ISSN: 0973-7510
E-ISSN: 2581-690X
Piriformospora indica is a root endophytic fungus that build a symbiotic association with the roots of a vascular host plants and induces overall plant growth. The P.indica can grown on a variety of synthetic and complex media. But these readymade medium consist of about 20-25 expensive chemicals. To minimise the cost for P.indica production (Arora et al 2014 and Varma et al, 2015) have already standardized a cost effective Jaggery medium (4% w/v) as a energy nutrient source for mass cultivation of P. indica with no compromise on quality. But growth of fungus varies substantially with different sources of Jaggery. So we collect Jaggery from four different sources i.e from 1. Local shop, 2. Indian Institute of Sugarcane Research, Lucknow, 3. Safal, New Delhi and 4.Organic Jaggery to check its impact on fungal growth and also check its efficacy on Spinach (Spinacia oleracea). The data observed from the present study shows that maximum growth of P.indica in terms of dry cell weight, radial diameter and spore count was recorded on Safal Jaggery medium as compared to other Jaggery medium. Moreover, fungal biomass grown on this medium showed positive effect on growth of spinach as it leads to increase in plant height and its dry and fresh weight. This finding suggest that Safal Jaggery medium is a cheaper and best source for commercial cultivation of P.indica.
P.indica, Safal Jaggery, Spinach, cultivation, growth.
Piriformospora indica is a root endophyte which has all properties of a typical arbscular mycorrhizal fungus1. But unlike other AM fungus it can easily cultivate in laboratory on a variety of synthetic submerged and solid media like Hill and Kaefer, Potato Dextrose, YMP, MS etc2. The fungus isolated from the soil of thar desserts of north western Rajasthan, India1. It belongs to phylum Hymenomycetes and order Sebacinales (Basidiomycota). It can colonize with roots of variety of plants and resistant to many fungal pathogens so it is capable to enhance overall plant growth3,4. This fungus has a multifarious activities as it can form a symbiotic association with variety of plants including legumes, flowering plants5, thale cress6,variety of grasses7, tobacco8. Due to this symbiotic association it has a strong potential to use as a biofertilizer in the field of agriculture9.
The fungus can grow inter- and intracellularly and produces autoûuorescent and pear shaped chlamydospores with thick walled hypahe with length and width of 15-24 and 11-16 µm respectively. Its shelf life is about 3 months at room temperature. The chlamydospores can be present in clusters of 2-3 or as a single spore also9,10. The fungus enters in root cortex of inoculated plant but it does not enter in the aerial part and endodermis of plants11,12. The fungus helps in nutrient uptake, protect plants from salt, water and temperature stresses and provide complete resistance to acidity, heavy metal toxins, pathogens etc13. It can use as a plant regulator, bio fertilizer, bioregulator because it promotes early flowering, seed production, excessive production of biomass. On solid media P.indica forms round ring like structures which are formed by chlamydospores or mycelium14.
Hill and Kaefer was most optimum media out of all other tested media used for growth and maintenance of P.indica15. But this synthetic media is consist of very expensive chemicals so in previous study cheap and easily available media was used for the mass cultivation of fungi16. In this study, we report about the mass production of P.indica by using a easily available, cost effective and indigenous nutritional source of energy which is known as Safal Jaggery medium. It is commercially and quantitatively competitive to Hill and Kaefer. Further its efficacy is also checked by inoculating a plant of Spinach (Spinacea oleracea) with the fungal biomass obtained on a Safal Jaggery medium.
Spinach which is popularly known as ‘‘palak’’ is belongs to a family chenopodiaceae family.
It is a nutritive plant which is rich in riboflavin, ascorbic acid, vitamin A, thiamine and also essential minerals like calcium and iron. So, it is an important and common part of human food for daily intake as it fullfill all the requirements of healthy nutrients. Moreover, this plant has a broad surface area and fast growth rate. Because of all these unique features S. oleracea is used in a number of scientific researches17,18.
