ISSN: 0973-7510
E-ISSN: 2581-690X
A study observing endophytic fungi from medicinal plant Melochia umbellata and their antibacterial potencies has been done. Twelve species of endophytic fungi were successfully isolated from Melochia umbellata leaves and twigs. Four of them showed an activity in the antagonist test against Escherichia coli, Pseudomonas aeruginosa, Vibrio cholerae and Shigella dysentriae. The active isolates were fermented in potato dextrose yeast (PDY) medium at 25oC for 21 days with agitation of 150rpm. The secondary metabolites were extracted from the fermentation medium using ethyl acetate and the mycelia were extracted using methanol. The ethyl acetate and methanol extracts were assessed for their antibacterial activity against the pathogenic bacteria and some of the isolates extract showed antibacterial activity with inhibition zone obtained were between 0 and 10.51mm. From ethyl acetate extracts, We were obtained MUD5 94mg with inhibition zone 8.08mm against E. coli, 10.51 against S. dysentriae, 7.81mm against P. aeruginosa, 9.00mm against V. cholerae, MUR4 116mg with inhibition zone 8.61 mm against E. Coli, MUR5 was obtained 93mg with inhibition zone 7.54mm against E. coli, and MUR6 96mg with inhibition zone 7.44mg against P. aeruginosa. While from methanol extracts were obtained MUR6 516mg with inhibition zone 9.3mm against P. aeruginosa. Phytochemical identification revealed that the active extracts contain alkaloids, flavonoids and steroids. From this present work, it can be concluded that these endophytic fungi could be promising source of bioactive compounds and can be used for further study.
Antibacterial Activity; Endophytic fungi; Melochia umbellata; Mycelia.
The discovery of natural products and novel bioactive molecules have played major role in the search for new drugs.2 The existence of microorganisms in various plant tissues (such as root, fruit, stem, seed, leaf, etc.) provide a mutual relationship without causing any symptom of diseases are called endophytes.1 Generally, the plant tissues are protected from infectious agents by the endophytic fungi by producing various bioactive compounds. The endophytic fungi reside asymptomatically in the internal tissues beneath epidermal cell layers and lives within the intercellular spaces of the tissues. The endophytic fungi also may penetrate to the living cells of all higher plants2. They are good and interesting source of antibiotics. Natural products from endophytic microbes have been observed to be able to inhibit or kill a wide variety of harmful disease-causing agents but not limited to Phytopathogens, as well as bacteria, fungi, viruses and protozoan that affect humans and animals1.
Isolation of Endophytes is a critical step which requires sensitivity to recover a maximum number of colonized Endophytes and should remove the epiphytic microbes which are present on the plant surface. The collected plants for studying endophytic communities should look apparently healthy and disease-free plant to decrease the possibility of pathogenic and saprobe species contaminant, and to avoid the isolation of pathogenic endophytic microorganisms around it2.
Studies about the endophytic fungi have been widely conducted in some countries. Recent study showed that Melochia umbellata leave extract triggered antibacterial activity against Staphylococcus aureus, and Shigella dysentriae with inhibition zone of 10.5mm (2500 ppm) and 9.36mm (2500ppm), respectively3. In this study, we focus on the isolation and identification of endophytic fungi of Melochia umbellata and the screening of their antibacterial activity. We are also determining for the phytochemical compounds in the extracts of endophytic fungi. We hope this study will contribute to the invention of new antibiotic candidate which can provide benefits to the health problems.
Sources for Endophytic Fungi
Leaves and twigs were thoroughly washed with mild detergent and running tap water and then air–dried. After which they were surface sterilized by using 3 step surface sterilization start with submerging them in 75% ethanol for 3min. Further sterilization was performed by using 5.3% sodium hypochlorite solution for 5min, and 75% ethanol for 0.5 min, sequentially. After sterilization, samples were washed with sterile water to remove ethanol residues. Each leaf was cut into 1 cm in size and twigs were cut into two pieces. Samples were placed at the surface of sterile potato dextrose agar (PDA) medium and incubated for 3-5 days, at 25oC. The fungal isolates were identified based on their morphological characters6, 7, 8, 9.
