ISSN: 0973-7510
E-ISSN: 2581-690X
Bio-ethanol used today is mainly produced from sugar cane and cereals, but reducing the production costs of ethanol is still crucial for a viable economic process. Cellulose from plant biomass will be the next cheaper raw material for second generation fuel ethanol production. Saccharomyces cerevisiae and Schizosaccharomyces pombae are the common industrial yeast strains used for the production of ethanol from various substrates derived from agricultural commodities. In the present study, ethanol was produced from various substrates such as molasses, sugarcane juice, sugarcane syrup, jaggery, grapes, beet root, orange and rice substrates by using Saccharomyces cerevisiae and Schizosaccharomyces pombae. The experimental study shows that the biomass production declines progressively when the ethanol accumulates in the surrounding medium after the production of ethanol. However, the conversion of ethanol was achieved in batch fermentation process at optimum temperature and the internal pH of the fermentation medium at laboratory level. The yield percentage of ethanol for the above substrates were analyzed and compared for different carbon sources. Among different substrates, Jaggery and Molases showed higher ethanol yield as 95.2% and 93.5% respectively. Among the two species of yeast studied, Saccharomyces cerevisiae yielded higher percentage of ethanol than Schizosaccharomyces pombae in batch fermentation process. The present study concludes the effect of various carbon sources on production of ethanol with two different industrial yeast strains such as Saccharomyces cerevisiae and Schizosaccharomyces pombae are vary in similar conditions of ethanol production process.
Ethanol fermentation, Saccharomyces cerevisiae, Schizosaccharomyces pombe, molasses, cane juice, rice, grapes, amylase, beetroot and Biofuel.
Ethanol has been used by human since prehistorically as the intoxicating ingredient in alcoholic beverages and used as solvents, in perfumes, paints and in medical applications (Kothari’s and Sons, 1987). During the last few years, there has been an increasing interest and demand in using ethanol as a substitute for fossil fuels and various other industrial applications Report of the committee of technical (Experts on Alcohol and Alcohol Based Industries, 1980; All India distillers association, 1997 – 1999). Ethanol production is among the oldest technology and is produced commercially by fermentation of molasses, cereal grains and other materials with high starch and/or sugar contents (Mahalingam, 1977; Yadav et. al., 1997a, 1997b). The fermentation process involves conversion of sugars to alcohol and carbon dioxide by the action of yeast species. The great majority of ethanol produced in the world is from sugarcane molasses (Buzz et. al., 1989) and the common strains of yeast used are Saccharomyces cerevisiae, Saccharomyces ellipsoids, Saccharomyces carlsbergensis, Saccharomyces fragilis, Schizosaccharomyces pombe and Saccharomyces ovaru (Kapadnis, 1999). The bacteria include Zymomonas mobilis also can also be used for alcoholic fermentation. In India, Production of ethanol is exclusively from cane molasses and every tone of sugar produce 190L of molasses (50-55% sugar) and one ton of molasses produced 280L of ethanol (Rao, UB, 1996).
There are many reports shows the production of ethanol from various fruits and its products worldwide. The production of ethanol from Orange fruits were achieved and Orange based ethanol is used as a Biodiesal (Sebastian Blancol, 2008). Grapes contain glucose and fructose are the major sugar components which is used to produce ethanol (Morris et. al., 1996). Saccharomyces Cerevesiae strains overcome osmotic stress and to yield ethanol fermenting high sugar concentration of grape in wine making and the wine making industry can use high sugar concentration musts (Malacrinò et. al., 2005). Sapota fruits also be used for ethanol fermentation. The composition of Sapota juice revealed that it is one of the rich sources of sugars, proteins, ascorbic acid, phenolics, carotenoids and minerals like iron, copper, zinc, calcium and potassium which can be used for the production of ethanol (Anand et. al., 2007).