Culturing and maintenance of mycobiont P. indica
The p. indica was cultivated on Hill-Kaefer medium19. One P.indica disc of 8mm in diameter from 8 days old P.indica plate is cut by using sterlized cork borer and then placed in the centre of fresh hill and kaefer agar medium plate. Now, after this the inoculated plates were kept in an incubater at 28 ± 2 °C for about 7-10 days. Then, these plates were stored at 4°C for further use in experiments.

Fig. 1. Four different sources jaggery : A. Local B. Indian Institute of Sugarcane Research, Lucknow C. Safal, New Delhi D. Organic
Co-cultivation of P.indica biomass with plants
Efficacy of P.indica spores grown on Safal Jaggery medium was checked on Spinach (Spinacia oleracea). Seeds were procured from National Seed Corporation, IARI, New Delhi, India. Seeds were sterilized and germinated by method mentioned in Arora et al 2014. For this experiment :1 mixture of Soil and peat with pH 6.8 was used.
For Mycorrhizal inoculation the 2gm of fungal mycelium is mixed carefully with 100g of sterile soil (w/w). Now, the upper surface of soil was covered with a thin layer of fungal inoculums which is further covered by a very thin layer of soil i.e forming a sandwich like model. Pre germinated seeds were transplanted in each pot. Each pot is consist of 3 seeds and were kept in biohardening lab at 28°C. Plants were fertilized with 1/10 Hoagland solution on every alternative week.
Estimation of plant growth parameters
The plants inoculated with fungus were harvested after about 20 days to check changes in its growth parameters like root and shoot length, root and shoot fresh and dry weight. Shoots and roots were separated from each other and then washed under running tap water to remove traces of soil and other materials. Now, root and shoot length was measured by using scale. Fresh weight was measured immediately and dry weight was measured by drying the root and shoot sample in an hot air oven at 80°C for 24h.
Root colonization
To check the presence of P.indica spores in plants the root sample of each inoculated and uninoculated plants were collected and boiled with 10% potaasium hydroxide to make it soft and then treated with 2% hydrochloric acid for atleast 4 mins. These samples were further stained with 0.5% cotton-blue dye and then observed under a light microscope at 40X magnification.
Statistical analysis
Each experiment was performed in tprilicate and results presented as a mean±standard error (SE) and differences were were considered to be significant at p<0.05.
Growth of P. indica on different sources of jaggery
P. indica can be grown on variety of complex synthetic media for example Potato Dextrose, Hill and Kaefer, YPG media but all these complex synthetic medium consisting of about 20-25 expensive chemicals. So, to minimize the use of these expensive nutrients we have standardized Jaggery (Gur) as energy nutrient source obtain from Sugarcane (Saccarum officanarum). With no compromise on its quality. But Growth of Fungus varies substantially with different sources of Jaggery. For this purpose we collected 4 different types of Jaggery from different places i.e From local shop, Indian Institute of Sugarcane Research, Lucknow, Safal, New Delhi and Organic Jaggery (Fig 1). Now, jaggery obtained was convert into powder form and dissolved in distilled water at 4% (w/v).This concept is based on preliminary success of the fungus on 4 % (w/v) using identical parameters20. Hill-Kaefer medium is taken as a control in all experiments.
To check growth pattern of fungus on solid media one P. indica disc having live spores of 8mm in diameter from 8 days old P. indica plate is cut by using sterlized cork borer and then placed in the centre of freshly prepared Jaggery agar medium plate. Now , after this the inoculated plates were kept in a incubater at 28 ± 2 °C for about 7-10 days.
For mass multiplication P. indica was grown in shaking flasks containing Jaggery medium without agar. Media with pH 6.5 was sterilized at 15 psi pressure for 20 minutes in an autoclave. The focus was on excessive production of chlamydospores for better formulation and storage for transfer to field at room temperature. For this puepose the bottles containing liquid broth media were inoculated with P. indica disc and then kept on rotator shaker at 120 rpm in dark at 28°C for about 10days.
Measurement of cell and radial growth
The P. indica growth was measured in terms of DCW i.e dry cell weight in per liter of culture Broth. After about 9-10 d of growth a known volume of biomass was filtered by using Whatmann filter paper No. 1 and then dried in a hot air oven for overnight at about 80°C. After removing all moisture the weight of dry mass was determined. To check the DCW 3 flasks were removed everyday. The scale was used to measure the mycelium radial growth from inoculated disc and it was measured every alternate day.