Mass cultivation and extraction of metabolites of endophytic fungi
The fungal endophytes were mass cultivated on potato dextrose yeast (PDY) by placing agar blocks of actively growing pure culture (3mm in diameter) in 250ml Erlenmeyer flasks that contain 100ml medium. The flasks were incubated at room temperature for 21 days with periodical shaking at 150rpm. After incubation period, culture media and mycelia were separated by filtration and extracted with the method described by Dasale et al (2013). The Fungi mycelia was sonicated in 100ml methanol for 30 minutes. The mycelia extract was filtered and evaporated. The culture media was extracted by liquid-liquid extraction using ethyl acetate in the same volume with the media. Extraction was repeated three times and the organic solvent of collected extract was evaporated under reduced pressure. The crude extract was then dissolved in Dimethyl sulphoxide (DMSO) for antibacterial bioassay.
Antibacterial Activity
Test Organisms
There were 4 strains of pathogenic bacteria which were used for the antibacterial activity of fungi. They were E. coli, S. dysentriae, P. aeruginosa, and V. cholerae.
Antagonist test
Endophytic fungi cultures were transferred to petri dish which consisted of nutrient agar medium and pathogenic bacteria. The dishes were incubated for 24hrs, at 37oC.
Antibacterial activity test
The agar well diffusion assay method was used to examine the antibacterial activity of fungi. In this method, wells were aseptically made in seeded media using sterile cork borer and an amount of 20µl bioactive metabolite was dropped in the previously prepared wells and incubated at 37oC in bacteriological incubator for 24hrs. Finally, plates were observed for zones of inhibition and their diameter was measured with Antibiotic zone scale.
Phytochemistry identification
The active antibacterial isolate extracts were identified for their phytochemistry compounds. These were done by thin layer chromatography method. The extracts were eluted using hexane: ethyl acetate (2:1) and the results were observed under UV 254, 366 nm and visible (after sprayed with 10% H2SO4). For Alkaloid identification, the spots were sprayed with dragendorf reagent and for flavonoids identification by using sitroboric reagents11, 12.
Table (1):
Zone of Inhibition produced by endophytic fungi on human bacerial pathogens.
| Extract | Endophytic Fungi | Diameters of Inhibition zone (mm) | Amount of extract (mg) | |||
|---|---|---|---|---|---|---|
| Escherichia coli | Shigelladysentriae | Pseudomonas aeruginosa | Vibrio cholerae | |||
| Ethyl acetate | MUD1 | – | – | – | – | – |
| MUD2 | – | – | – | – | – | |
| MUD3 | – | – | – | – | – | |
| MUD4 | – | – | – | – | – | |
| MUD5 | 8.08±0.053 | 10.51±0.153 | 7.81±0.0781 | 9.00±0.097 | 94 | |
| MUD6 | – | – | – | – | – | |
| MUR1 | – | – | – | – | – | |
| MUR2 | – | – | – | – | – | |
| MUR3 | – | – | – | – | – | |
| MUR4 | 8.61±0.215 | – | – | – | 116 | |
| MUR5 | 7.54±0.262 | – | – | – | 93 | |
| MUR6 | – | – | 7.44±0.301 | – | 96 | |
| Methanol | MUD1 | – | – | – | – | – |
| MUD2 | – | – | – | – | – | |
| MUD3 | – | – | – | – | – | |
| MUD4 | – | – | – | – | – | |
| MUD5 | – | – | – | – | 257 | |
| MUD6 | – | – | – | – | – | |
| MUR1 | – | – | – | – | – | |
| MUR2 | – | – | – | – | – | |
| MUR3 | – | – | – | – | – | |
| MUR4 | – | – | – | – | 97 | |
| MUR5 | – | – | – | – | 614 | |
| MUR6 | – | – | 9.30±0.106 | – | 516 | |
Isolation of Endophytic fungi
In the present study, fungal strains were isolated from leaves and twigs of Melochia umbellata. A total of 12 fungi was isolated. Four most active isolates from antagonist test which showed the biggest inhibition zone were fermented. The active endophytic fungi were fermented for 21 days using PDY to obtain the secondary metabolites. The fermentation broth or supernatant was extracted with ethyl acetate and the biomass was extracted with methanol. Screening of endophytic fungi to determine antibacterial activity was executed by agar well diffusion method against 4 pathogenic human bacteria (Escherichia coli, Shigella dysentriae, Pseudomonas aeruginosa, and Vibrio cholerae).