All micro-organisms including ethanol producing yeasts, display an optimum growth temperatures which is usually close to the maximum temperature allowing growth of cells. The fermentation of molasses was optimized with respect to temperature, pH and sugar concentration and the results revealed a temperature of 30°C, pH 6.0 and 20% sugar concentration as optimum for fermentation (Anoop Verma, 2006). The temperature and pH for maximum were found to be 30°C, and pH 5 which is also optimum temperature and pH for the growth of the organism (Benerji et. al., 2010). The pH of the media is generally made acidic for yeast (4.5-5.5) growth and production of ethanol achieved. During batch fermentation, the rate of ethanol production per milligram of cell protein is maximal for a brief early period and declines progressively as ethanol accumulated in the surrounding broth (Dombek and Ingram, 1987). However, with the gradual development of beet sugar and cane sugar factories throughout the world, production of ethanol from molasses is the cheapest raw material; hence cellulose hydrolysis cannot compete with these. Sugar cane, sweet sorghum and cassava are considered to be the efficient converters of solar energy into stored energy in the form of sugar and carbohydrates and are ideal crops for the production of ethyl alcohol from renewable sources of energy (Mathur, 2002). Hence these are called energy crops. Sugar, carbohydrates and cellulose are products of photosynthesis and known as biomass and can theoretically converted into ethyl alcohol (Alcohol based industries, 1981). In view of the expected global shortage of petroleum products and the advantages ethyl alcohol to replace gasoline, it may be imperative for all the countries to think of producing ethyl alcohol from any agricultural raw materials, as economically as possible, particularly to reduce the dependence on other countries.
Therefore, the present study is carried out with the objectives of studying the yield and efficiency of the alcohol fermentation by using various carbon substrates like molasses, sugarcane juice, sugarcane syrup, jaggery, grapes, orange juice, beetroot mash and rice in the laboratory scale level. The yield of alcohol and fermentation efficiency by different yeast strains like Saccharomyces cerevisiae and Schizosaccharomyces pombe were compared for future process development and economical viability of alcohol production. In addition to the alcohol production efficiency, the fermentable sugars and non-fermentable sugars also analyzed for comparison.
Chemicals, Glassware and Lab procedures
The chemicals and reagents used in the present study were analytical grade of Emerck, Loba and BDH purchased from licensed Scientific companies, Chennai. The glassware including Distillation apparatus, conical flask, beakers, test tubes, boiling tubes, eppendorf tubes, measuring jars, micropippetes etc. used in the present study are belong to Borosil.
The glassware were first soaked in chromic acid solution (10% Potassium dichromate solution with 25% Con. Sulphuric acid) for few hours to remove tough residues and washed with tap water subsequent to rinsing with distilled water. After draining the water completely, it was dried in a drying chamber at 80oC and cooled before it being taken for further use in the experiments (Mahadevan and Sridhar, 1996). The general laboratory and microbiological methods used in the present study is followed after (Purvis et. al., 1996). Sterilization of culture media, glassware and miscellaneous articles were carried out in an autoclave at 121oC, 15 psi for 15 minutes. All the microbial culture and fermentation experiments were conducted under laminar air flow inoculation chamber with strict aseptic conditions. However, the glassware was sterilized by using hot air oven at 120oC for 3hrs period.
Yeast Cultures and enzymes
The yeast species used in the present study for production of alcohol were Saccharomyces cerevisiae and Schizosaccharomyces pombe which were obtained from E.I.D Parry (India) Ltd. Nellikuppam, Cuddalore District, Tamil Nadu, India. The Potato dextrose agar (PDA) slants were used for propagation and preservation of yeast cultures in the laboratory for the experimental study. The enzyme á – Amylase obtained from Meera Labs (p) Ltd., Chennai was used for hydrolysis of starch present in rice.
Sample Substrates for analysis
Various carbon sources containing substrates used in the present study are molasses, sugarcane juice, sugarcane syrup, jaggery, grapes, beet root, orange and rice substrates for the production of alcohol by using above yeast species. The substrates were analysed for its total reducing sugars for the alcohol producing capacity and non-fermentable sugars.
