ISSN: 0973-7510
E-ISSN: 2581-690X
Twenty four adult female of Swiss white mice (aged 60 days each) and weighing 42-48gm were selected for this experimental study. They were divided into four groups (4 mice each) A, B, C, and D inoculated with mammary gland carcinoma and irradiated with laser, they were considered as irradiated groups of mice. The remaining animals were divided also into four groups a, b, c, and d inoculated with mammary gland carcinoma not irradiated with laser, they were considered as control groups of mice. The laser of 650nm, 50 mw power, 25 mw/cm2 power density was focused on the pancreas of the targeted mice which represent irradiated groups. The laser caused a reduction in the damage that occurred in the architecture of the cells of exocrine pancreas because of the negative effects caused by cancer on the mentioned cells such as decreased in size, dense fragmentation of the content of the nucleus, and dark appearance of the cytoplasm which represent slight degenerative alterations. In the same time, the laser caused a decrease in the mean of the apoptotic cells of the exocrine pancreas gradually for the same reason that above mentioned. Whereas in the control groups of mice, the mean of apoptotic cells of the exocrine pancreas displayed a gradual increase because the cancer caused activation in cellular apoptosis. Besides, there were diagnostic markers in the examination of the histology of the cells of exocrine pancreas which showed that there were abundant degenerative alterations in the architecture of the cells such as decreased in size, dense fragmentation of the content of the nucleus, and dark appearance of the cytoplasm because of the suppressive activity of the cancer which caused these damages in the architecture of the cells.
Apoptosis, Pancreas, Laser in medicine
The cells of a multicellular organism are members of a highly organized community. The number of cells in this community is tightly regulated—not simply by controlling the rate of cell division, but also by controlling the rate of cell death. If cells are no longer needed, they commit suicide by activating an intracellular death program. This process is therefore called (programing cell death)1, although it is more commonly called apoptosis (from a Greek word meaning “falling off,” as leaves from a tree)2.
The amount of apoptosis that occurs in developing and adult animal tissues can be astonishing. In the developing vertebrate nervous system, for example, up to half or more of the nerve cells normally die soon after they are formed. In a healthy adult human, billions of cells die in the bone marrow and intestine every hour3, 4. It seems remarkably wasteful for so many cells to die, especially as the vast majority are perfectly healthy at the time they kill themselves5, 6.
The mechanism of low level laser therapy at the cellular level is based on the increase of oxidative metabolism of mitochondria, which is caused by electronic excitation of components of the respiratory chain (7). Lasers improve the flow of electrons through the respiratory chain to increase the mitochondria production of ATP without increasing the production of free radicals (8). Cell stress results in mitochondrial inhibition which reduces ATP energy output and increases free radical production or reactive oxygen species (ROS) to accelerate common signs of normal ageing9,10,11.
Low level laser therapy (LLLT) has been used successfully in biomedicine and some of the results are thought to be related to cell proliferation. The effects of LLLT on cell proliferation is debatable because studies have found both an increase and a decrease in proliferation of cell cultures12. Cell culture is an excellent method to assess both effects and dose of treatment13. In both soft tissue and connective tissue injuries, LLLT can increase the final tensile strength of the healed tissue. By increasing the amount of collagen production/synthesis and by increasing the intra and inter-molecular hydrogen bonding in the collagen molecules, laser therapy contributes to improved tensile strength14, 15.
Twenty four adult female of Swiss white mice (aged 60 days each) and weighing 42-48gm were selected to be the projects for this experimental study. They were divided into four groups (4 mice each) A, B, C, and D inoculated with mammary gland carcinoma and irradiated with laser, they were considered as irradiated groups of mice as shown in table (1). The remaining animals were divided also into four groups a, b, c, and d inoculated with mammary gland carcinoma not irradiated with laser, they were considered as control groups of mice as shown in table (2). The laser of 650nm, 25mw/cm2 power density was selected for this experiment, and the laser was focused on the pancreas of targeted groups of mice that mentioned in table (1) after anaesthetizing them. The duration time of irradiation with laser and the time interval were explained as in the following:
- Group A 1rradiated for 15 minutes twice daily with time interval of two hours.
- Group B irradiated for 20 minutes twice daily with time interval of two hours.
- Group C irradiated for 25 minutes twice daily with time interval of two hours.
- Group D irradiated for 30 minutes twice daily with time interval of two hours.