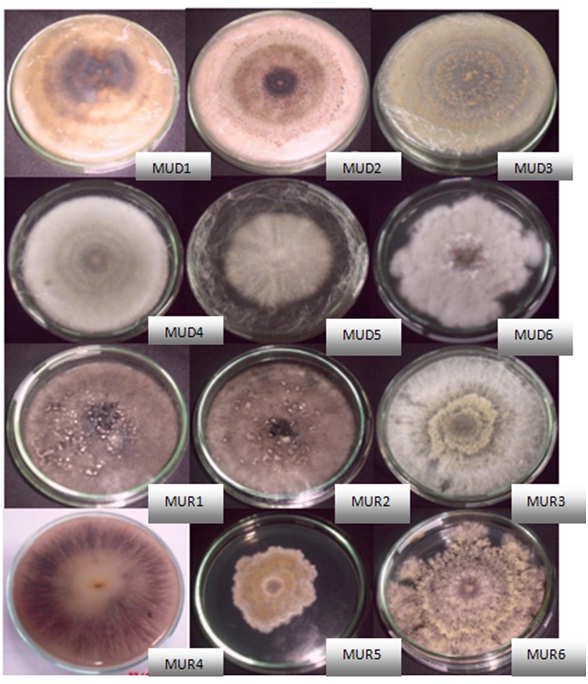 Fig. 1. Endophytic Fungi Isolates
Fig. 1. Endophytic Fungi IsolatesExtraction of isolates in 200ml PDY medium resulted in 94 mg ethyl acetate extract from MUD5 isolates, 116mg extract MUR4, 93mg extract MUR5 and 96 mg extract MUR6. The methanol extract obtained were 257mg from MUD5 isolates, 97mg extract MUR4, 614mg extract MUR5 and 516 mg extract MUR6.
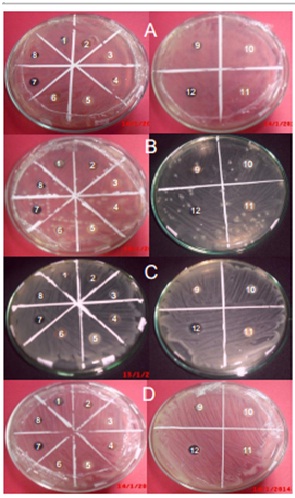 A. Antagonist test against Escherichia coli
A. Antagonist test against Escherichia coliB. Antagonist test against Pseudomonas aeruginosa
C. Antagonist test against Shigella dysentriae
D. Antagonist test against Vibrio cholera
1-12 are the 12 endophytic fungi isolate examined in the antagonist test
1 : MUD1 7 : MUR1
2 : MUD2 8 : MUR2
3 : MUD3 9 : MUR3
4 : MUD4 10 : MUR4
5 : MUD5 11 : MUR5
6 : MUD6 12 : MUR6
Fig. 2. Antagonist test result
In antibacterial activity, MUD5 showed high inhibition zone, 8,08 mm against E. coli, 10,51 against S. dysentriae, 7,81mm against P. aeruginosa, 9,00 mm against V. Cholerae. Diameter of inhibition zone of MUR4 was 8,61mm against E. Coli, MUR5 was 7,54mm against E. Coli, and MUR6 was 7,44mg against P. Aeruginosa. While from methanol extracts were obtained MUR6 with inhibition zone 9,3mm against P. Aeruginosa.
Phytochemical identification were carried out for all the active extracts and showed that ethyl acetate extract of MUD5, MUR4, MUR5, MUR6 contained alkaloid compound based on red spot formation on thin layer chromatography after spraying of Potassium Bismuth Iodide solution. Flavonoids content of all active extracts showed by the light green spot on uv 366 after being sprayed with sitroboric. From the preliminary phytochemical screening also revealed the presence of steroids in ethyl acetate extract of MUR6 based on the formation of red spot after the spot being sprayed with H2SO4 10%. (Figure 3).