Determination of total reducing sugars / Fermentable sugars (Lane Eynon, 1923)
The chemical method for invert sugar estimation depends on properties of reducing sugars as glucose and fructose to reduce copper in the cupric state (Cu2+) to cuprous (Cu+) in alkaline solutions as mentioned in the following procedure.
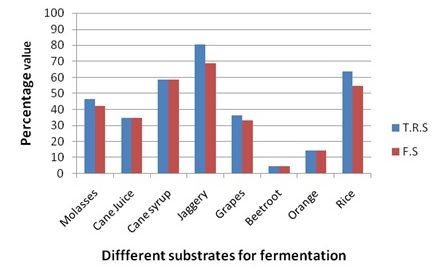
Graph 1. Details of Total reducing sugars (TRS), Fermentable sugars (FS) of different substrates
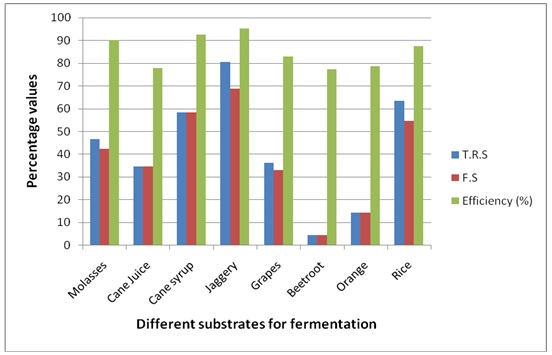
Graph 2. Details of Total reducing sugars (TRS), Fermentable sugars (FS) and alcohol effieciency (%) of substrates fermented by Sacharomyces cerevieseae
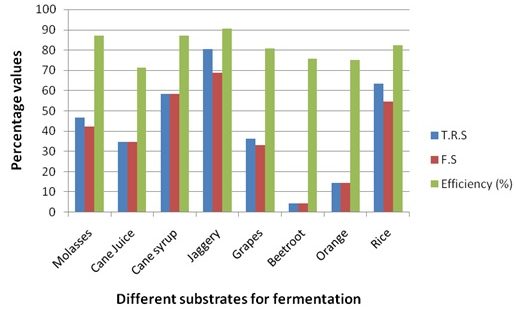
Graph 3. Details of Total reducing sugars (TRS), Fermentable sugars (FS) and alcohol effieciency (%) of substrates fermented by Sizosacharomyces pombe
Fehling’s solution (A and B)
- Fehling’s solution was prepared by mixing equal volumes of (i) and (ii) just before use.
- Copper sulphate solution (A): Exactly 34.639g of Copper sulphate (CuSo4, 5H2O) was dissolved in water and 0.5 ml of concentrated sulphuric acid of specific gravity 1.84 was added. This solution was diluted to 500 ml in a volumetric flask and filtered.
- Alkaline tartarate solution (B): Rochelle Salt or Potassium sodium tartarate (KNaC4H4O64H2O) 173 g and 50g of sodium hydroxide (NaOH) were dissolved in water and diluted to 500 ml. It was let to stand for 2 days and filtered.
- Invert sugars stock solution (1%): About 9.5g of pure sucrose was weighed in a standard flask. To this 5ml of concentrated H2SO4 was added and diluted with water of about 100ml. This was left to stand for 3 days at 20 to 25oC and then diluted to 1000ml.
- Working standard solution: About 50 ml of the stock solution of invert sugar was pipetted out in a 250 ml volumetric flask. It was neutralized carefully with NaOH of about 1% (w / v) and made up to the volume.
- Methylene blue indicator solution: Methylene blue indicator (1%) was prepared in distilled water.
- Sodium hydroxide solution (6N): About 60 g of sodium hydroxide was dissolved in 250ml of distilled water.
- EDTA solution: About 40g of ethylene di-amine tetra acetic acid (EDTA) was dissolved in 1000 ml of distilled water.
- Amylase enzyme: Liquid Amylase enzyme was used with sufficient concentration to convert starch into maximum reducing sugars.