Then, the mice under experimentation were sacrificed after the end of each experiment, the pancreas of the sacrificed mice were rapidly obtained. Sections of exocrine pancreas were prepared by using a routine procedure. The overall sections of the exocrine pancreas including control and irradiated groups of mice were evaluated histopathologically by using light microscopy, and photographs were made at special magnification. The number of apoptotic cells was estimated by using mesh lens inserted into eyepiece of the light microscopy.
Table (1):
Shows the number of mice per group, the time of irradiation with laser, and the time interval of the irradiated groups of mice inoculated with mammary gland carcinoma
| Group A | Group B | Group C | Group D |
|---|---|---|---|
| Inoculated with mammary gland carcinoma irradiated with laser for 15 minutes twice daily | Inoculated with mammary gland carcinoma irradiated with laser for 20 minutes twice daily | Inoculated with mammary gland carcinoma irradiated with laser for 25 minutes twice daily | Inoculated with mammary gland carcinoma irradiated with laser for 30 minutes twice daily |
| Number of mice per group = 4 | |||
Table (2):
Shows the number of mice per group inoculated with mammary gland carcinoma not irradiated with laser (control groups)
| Group a | Group b | Group c | Group d |
|---|---|---|---|
| Inoculated with mammary gland carcinoma not irradiated with laser | Inoculated with mammary gland carcinoma not irradiated with laser | Inoculated with mammary gland carcinoma not irradiated with laser | Inoculated with mammary gland carcinoma not irradiated with laser |
| Number of mice per group = 2 | |||
After the examination of the histology of the overall stained sections with hematoxylin and eosin of the cells of exocrine pancreas including both control and irradiated groups of mice, the results of our work emphasized the remarkable role of soft laser in both decreasing the mean of apoptotic cells of the exocrine pancreas in irradiated mice with laser inoculated with mammary gland carcinoma with the increasing in the time of irradiation gradually as shown in table (3), and in reducing the damage that occurred in the architecture of the cells because of the negative effects caused by cancer on the cells such as decreased in size, dense fragmentation of the content of the nucleus, and dark appearance of the cytoplasm which represent slight degenerative alterations as shown in figure (1). Whereas the mean of apoptotic cells of the exocrine pancreas in control groups of mice inoculated with mammary gland carcinoma not irradiated with laser displayed a gradual increase because the cancer caused activation in cellular apoptosis as shown in table (4). There were diagnostic markers in the examination of the histology of the cells of exocrine pancreas in control groups of mice which showed that there were abundant degenerative alterations in the architecture of the cells such as decreased in size, dense fragmentation of the content of the nucleus, and dark appearance of the cytoplasm because of the suppressive activity of the cancer which caused these damages in the architecture of the cells as shown in figure (2).
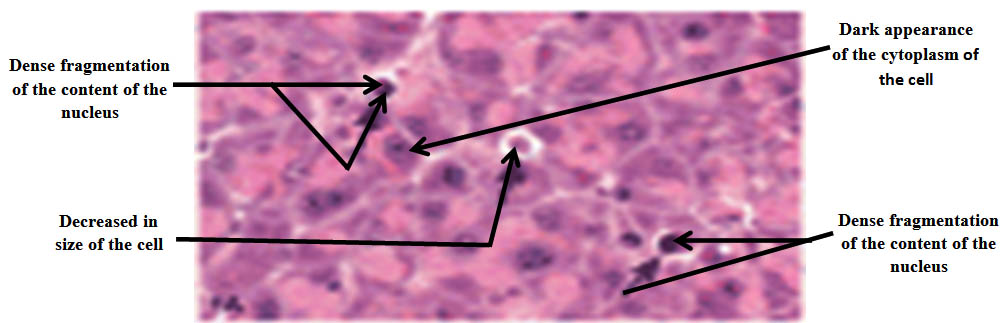
Fig. 1. The histology of the cells of exocrine pancreas of the irradiated groups of mice with laser inoculated with mammary gland carcinoma showing slight degenerative alterations in the mentioned cells such as decreased in size, dense fragmentation of the content of the nucleus, and dark appearance of the cytoplasm. H&E, 40X
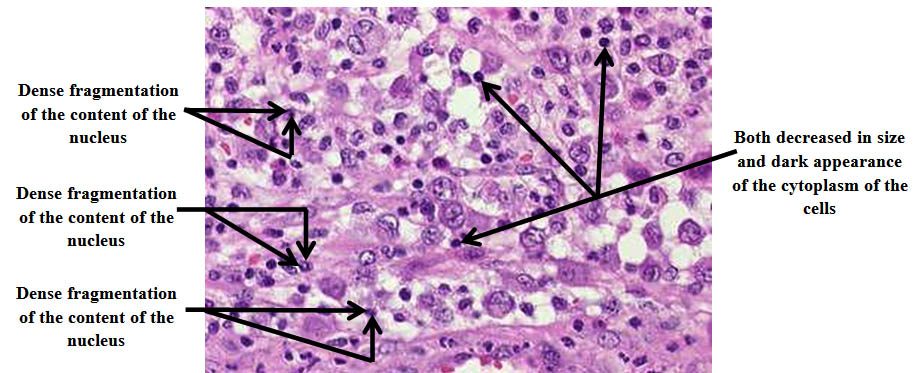
Fig. 2. The histology of the cells of exocrine pancreas of control groups of mice inoculated with mammary gland carcinoma not irradiated with laser showing abundant degenerative alterations in the mentioned cells such as de-creased in size, dense fragmentation of the content of the nucleus, and dark appearance of the cytoplasm. H&E, 40X
Table (3):
Shows the mean of apoptotic cells in exocrine pancreas of mice per each group inoculated with mammary gland carcinoma and irradiated with laser according to the time of irradiation and the time that above mentioned in table (1) respectively
