ISSN: 0973-7510
E-ISSN: 2581-690X
Indiscriminate application of pesticides in paddy crop field soil to enhance paddy production could cause adverse impact on the both soil fertility and bacterial communities. The present study aimed to isolate bacteria prevalent in Lambdacyhalothrin exposed soil to determine their Lambdacyhalothrin degrading ability and assay the degraded metabolites. The observations registered in this study revealed that the dominant bacteria isolated from paddy crop field soil were Bacillus cereus and Aneurinibacillus migulanus. These bacteria were inoculated in soil spiked with Lambdacyhalothrin. After 36 hours of inocubation, the extract were analysed by GCMS. Lambdacyhalothrin degradation was accelerated by Aneurinibacillus migulanus (100 % of pesticide degraded compared to Bacillus cereus 94.09 % and control 55.2 %). Thus Aneurinibacillus migulanus could be the used as bioagents to degrade lambdacyhalothrin.
Lambdacyhalothrin, Bacillus cereus, Aneurinibacillus migulanus, GCMS, Biodegradation.
The application of pesticides is a pivotal strategy to control pest. In Tiruchirappalli district of Tamil Nadu, India, commonly applied pesticide in paddy cultivation is Lambda cyhalothrin. The persistence of Lambdacyhalothrin and its degradative products in paddy crop field soil could deteriorate the soil quality. Hence, protocols have to be evolved to eliminate these residues from the soil. In this study, we have aimed to tap the potential of autochthonous bacteria inhabiting the paddy crop field soil to degrade lambdacyhalothrin.
Isolation of pesticide resistant bacteria from paddy crop field soil
1gm of Lambda cyhalothrin exposed paddy crop field soil were aseptically inoculated in 100ml of sterile minimal salt media (MSM) into cotton plugged flasks in triplicates. Conical flasks were kept under continuous shaking at room temperature for one week. Minimal salt media containing the following salts: CaCl2 – 0.002 g , MgCl2 – 0.02 g, K2 HPO4 – 0.1 g, KH2PO4– 0.1 g, NH4NO3 – 0.1 g and FeCl3 trace amount in distilled water (pH 7.2 – 7.4) upto 1L were used for inoculation of soil sample. Total heterotrophic bacteria were isolated and identified following Bergeys manual of Determinative Biology (Sneath, et al., 1994). The dominant bacteria Bacillus cereus and Aneurinibacillus migulanus were selected for pesticide resistant studies.
Lambda cyhalothrin in soil inoculated with Bacillus cereus and Aneurinibacillus migulanus
Bacillus cereus was subcultured in autoclaved nutrient broth for 48 hours at 30ºC in a rotatory shaker. After 48 hours, 1ml (45 x 1015 cfu/ ml) of Bacillus cereus broth was inoculated in 50 g of sterile paddy crop field soil in 250 ml of cotton plugged conical flask containing 500 ppm Lambda cyhalothrin in triplicates. 20 ml of autoclaved minimal salt medium was added to maintain 60 % of humidity. Control (without Bacillus cereus ) was maintained simultaneously. Similar procedure was followed for Aneurinibacillus migulanus ( 29 X 1015 cfu/ ml).
GC-MS analysis of degraded metabolites of Lambda cyhalothrin
After 36 hours of incubation, the samples were subjected to GC-MS analysis. The control and the Lambda cyhalothrin treated soil were extracted for GCMS analysis based on the method of Malghani et al., (2009) with minor modifications. The pesticides in the control and treatment were extracted using organic solvent extraction three times with acetone and hexane (1:1) mixture, then the extract was concentrated using rotary vaccum evaporator (Buchi R-210, Surkzer ) and cleaned up with silica gel column (1:3cm dimeter x 243cm length). The pesticide extract were eluted with n-hexane collected in a glass vial and subjected to gas chromatograph- Mass spectrometer (GC-MS) analysis.