Working standard preparation
The working standard invert sugar solutions were poured into a 50ml burette. Exactly 10ml of Fehling’s solution was pipetted out into a 250ml Erlenmeyer flask. The standard invert sugar solution from the burette was titrated against the flask containing Fehlings solution, in order to obtain reduction of all copper. The sugar solution from the burette was added drop by drop till the blue colour of the solution just disappears and appearance orange to brick red colour. The titer was noted and the values were plotted in the graph and working standard curve was prepared for comparison of sample values.
Preparation of samples
About 12.5g of individual substrates were weighed accurately and transferred to a 250ml volumetric flask. To this 40ml of EDTA solution was added and made up to the volume of 250ml and mixed well. From this solution 100 ml was taken in a 500ml volumetric flask and made up to volume with water and mixed.
From the above sample, 50ml was taken in a 100ml volumetric flask, to this 2.5ml of concentrated HCL was added. The flask was kept in the water bath with constant agitation till the temperature raised to 70oC and allowed to keep for 5 minutes. Then the flask was kept in cold water at 20oC till the contents reached to 35oC. Then about 2 – 3 drops of phenolphthalein was added to the flask and neutralized the solution with 6N sodium hydroxide till the solution turned slightly pink. The flask was kept at 20oC for 30 minutes and made up to the volume with water and mixed the solution well.
Preparation of Sample solution of rice
Twenty gram of rice was mixed in 100ml of distilled water and 2ml of amylase enzyme was added. The solution was stirred well and added about 900ml of gently boiling water in a large beaker and cooked well. The cooked solutions were mixed well and the gelatinized starch solutions were kept in room temperature for hydrolysis reaction. Then the hydrolyzed solutions were used as invert solutions of rice substrate for analysis of reducing sugars (Dalel Singh et. al., 2007).
Method of Titration in samples
The diluted inverted solutions were taken in 50ml burette individually. About 10ml of Fehling’s solution was pipetted out in a 250ml conical flask and enough quantity of water was added to it. The contents of the flask was heated and allowed to boil for 1 to 2 minutes. Then the sample solutions containing invert sugar from the burette was added in small quantities and the liquid was allowed to boiled, till the blue colour disappeared. As the original orange to brick red colour reappears the titer value was noted.
Calculation
Total reducing sugars in 100ml of sample = Fehling’s factor (F) X 100 X 2500 mg/ Titer x Weight of substrate taken
Determination of Non-fermentable sugars (Lane Eynon, 1923)
Non fermentable sugars are the fraction of dissolved solids of the molasses and other substrates which cannot be fermented. The yeast utilizes all the fermentable sugars during exhaustive fermentation. The sugars, which are not utilized during alcohol fermentation, are determined by the method mentioned earlier for determination of reducing sugars.
Preparation of samples for estimation of Non-fermentable sugars
All the substrates like Molases, Sugarcane juice, sugarcane syrup, Jaggery, Grapes, Beet root, Orange and Rice after yeast fermentation for alcohol production were taken for analysis of non-fermentable sugars. The above substrates were weighed and converted into reducing sugars by acid hydrolysis as mentioned earlier of sulphuric acid and the samples were prepared for further titration by Fehling’s method.
Table (1):
Details of Total reducing sugars, Non-fermentable sugars and Fermentable Sugars of various substrates.
Sl. No. |
Substrate |
T.R.S |
N.F.S |
F.S |
|---|---|---|---|---|
1 |
Molasses |
46.61 |
4.27 |
42.34 |
2 |
Cane Juice |
34.76 |
Nil |
34.76 |
3 |
Cane syrup |
58.5 |
Nil |
58.5 |
4 |
Jaggery |
80.4 |
11.6 |
68.8 |
5 |
Grapes |
36.26 |
3.2 |
33.06 |
6 |
Beetroot |
4.5 |
Nil |
4.5 |
7 |
Orange |
14.5 |
Nil |
14.5 |
8 |
Rice |
63.5 |
8.8 |
54.7 |
Preparation of sample solution with invert sugars
Exactly 12.5g of substrate was weighed and 150 ml of distilled water was added to it. About 15ml of amylase is added and the pH was adjusted to 4.5- 4.6 with diluted acid. The hydrolysed solution was transferred to 250 ml flask. Forty ml of EDTA was added to the flask and the solution was made up to 250 ml with distilled water.