The groups |
Group A |
Group B |
Group C |
Group D |
|---|---|---|---|---|
The mean of apoptotic cells after irradiation with laser |
11 |
8 |
5 |
3 |
Table (4):
Shows the mean of apoptotic cells in exocrine pancreas per each group of mice inoculated with mammary gland carcinoma not irradiated with laser (control groups)
The groups |
Group a |
Group b |
Group c |
Group d |
|---|---|---|---|---|
The mean of apoptotic cells not irradiated with laser |
5 |
11 |
16 |
21 |
The process of programmed cell death, or apoptosis, is generally characterized by distinct morphological characteristics and energy-dependent biochemical mechanisms (1). Apoptosis is considered as a vital component of various processes including normal cell turnover, proper development and functioning of the immune system, hormone-dependent atrophy, embryonic development, and chemical-induced cell death. Inappropriate apoptosis (either too little or too much) is a factor in many human conditions including neurodegenerative diseases, ischemic damage, autoimmune disorders, and many types of cancer (2). The ability to modulate the life or death of a cell is recognized for its immense therapeutic potential (3). Therefore, research continues to focus on the elucidation and analysis of the cell cycle machinery and signaling pathways that control cell cycle arrest and apoptosis. To that end, the field of apoptosis research has been moving forward at an alarmingly rapid rate. Although many of the key apoptotic proteins have been identified, the molecular mechanisms of action or inaction of these proteins need to be elucidated (4). The goal of this experimental work is to provide basic facts which were not existed of current knowledge about the process of apoptosis including morphology, biochemistry, the role of apoptosis in health and disease, detection methods, as well as a discussion of potential alternative forms of apoptosis (5, 6).
Our study showed that the laser effect was on the cells directly and caused an increase in their activity, enhance the action of the cells by secretion hormones and enzymes that made the cells more healthy. That was clear from the decreasing in the mean of the apoptotic cells due to laser action as shown in table (3) and this gradual decreasing was noticeable with the increasing of irradiation time as shown in table (1) in comparison with the mean of apoptotic cells in control groups of mice which increased noticeably also as a result of suppressive activity of the cancerous cells which caused in turn an increase in the apoptotic action of the exocrine pancreas of mice as shown in table (4).
The mean of apoptotic cells of the control groups tables (2 and 4) were increasing with time until the end of the experimentation period because the apoptotic cells affected with the circumstances of the cells like cancer mouse paws, for example, are sculpted by cell death during embryonic development: (they start out as spade like structures, and the individual digits separate only as the cells between them die). In other cases, cells die when the structure they form is no longer needed. When a tadpole changes into a frog, the cells in the tail die, and the tail, which is not needed in the frog, disappears. In many other cases, cell death helps regulate cell numbers. In the developing nervous system, for example, cell death adjusts the number of nerve cells to match the number of target cells that require innervation. In all these cases, the cells die by apoptosis. In appropriate apoptosis (either too little or too much) is a factor in many human conditions including neurodegenerative diseases, ischemic damage, autoimmune disorders and many types of cancer. The ability to modulate the life or death of a cell is recognized for its immense therapeutic potential. Therefore, research continues to focus on the elucidation and analysis of the cell cycle machinery and signaling pathways that control cell cycle arrest and apoptosis. To that end, the field of apoptosis research has been moved forward at an alarmingly rapid rate.