Instrumental Analyses
The qualitative and quantitative determination of Lambda cyhalothrin was performed by GC-MS (45 X GC – 44, Bruker) equipped with auto injector (8410). The analyses separation was performed in a 60 mm x 0.25 mm I.D x 0.25 µm film thickness BR 5 ms column (made in USA) and Helium was used as a carrier gas at a flow rate of 1 ml / min. The column temperature was programmed as 70ºC to 150ºC at 10ºC / min, to 250ºC at 5ºC/ min, to 280ºC at 2ºC / min, finally to 320 ºC at 5ºC/ min and hold for 10 minutes. 1 µl of the extract was injected into the injection port (at 280ºC) using auto injector. The mass spectrometer was operated in scan mode and the ion source temperature was kept at 250 ºC.
The electron ionisation (EI) unit was operated at 70 ev and at an emission current of 60 µA. Full scan data was obtained in a mass range of m/ z 50-650. Scanning interval and sample rate were 0.5 and 0.28, respectively.
Bacteria was isolated from paddy crop field soil exposed to lambdacyhalothrin. The 16s rRNA gene sequencing was performed and identified as Bacillus cereus (Gene Bank Accession Number : KY293394) and Aneurinibacillus migulanus (Gene Bank Accession Number: KY293393).
Residual quantification analysis of Lambdacyhalothrin by gas chromatography due to bacterial activity is presented in table 1. Relative to the control (55.2%), Bacillus cereus and Aneurinibacillus migulanus accelerated the degradation of Lambdacyhalothrin by 94.09% and 100%, respectively. These results indicate that Bacillus cereus and Aneurinibacillus migulanus play a vital role in accelerating the degradation of Lambdacyhalothrin.
In the control soil, apart from Lambdacyhalothrin the various metabolites obtained were Isopropyl benzene, 2-ethyl-1, 3- dimethyl benzene, 1, 2, 3, 4-tetra methyl-5-methylene 1,3-cyclopentadiene, 4- methyl benzoic acid pentafluoro phenyl ester, 1 methyl-4- (1-methyl ethyl)- cyclohexane (Fig 1, table 2). Lambdacyhalothrin residue also was persistence in Bacillus cereus inoculated soil containing 500 ppm Lambdacyhalothrin. In addition, only Lambdacyhalothrin persisted and no other metabolites were present (fig 2, table 2). Aneurinibacillus migulanus resulted in the formation of Isopropyl benzene, 1, 2, 4-Trimethyl benzene, 1, 2, 4- tris (methylene) cyclohexane, 1- Ethyl-2,4-dimethyl benzene, methyl ester 1, 4-Benzene dicarboxylic acid and 3-Chloro propanic acid (Fig 3, table 2).
Table (1):
GCMS result showing the biodegradation of Lambdacyhalothrin by Bacillus cereus and Aneurinibacillus migulanus after 36 hours of exposure.
Treatments |
RT |
Area |
Remaining pesticide (ppm) |
% of Pesticide degraded |
|---|---|---|---|---|
Control Soil+ 500 ppm Lambda cyhalothrin |
20.487 |
23940000000 |
223.765 |
55.2 |
Test soil+ 500 ppm Lambda cyhalothrin + 1ml (45 x 10 15 cfu/ ml) Bacillus cereus |
20.353 |
3160000000 |
29.536 |
94.09 |
Test soil+ 500 ppm Lambda cyhalothrin
1ml (29x 10 15 cfu/ ml) Aneurinibacillus migulanus |
– |
– |
– |
100% |
RT -Retention time
-No Peak
Table (2):
Biodegraded metabolites of Lambdacyhalothrin in soil detected by GC-MS on inoculation with bacteria.