Method of Titration
The titration of above prepared samples for determination of reducing sugars was done by the method used as earlier and calculated.
Calculation of Fermentable sugars
The difference between total reducing sugars (TRS) and non-Fermentable sugar (NFS) gives the value of total fermentable sugars.
Total fermentable sugars = Total reducing substances – Non Fermentable sugar
The fermentable sugar content of substrates was very important in determining the yield and the efficiency of fermentation.

Plate 1. Beetroot Fermentation

Plate 2. Rice Fermentation Plate

Plate 3. Grape Fermentation

Plate 4. Cane Juice Fermentation

Plate 5. Orange Fermentation

Plate 6. Molasses Fermentation

Plate 7. Distillation of fermented broth
Alcohol Fermentation
Different substrates like Molases, Sugarcane juice, sugarcane syrup, jaggery, Grapes, Beet root, Orange juice and Rice were taken for fermentation of alcohol by both the yeast species like S. cereviseae and S. bombe. Eight different 500 ml conical flasks were taken and filled with 200ml of distilled water. To these, about 30 gm of substrate was dissolved in each conical flask and mixed well. The cooked rice was weighed about 30 g and 200 ml of water was added. About 10ml of 48 hour old yeast culture suspension was added and stirred well. Then the individual flasks containing all the contents for fermentation were incubated at 34oC for 48 – 72 hours. After the incubation, the fermented broth was analyzed for the yield of alcohol by various tests.
Distillation and recovery of Alcohol
Exactly 150ml of fermented sample broth was measured in a volumetric flask. The content was transferred to 500ml flat bottomed flask. The standard flask was rinsed with 25ml of distilled water twice and combined the washings with sample. The flask was connected to the still head and condenser. About 125ml of the sample was distilled out and the distilled sample was made up to 150ml with distilled water.
Determination of Ethanol by Skye’s Hydrometer
The ethanol content was determined as a function of the density of the distilled fluid. The distilled fluid contains some ethanol which correspondingly reduces the density of the mixture of water and ethanol that is distilled out.
The distillate was poured into 250 ml measuring cylinder. Sykes hydrometer was slowly put into the cylinder. The reading was rated on the hydrometer corresponding to the lower meniscus. The temperature was rated with the help of Fahrenheit thermometer and the strength was found out from the table.
Calculation
The strength obtained from the sykes hydrometer is in proof table. Proof is multiplied by 0.571 to get strength in % V/V.
Ethanol content = Proof x 0.571
Determination of Efficiency
Expected value = ((Weight of substrate taken x Fermentable sugar )/100) X 0.644
Observed value = Ethanol % X Total volume/100
Efficiency = Observed value/Expected value
Table (2):
Alcohol Content and Efficiency of Various Substrates using Saccharomyces cerevisiae.
Sl. No. |
Substrate |
Fermentable Sugars |
Alcohol (%) |
Efficiency (%) |
|---|---|---|---|---|
1 |
Molasses |
42.34 |
3.82 |
90.20 |
2 |
Cane Juice |
34.76 |
2.61 |
77.90 |
3 |
Cane syrup |
58.50 |
5.22 |
92.50 |
4 |
Jaggery |
68.80 |
6.32 |
95.20 |
5 |
Grapes |
33.06 |
2.65 |
83.00 |
6 |
Beetroot |
4.50 |
3.36 |
77.30 |
7 |
Orange |
14.50 |
1.10 |
78.70 |
8 |
Rice |
54.70 |
4.62 |
87.60 |
Table (3):
Alcohol Content and Efficiency of Various Substrates using Schizosaccharomyces pombe.