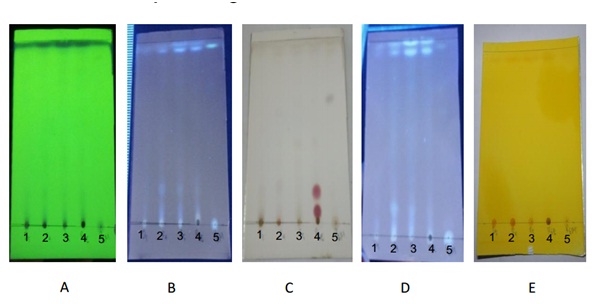 A. UV 254 1. Ethyl acetate extract of MUD5
A. UV 254 1. Ethyl acetate extract of MUD5B. UV 366 2. Ethyl acetate extract of MUR4
C. H2SO4 10% 3. Ethyl acetate extract of MUR5
D. Sitroboric 4. Ethyl acetate extract of MUR6
E. Dragendorf 5. Methanol extract of MUR6
Fig. 3. Phytochemical identification
Various species of endophytic fungi made an ecological niche in the inner space of plants. The fungi interact with their environment in a positive manner in the role of improving plant defense and disease control. Endophytic fungi isolation from medicinal plant results in the production of bioactive metabolites which has a great activity against microbes. Hence, scaling up the production of bioactive metabolites is necessary to fulfill the demand of agriculture and pharmaceutical industries. In this study, we have isolated 12 endophytic fungi from Melochia umbellata. Ethyl acetate extract from fungi isolate (MUD5) shows activities against E. coli, S. dysentriae, P. aeruginosa, and V. cholerae. MUR6 produce active secondary metabolites against P. aeruginosa, both in the supernatant and the biomass. Phytochemical identification showed that all active extract consist of alkaloids, steroids and flavonoids compound.
The present investigation was an attempt to search for potential endophytic fungi from Melochia umbellata. The study revealed the active antibacterial extracts which were extracted with ethyl acetate and methanol. Four active ethyl acetates and 1 active methanol extract were examined for their antibacterial activities based on their inhibition zone diameters. Therefore, further determination of active metabolites content in the extract needs to be investigated quantatively and completely. Besides that, TLC bioautography need to be developed in case to investigate the active antibacterial fraction.
- Sandhu SS, Kumar S and Aharwal RP. Isolation and Identification of Endophytic Fungi from Ricinus Communis Linn. And Their Antibacterial Activity. International Journal of Research in Pharmacy and Chemistry, 2014; 4(3): 611-618.
- Selim KA, El-Beih AA, AbdEl-Rahman TM, and El-Diwany AI. Biology of Endophytic Fungi. Current Research in Environmental & Applied Mycology. 2012; 2(1): 31–82.
- Wullur S, Firdaus, Natsir H, and Soekamto NH. Study of Compounds from Extract of Melochia umbellata (Houtt.) Stapf var. Degrabrata K. (Paliasa) Leaves that has Potential as Antibacterial. Indonesia Chimica Acta. 2015; 8(1).
- Selvi KB & Balagengatharathilagam P. Isolation and Screening of Endophytic Fungi From Medicinal Plants of Virudhunagar District for Antimicrobioal Acitivity. International Journal of Science and Nature. 2014; 5(1): 147-15.
- Dasale MG and Bodhankar MG. Animicrobial Activity of Endophytic Fungi Isolated From Vitex negundo Linn. International Journal of Current Microbiology and Applied Sciences. 2013; 2(12): 389-395.
- D‘Sauza MA and Hiremath KG. Isolation and Bioassay screening of Medicinal Plant Endophytes From Western Ghats Forest, Goa, India. Interntional Journal of Advanced Research in Biological Sciences. 2015; 2(8): 176-190.
- Pavithra N, Sathish, and Ananda K. Antimicrobial and Enzyme Activity of Endophytic Fungi Isolated from Tulsi. Joutnal of Pharmaceutical and Biomedical Scienes. 2012; 16(12) : 1-2.
- Verma SK, Kumar A, Ashish, Debnath M. Antimicrobial Activity of Endophytic Fungal Islate in Argemone Maxicana; A Traditional Indian Medicinal Plant. International Journal of Innovative Research in Science. Engineering and Technology. 2014; 3(3): 10152.
- Muzzamal H, Sarwar R, Sajid I, and Hasnain S. Isolation, Identification and Edophytic Bacteri Antagonistic to Biofilm Former. Pakistan Journal of Zoology. 2012; 44(1): 250.
- Polonio JC, Almeida TT, Garcia A, at al. Biotecnological Prospecting of Foliar Endophytic Fungi of Guaco (Mikania glomerata Spreng.) with Antibacterial and Antagonistic Activity Against Phytopathogens. Genetic and Molecular Research. 2015; 14(3): 7293-7309. DOI http://dx.doi.org/10.4238/2015.July.3.5
- Tiwari P, Kumar B, Kaur M, et al. Phytochemical Screening and Extraction : A Review. Internationale Pharmaceutica Sciencia. 2011; 1(1) : 98-106
- Huwaitat S, Al-khateeb E, Finjan S. Isolation and Identification of some Phytochemical Compunds from Different Parts of Iris Nigricans. Europan Scientific Journal. 2013; 9(6) : 32-37.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