| Treatment | R.T | Degraded metabolites |
|---|---|---|
| Control (soil + 500 ppm Lambdacyhalothrin) | 6.918 | Isopropylbenzene |
| 9.462 | 2-ethyl-1,3-dimethyl benzene | |
| 10.454 | 1,2,3,4-tetramethyl-5-methylene 1,3- cyclopentadiene | |
| 14.532 | 4-methyl benzoic acid pentafluoro phenyl ester | |
| 16.094 | 1-methyl-4-(1-methylethyl)- cyclohexane | |
| 20.487 | Lambdacyhalothrin | |
| Test (soil + 500 ppm Lambdacyhalothrin +Bacillus cereus) | 20.353 | Lambdacyhalothrin |
| Test( Soil + 500 ppm + lambdacyhalothrin + Aneurinibacillus migulanus) | 6.539 | Isopropylbenzene |
| 7.626 | 1,2,4-trimethyl benzene | |
| 8.584 | 1,2,4-tris(methylene) cyclohexane | |
| 9.182 | 1-ethyl-2,4-dimethyl-benzene | |
| 20.518 | methyl ester 1,4-benzenedicarboxylic acid | |
| 21.972 | 3-Chloro propanoic acid, |
R.T – Retention Time
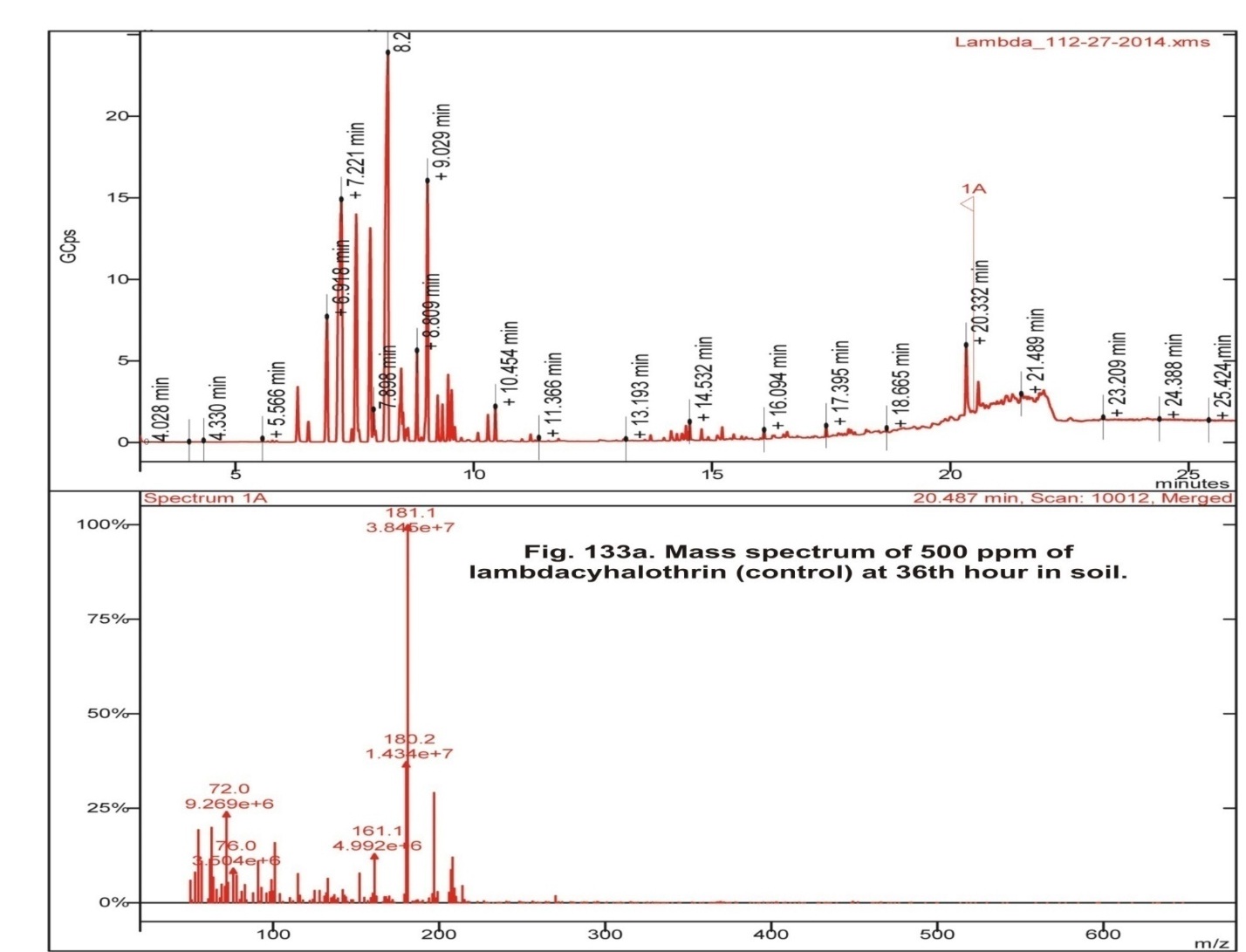 Fig. 1: Gas chromatogram of 500 ppm of lambdacyhalothrin (control) at 36th hour in soil
Fig. 1: Gas chromatogram of 500 ppm of lambdacyhalothrin (control) at 36th hour in soil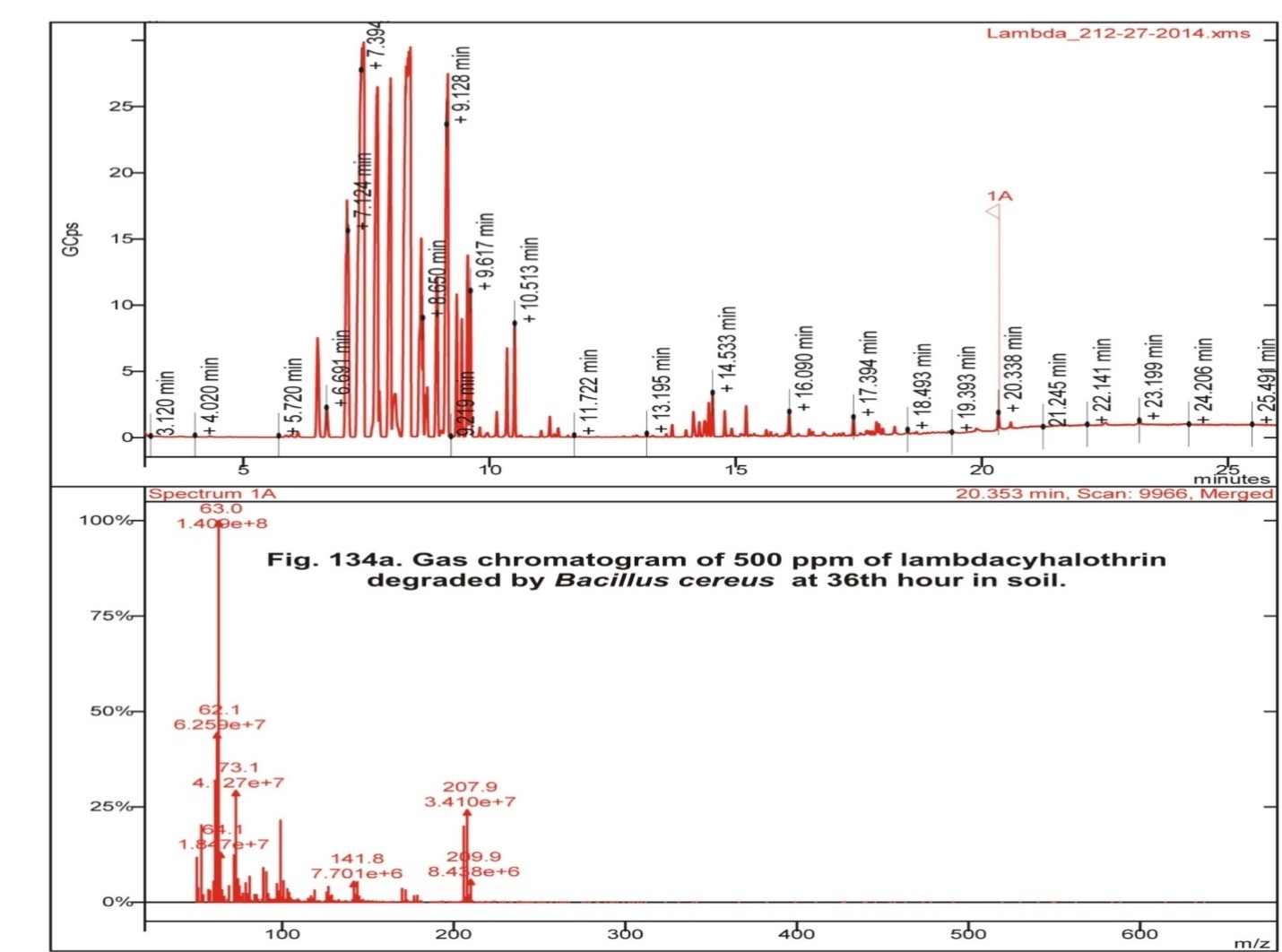 Fig. 2: Gas chromatogram of 500 ppm of lambdacyhalothrin degraded byBacillus cereus at 36th hour in soil