Sl. No. |
Substrate |
Fermentable Sugars |
Alcohol % |
Efficiency (%) |
|---|---|---|---|---|
1 |
Molasses |
42.34 |
3.68 |
86.95 |
2 |
Cane Juice |
34.76 |
2.48 |
71.30 |
3 |
Cane syrup |
58.50 |
5.10 |
87.17 |
4 |
Jaggery |
68.80 |
6.22 |
90.40 |
5 |
Grapes |
33.06 |
2.57 |
80.66 |
6 |
Beetroot |
4.50 |
3.29 |
75.80 |
7 |
Orange |
14.50 |
1.05 |
75.00 |
8 |
Rice |
54.70 |
4.34 |
82.20 |
Determination of Fermentable and non-fermentable sugars of substrates
The total reducing sugar content of Jaggery was very high 80.4% followed by Rice (63.5%), Cane syrup (58.5%), Molases (46.6%), Cane juice (34.8%), Grapes (36.3%), Orange (14.5%) and Beet (4.5%) root. The Non-fermentable sugars which are determined after completion of alcohol fermentation by yeast observed to be Jaggery (11.6%) followed by rice (8.8%), Molases (4.27%) and Grapes (3.2%). Other substrates like Cane juice, cane syrup, Beet root and orange not contain any reducing sugars after fermentation which is considered to be non-fermentable sugars. The amount of non-fermentable sugars subtracted from total reducing sugars designated as fermentable sugars which are in the range of Jaggery 68.8%, Cane syrub 58.5%, Rice 54.7%, Molases 42.34%, Can juice 34.76%, Grapes 33.06%, Orange 14.5% and Beet root 4.5% respectively. The amount of total reducing sugars, non-fermentable sugars and fermentable sugars of various substrates are indicated in Table 1.
The ethanol fermentation process is depending on environmental parameters like temperature, pH of the substrate and nutrients and cellular characteristics like metabolism of the organisms used. It is needed to understand the physiological regulation of yeast for ethanol production and has been studied for many years (Elena Patrascu et. al., 2009). Therefore, the present study carried out to explain suitable carbon substrates and strains for the maximum ethanol production. The fermentation efficiency is based on the carbon substrates used in the fermentation process and the yeast convert the sugar molecules in to ethanol which showed in the present study as the Jaggery, Cane syrup, Molases and rice contain higher fermentable sugars yielded higher percentage of alcohol respectively. Although the rice showed higher carbohydrate content, the yield percentage of alcohol is not comparatively higher than Jaggery, Cane syrup and Molases. This variance is based on the total reducing sugars (TRS) and fermentable sugars present in each carbon substrates and also the efficiency of the yeast utilizing the sugars for fermentation.
Among raw cane syrup and Cane juice, Cane syrup showed more than 90% of efficiency on inoculation with Saccharuomyces cerevisiae and also Schizosaccharomyces pombe in ehanol production. This may be due to the presence of other materials including additives which may not favour for complete conversion of alcohol by yeast fermentation. Considering the effect of fruits, grapes showed more than 80% efficiency in ethanol production than orange fruits which might be the grapes contain larger amount of reducing sugars which was mostly converted into ethanol. Among Jaggery and Molasses used for fermentation, Jaggery showed more than 93% efficiency than molasses which contain slightly higher residual sugars. The usage of cheapest raw material like Cellulose from vegetable biomass and agricultural by-products with a low commercial value, as corn stover, corn fiber and cane bagasses would become an attractive feedstock for bioethanol production (José Duarte et. al. 2009). Even though it showed better efficiency and better yield, molasses is commercially used as carbon substrate in many industries because it is comparatively less expensive and yields more than 90% efficiency i.e. 4-6% alcohol content (Rao, UB, 1996). .