Light and electron microscopy have identified the various morphological changes that occur during apoptosis. During the early process of apoptosis, cell shrinkage and pyknosis are visible by light microscopy (7).
The examination of the overall stained sections with hematoxylin and eosin of the cells of exocrine pancreas including both control and irradiated groups of mice revealed the following facts:
- The apoptotic cells of exocrine pancreas of the irradiated groups of mice inoculated with mammary gland carcinoma showed slight degenerative alterations in the architecture of the mentioned cells such as decreased in size, dense fragmentation of the content of the nucleus, and dark appearance of the cytoplasm as shown in figure (1). These slight alterations that occurred in the architecture of the mentioned cells may be explained that the laser affected the cells positively and in turn resulted in a reduction in the damage of the architecture of the cells because the laser inhibited the action of the cancer.
- The apoptotic cells of exocrine pancreas of the control groups of mice inoculated with mammary gland carcinoma not irradiated with laser showed abundant degenerative alterations in the architecture of the mentioned cells such as decreased in size, dense fragmentation of the content of the nucleus, and dark appearance of the cytoplasm as shown in figure (2). These abundant alterations that occurred in the architecture of the mentioned cells were due to the suppressive activity of the cancer which caused a noticeable damage in the architecture of the cells.
We reckon that this experimental work as a whole represents a new method that may be added to the methods in the field of medical applications of laser especially in the histopathology such as treatment of cancer.
- Soraya, Coelho and Orlando, Ayrton de Toledo. “Morphological alterations of the parotid gland maintained on liquid deite”, Brazilian Dental Journal. 2005; 2: 45-51.
- Nagata, S., Hanayama, R. and Kawane, K. Autoimmunity and the clearance of dead cells. Cell 2010; 140, 619–630.
- Poon, I.K., Lucas, C.D., Rossi, A.G. and Ravichandran, K.S. Apoptotic cell clearance: basic biology and therapeutic potential. Nat. Rev. Immunol. 2014; 14, 166–180.
- Monks, J. et al. Epithelial cells as phagocytes: apoptotic epithelial cells are engulfed by mammary alveolar epithelial cells and repress inflammatory mediator release. Cell Death Differ. 2005; 12, 107–114.
- Nagata, S. Autoimmune diseases caused by defects in clearing dead cells and nuclei expelled from erythroid precursors. Immunol. Rev. 2007; 220, 237–250.
- Metchnikoff, E. in Lectures on the Comparative Pathology of Inflammation (Dover, New York, 1968).
- Van Furth, R. et al. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull. World Health Organ. 1972; 46, 845–852.
- Shafik, SS., Kheir, AO., Kany, F. and Omran, M. “Effect of Nd: YAG laser on dental cementum: A scanning electron microscopic study”. J. Oral Laser. 2002; 2, 95-99.
- Chen, Y-J., Jeng, J-H., Yao C-CJ., Chen, M-H., Hou, L –T. and Lan, W-H. “Long-term effect of pulsed Nd: YAG laser irradiation on cultured human periodontal fibroplasts”, J. Laser Surg. Med. 2005; 36, 225-233.
- Edgar, WM., and Jenkins, GN. “Can salivary function in man be enhanced by increased mastication?”. Jornal of Dent. Res. 1981; 60(B), 1172.
- Fayad, MI., Hawkinson, R., Daniel, J. and Hao, J. “The effect of Co2 laser irradiation on PDL cell attachment to resected root surfaces”. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2004; 97, 518-523.
- Pourzarandian, A., Watanabe, H., Ruwanpura, SMPM., Aoki, A. Noguchi, K. and Ishikawa” I, Er: YAG laser irradiation increases prostaglandin E2 production via the induction of cyclooxygenase-2 mRNA in human gingival fibroblasts”. J. Periodontal Res. 2005; 40, 182-186.
- Kreisler, M., Meyer, C., Stender, E, Daublander, M., Willershausen-Zonnchen, B.d. and Hoedt, B. “Effect of diode laser irradiation on the attachment rate of periodontal ligament cells: An in vitro study”. J. Periodontal. 2001; 72, 1312-1317.
- Reddy GK, Stehno-Bittel L, and Enwemeka CS. Laser photo stimulation accelerates wound healing in diabetic rats. Wound Repair Regen. 2001; 9, 248-255.
- Nes, AG. and Posso M B. Patients with moderate chemotherapy-induced mucositis: pain therapy using low intensity lasers. Int. Nurs. Rev., 2005; 52, 68-72.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