Fig. 2: Gas chromatogram of 500 ppm of lambdacyhalothrin degraded byBacillus cereus at 36th hour in soil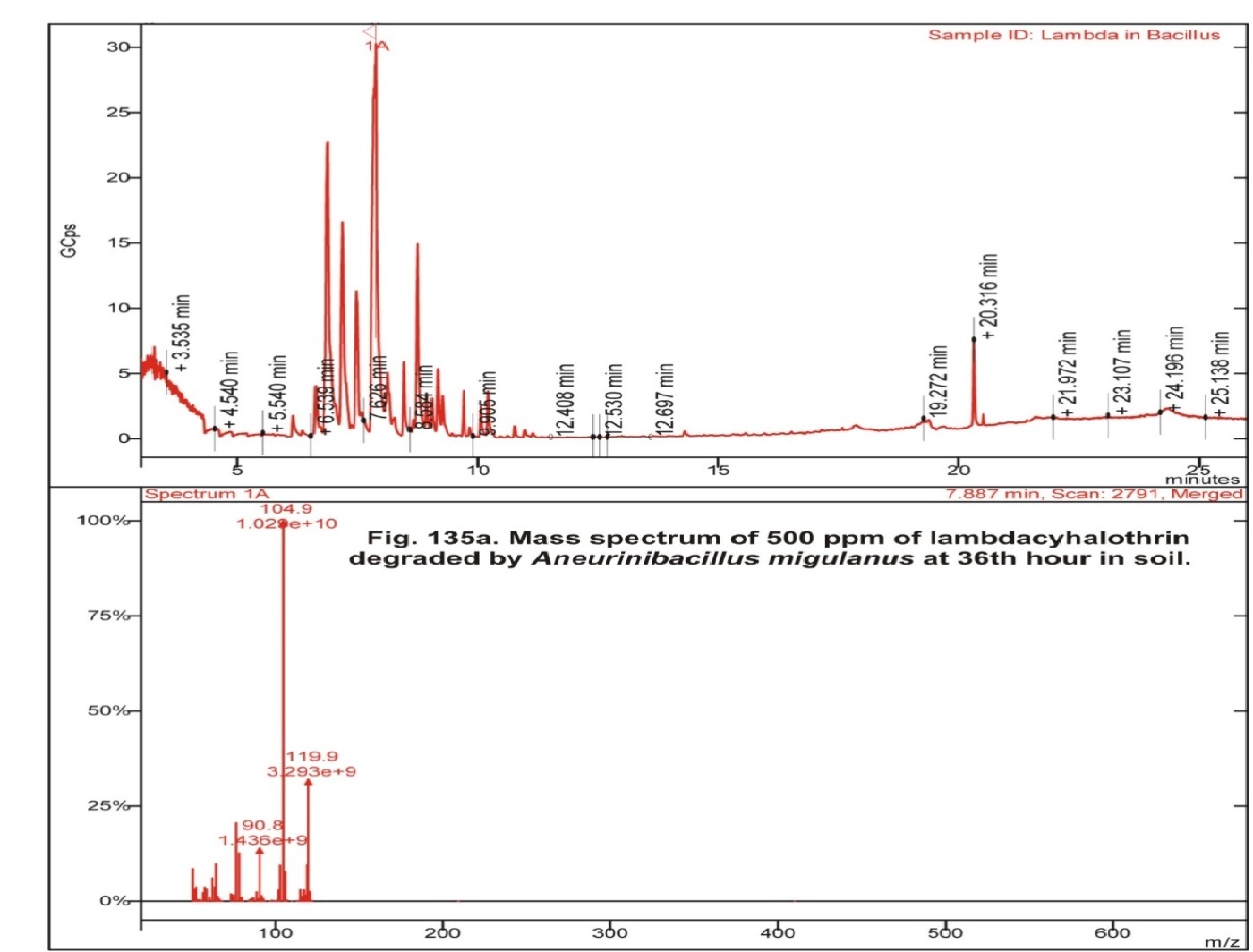 Fig. 3: Gas chromatogram of 500 ppm of lambdacyhalothrin degraded byAneurinibacillus migulanus at 36th hour in soil
Fig. 3: Gas chromatogram of 500 ppm of lambdacyhalothrin degraded byAneurinibacillus migulanus at 36th hour in soilAs evinced in this study, Geeta et al., (2014) have demonstrated that bacteria accelerated the degradation of pesticides in soil (arbendazim: Bacillus sp., Exiguabacterium, Achromobacter, imidacloprid: Achromobacter; Microbacterium, Pseudomonas sp. and a and b – endosulfan :, Xanthomonas sp., Microbacterium sp., Bacillus sp.,). They have also observed dose – dependent degradation of Carbendazim by Exiguobacterium. In addition, they have reported that comparatively, consortium of bacteria enhanced the degradation of pesticides.
The results of the present finding are in corroboration with the work done by Agarry et al., (2013) who have demonstrated that bacterial consortium (Proteus vulgaris, Vibrio sp., Serratia sp., and Acinetobacter sp.,) isolated from agricultural farm soil from Ladike Akintola University of