During batch fermentation, the rate of ethanol production per milligram of cell protein is maximal for a brief period early in this process and declines progressively as ethanol accumulated in the surrounding broth. This study demonstrates that the removal of this accumulated ethanol that the decline in metabolic rate is due to physiological changes rather than to the presence of ethanol. Several potential causes for the decline in fermentative activity have been investigated (Belkis Caylak and Fazilet Vardar Sukan, 1998). Viability of cell products remained at or above 90% internal pH remained near neutrality, and the specific activities of the glycolytic and alchologenic enzymes remained high throughout batch fermentation. None of these factors appears to be causally related to the fall in fermentative activity during batch fermentation. (Dombek and Ingram, 1987).
When comparing the Yeast strains used, Saccharomyces cerevisiae is having higher efficiency than Schizosaccharomyces pombe in batch fermentation of ethanol production. It produced almost 80% and above efficiency for alcohol production in all carbon substrates used. Rice is produced about 82% and 85% ethanol by both the strains Saccharomyces cerevisiae and Schizosaccharomyces pombe respectively and S. cerevisiae is having higher portential of alcohol fermentation with rice. The growth and metabolism of yeast species may vary which was reported as Saccharomyces cerevisiae reproduce through budding while Schizosaccharomyces pombe reproduce through cell fission by the formation of crosswall (septum) in which the incubation time of S. Cerevisiae yield better results only in batch fermentation and cannot be recycled after fermentation, while S. pombe gives better results in continuous fermentation and it can be recycled for further fermentations (Chardhary and Chincholkar, 1996; Hafiz O. Abubaker et. al., 2012). However, both the strains are suitable for fermentation of ethanol in individual process of fermentation technology.
In recent years, the fermentation of alcohol with cane molasses, grapes and other fruits such as orange having increased fermentable sugars are reported. In the manufacture of sugars, molasses the valuable by- product of sugar industry is considered as a waste product; hence molasses becomes a greatest disposable problem for sugar industries. However molasses can be utilized as the raw material for the production of ethanol, which is a very versatile chemical and has great demand all over the world.
This study demonstrates that the removal of this accumulated ethanol that the decline in metabolic rate is due to physiological changes rather than to the presence of ethanol. Several potential causes for the decline in fermentative activity have been investigated. Even though S. cerevisea giving higher yield, it cannot be used for continuous fermentation as it is unable to recycle for further fermentation, Schizosaccharomyces pombe is mostly used for continuous fermentation which gives moderately higher yield and having many advantages. Because of increasing demand for fuel ethanol, there is a need to search for high yielding carbon substrates and easily accessible technology for the production of ethanol at reduced cost.
ACKNOWLEDGMENTS
The Authors were thankful to The General Manager, EID Parry sugars (p) Ltd., Nellikuppam, Cuddalore, Tamil Nadu for providing laboratory facilities and permission to carry out their final year project in their Sugar Industry.
- Alcohol Based industries, 1981. Annual Report.
- All India Distilleries association, News letter 1996, 1997, 1998 and 1999.
- Anand P. Kulkarni, R.S. Policegoudra, and S.M. Aradhya, 2007. Chemical Composition and Antioxidant Activity of Sapota (Achras Sapota Linn.) Fruit. Journal of Food Biochemistry, 2007; 31: 399–414.
- Anoop Verma, Process optimization for the production of ethanol via fermentation a dissertation Submitted in the partial fulfillment of the requirements Dissertation for the award of degree of Masters of Science In Biotechnology, Department of biotechnology and env. sciences thapar institute of Engg. & Technology (deemed university), Patiala 2006.
- Belkis Caylak and Fazilet Vardar Sukan, Comparison of Different Production Processes for Bioethanol, Turk J Chem. 1998; 22: 351 – 359.
- Benerji, DSN, K. Rajini, B. Srinivasa Rao, D.R.N. Banerjee, K. Swaroopa Rani, G. R ajkumar and C. Ayyanna, 2010. Studies on Physico-Chemical and Nutritional Parameters for the Production of Ethanol from Mahua Flower (Madhuca indica) Using Saccharomyces Cerevisiae – 3090 Through Submerged Fermentation (smf). Journal of Microbial & Biochemical Technology – Open Access, JMBT/Vol.2 Issue 2.
