarium in Norwegian grains of reduced quality – A matched pair sample study. Int. J. Food Microbiol., 2004; 93: 51–62. doi:10.1016/j.ijfoodmicro.2003.10.006
- Leach, J.E., White, F.F., Rhoads, M.L., Leung, H., A Repetitive DNA Sequence Differentiates Xanthomonas campestris pv. oryzae from Other Pathovars of X. campestris. Mol. Plant-Microbe Interact., 1990; 3: 238. doi:10.1094/MPMI-3-238
- Logrieco, a., Moretti, a., Solfrizzo, M., Alternaria toxins and plant diseases: an overview of origin, occurrence and risks. World Mycotoxin J., 2009; 2: 129–140. doi:10.3920/WMJ2009.1145
- Mahmoud, M.A., Al-Othman, M.R., Abd El-Aziz, A.R.M.A., Mycotoxigenic fungi contaminating corn and sorghum grains in Saudi Arabia. Pakistan J. Bot., 2013; 45: 1831–1839.
- Mine, Y., Zhang, J.W., Identification and fine mapping of IgG and IgE epitopes in ovomucoid. Biochem. Biophys. Res. Commun., 2002; 292: 1070–1074. doi:10.1006/bbrc.2002.6725
- Neergaard, P. 1977. Seed pathology Vols. 1 and 2. The MacMillan Press.
- Pitt, J.I., Toxigenic fungi and mycotoxins. Br. Med. Bull., 2000a; 56: 184–92. doi:10.1080/744118730
- Pitt, J.I., Toxigenic fungi: which are important? Med. Mycol. Off. Publ. Int. Soc. Hum. Anim. Mycol., 2000b; 38 Suppl 1, 17–22.
- Richard, E., Heutte, N., Sage, L., Pottier, D., Bouchart, V., Lebailly, P., Garon, D., Toxigenic fungi and mycotoxins in mature corn silage. Food Chem. Toxicol., 2007; 45: 2420–2425. doi:10.1016/j.fct.2007.06.018
- Rodrigues, A.A.C., Menezes, M., Identification and pathogenic characterization of endophytic Fusarium Species from cowpea seeds, in: Mycopathologia., 2005; pp. 79–85. doi:10.1007/s11046-004-7138-x
- Sambrook, J., Fritsch, E., Maniatis, T., Molecular Cloning: A Laboratory Manual., 1989; New York.
- Sánchez-Hervás, M., Gil, J. V., Bisbal, F., Ramón, D., Martínez-Culebras, P. V., Mycobiota and mycotoxin producing fungi from cocoa beans. Int. J. Food Microbiol., 2008; 125: 336–340. doi:10.1016/j.ijfoodmicro.2008.04.021
- Selcuk, M., Oksuz, L., Basaran, P., Decontamination of grains and legumes infected with Asppergillus spp. and Penicillum spp. by cold plasma treatment. Bioresour. Technol., 2008; 99: 5104–5109. doi:10.1016/j.biortech.2007.09.076
- Sette, A., Fikes, J., Epitope-based vaccines: An update on epitope identification, vaccine design and delivery. Curr. Opin. Immunol., 2003; doi:10.1016/S0952-7915(03)00083-9
- Thomma, B.P.H.J., Alternaria spp.: From general saprophyte to sppecific parasite. Mol. Plant Pathol., 2003: doi:10.1046/j.1364-3703.2003.00173.x
- Vuèkoviæ, J., Alternaria spp. on small grains. Food Feed Res., 2012; 39(2)79-88.
- Weder, J.K.P., Species Identification of Beans, Peas and Other Legumes by RAPD-PCR after DNA Isolation using Membrane Columns. LWT – Food Sci. Technol., 2002; 35: 277–283. doi:10.1006/fstl.2001.0857
- Yassin, M.A., El-Samawaty, A.R., Bahkali, A., Moslem, M., Abd-Elsalam, K.A., Hyde, K.D., Mycotoxin-producing fungi occurring in sorghum grains from Saudi Arabia. Fungal Divers., 2010; 44: 45–52. doi:10.1007/s13225-010-0058-9.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