Technology, Nigeria were able to grow in nutrient medium containing Dichlorvos as the only carbon source. Further, they have observed that the percentage removal of Dichlorvos pesticide from the soil was relatively higher in soil amended with NH4NO3 (75%) and KH2PO4 (85%), respectively.
Bacillus mediated degradation of Lambdacyhalothrin observed in this study is consistent with the findings of Osama el Gialanielsaid et al., (2010) who have compared the endosulfan degradation potential of mutant strains of Bacillus sp. (isolated through consecutive exposure to elevated concentrations of endosulfan under carbon free media) and wild type strain and have confirmed through GLC (Gas liquid Chromatography) that wild type of bacteria (from stock culture) caused 83% reduction in half lives of both ± and ² endosulfans. Enhanced degradation of Lambdacyhalothrin by Aneurinibacillus migulanus is well supported by Abdullah et al., (2016) who have also evinced acceleration of degradation of Lambdacyhalothrin by Pseudomonas putida.
- Sneath PHA, Mair SN, Elisabeth M, sharpeand G. Holt (1994) Bergeys manual of systematic bacteriology. Williams andailkins, Baltimore. USA .
- Malghani S, Chatteijee N, Yu H X, Luo Z. Isolation and identification of \y profenofos degrading bacteria. Brazilian Journal of Microbiology., 2009; 40: 893-900.
- Geeta, N., Pankaj, Anjana S., Anita S., In situ Biodegradation of Endosulfan, Imidacloprid, and Carbendazim Using Indigenous Bacterial Cultures of Agriculture Fields of Uttarakhand, India. International Journal of Biological, Biomolecular, Agricultural, Food and Biotechnological Engineering, 2014; 8(9): 973 – 981.
- Agarry, S. E., Olu-Arotiowa, O. A., Aremu. M. O., and Jimoa, L. A., “Biodegradation of Dichlorovos (Organophosphate Pesticide) in Soil by Bacterial Isolates,” Journal of Natural Sciences Research, 2013; 3(8): 12-16.
- Osama el gialani elsaid, Azhari Omer Abdelbagi, Elsiddig Ahmed Elmustafa Elsheikh, “ Pesticide-Resistant Bacterial Strain,” International Journal of Environmental Sciences, 2010; 1(2): 123-131.
- Abdullah R. R., Abdel Ghani, S. B., and Sukar, N. A., “Degradation of Profenofos and ³ – Cyhalothrin using endogenous bacterial isolates and detection of the responsible Genes”, Journal of Bioremediation and Biodegradation, 2016; 7(4): DOI 10.4172/ 2155-6199.1000360.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