- Buzaz ZS, Dallman and Szajani B, Best Alternatives Renewable Raw Material for Alcohol Production by R.A Yates Biotech. and Bioeng (1989), 34:882.
- Chardhary, A.B and Chincholkar, S.B. A critical approach to ethanol production by saccharomyces cerevisiae and schizosaccharomyces pombe, Indian Journal of Microbiology, 1996; 75-83.
- Dalel Singh, Jagroop S. Dahiya and Poonam Nigam, Simultaneous raw starch hydrolysis and ethanol fermentation by glucoamylase from Rhizoctonia solani and Saccharomyces cerevisiae, 2007; DOI: 10.1002/jobm.3620350209.
- Dombek KM and Ingram Lo, Jun; Ethanol production during batch fermentation with Scervisiae; changes in Glycolytic enzymes and internal pH; Appl Environ Microbial, 1987; 53(6): 1286-91.
- Elena Patrascu, Gabriela Rapeanu, Traian Hopulele, 2009, Current approaches to efficient biotechnological production of ethanol, Innovative Romanian Food Biotechnology 4, Issue of March, 2009, Pp. 1-11.
- Hafiz O. Abubaker, Abdel Moneim E. Sulieman, Hassan B. Elamin, 2012. Utilization of Schizosaccharomyces pombe for Production of Ethanol from Cane Molasses, Journal of Microbiology Research 2012, 2(2): 36-40.
- José Duarte, Vera Lourenço, Belina Ribeiro, Maria Céu Saagua, Joana Pereira and Lina Baeta-Hall. Ethanol Production from Different Substrates by a Flocculent Saccharomyces cerevisiae Strain, BioEnergy, 2009; 7: A58.
- Kapadnis, BP, 1999. Yeast Systematics – Department of Microbiology, University of Pune, New Letter, March 1999.
- Kothari and Sons, 1987. Kothari’s Economic and Industrial Guide of India 1979-80; 1980-81. Ghanshyamdas, C.B. Madras, 1987. Pp. 181.
- Lane JH and Eynon L,(1923), Soc.Chem Eng 42:327.
- Mahadevan, A and R. Sridhar [1996] Methods on physiological plant pathology (4th Edition). Sivakami Publications, Chennai.
- Mahalingam, PR, 1977. Manufacture of Industrial Alcohol, Chemical Age of India, Vol.28: Issue 4.
- Malacrinò, P, E. Tosi, G. Caramia, R. Prisco and G. Zapparoli, The vinification of partially dried grapes: a comparative fermentation study of Saccharomyces cerevisiae strains under high sugar stress. Letters in Applied Microbiology, 2005; 40(6): 466–472.
- Morris, J. R., Main, G. and Threfall, R. Fermentations: Problems, Solutions and Preventions. Vitic. Enol. Sci. 1996; 51(3), 210-213. Fraund Verlag, Mainz.
- Purvis, M. J., D. C. Collier, and D. Walls. 1966. Laboratory techniques in Botany. Second edition. Butterworth Company Ltd., London, Pp. 439.
- Rao, UB, 1996. New Development s in the Manufacture of Alcohol from Molasses. All India Distilleries association, News letter 1996.
- Report of the committee of technical Experts on Alcohol and Alcohol Based Industries; Ministry of Petroleum, Chemicals and Fertilizers, Department of Fertilizers, Government of India, New Delhi, January 1980.
- Sebastian Blancol, 2008. Autolog Green for 02.28.08. Febraury 28th , 2008. Biodiesel.
- Yadav A, Dilbaghi N and Sharma S. Pretreatment of sugarcane molasses for ethanol production by yeast. Indian j of Microbiol. 1997a; 37: 37-40.
- Yadav BS, Anita Sheoran, Usha Rani and Dalel Singh. High ethanol productivity in an immobilized cell reactor Indian J Microbiol, 19997b; 37: 65-67.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