Spore counting of fungus
To count the pear shaped chlamydospores of P. indica culture first separate these spores from mycelium. For this separation in case of solid media, 1 ml of tween 20 was spread on the agar plate and swirled gently by using sterilized spatula on the surface of agar plate. Now, the already detached spores are scratched and collected in a centrifuge tube. These spores are centrifuged at 3000 rpm for about 8 mins which is further followed by washing with distilled water containing sterilized tween 20. Number of spores is observed by using Hemocytometer with microscope.
Estimation of residual glucose
A 1ml sample diluted with 1ml of sterlized water was taken in a test tube, 3ml DNS reagent was added and heated for 15 min in a boiling water bath. Then the hot tube was supplemented with 1ml Rochelle salt solution and cooled to room temperature. The absorbance was measured at 575nm using Spectrophotometer (Systronics-20).
Confocal microscopy
P. indica spores grown on Safal Jaggery medium were examined under Confocal laser scanning microscope LSM-780 ((Carl-Zeiss, Inc., Jena, Germany). First these spores were separated from mycelium by the method explained in section 4. Now, these pure spores were placed on a glass slide and trichloroacetic acid/ethanol in the ratio of 1/0.15% v/w were used for the fungal biomass fixation. The spore sample was washed with distilled water and then with PBS (phosphate buûered saline) which contains 50 µg/mL WGA- AF488 and 0.2% Silwet L-77. Now, these samples were observed under confocal microscope.
Separation of Culture filterate
To prepare fungal formulation the fungal biomass was grown on liquid Safal Jaggery medium containing live chlamydospores and mycelium were collected through filteration after 9 days by using sterilized musclin cloth (400nm). It can be preserved at 4 °C if not required immediately.
Co-cultivation of P. indica biomass with plants
Efficacy of P. indica spores grown on Safal Jaggery medium was checked on Spinach (Spinacia oleracea). Seeds were procured from National Seed Corporation, IARI, New Delhi, India. Seeds were sterilized and germinated by method mentioned in Arora et al 2014. For this experiment: 1 mixture of Soil and peat with pH 6.8 was used.
For Mycorrhizal inoculation the 2gm of fungal mycelium is mixed carefully with 100g of sterile soil (w/w). Now, the upper surface of soil was covered with a thin layer of fungal inoculums which is further covered by a very thin layer of soil i.e forming a sandwich like model. Pre germinated seeds were transplanted in each pot. Each pot is consist of 3 seeds and were kept in biohardening lab at 28°C. Plants were fertilized with 1/10 Hoagland solution on every alternative week.
Estimation of plant growth parameters
The plants inoculated with fungus were harvested after about 20 days to check changes in its growth parameters like root and shoot length, root and shoot fresh and dry weight. Shoots and roots were separated from each other and then washed under running tap water to remove traces of soil and other materials. Now, root and shoot length was measured by using scale. Fresh weight was measured immediately and dry weight was measured by drying the root and shoot sample in an hot air oven at 80°C for 24h.
Root colonization
To check the presence of P. indica spores in plants the root sample of each inoculated and uninoculated plants were collected and boiled with 10% potaasium hydroxide to make it soft and then treated with 2% hydrochloric acid for atleast 4 mins. These samples were further stained with 0.5% cotton-blue dye and then observed under a light microscope at 40X magnification.
Statistical analysis
Each experiment was performed in tprilicate and results presented as a mean±standard error (SE) and differences were were considered to be significant at p<0.05.
Selection of media for P.indica cultivation on the basis of Dry cell weight and radial growth
Four different types of jaggery were used for P.indica cultivation. Hill and kaefer served as a control. P.indica was grown on both solid and liquid media to find a appropriate media for sporulation and growth of fungus. The P.indica growth in submerged and in solid media after 10d is shown in fig 2(a-e) and 3 (a-e) respectively.
Maximum DCW i.e dry cell weight of P.indica was observed in Safal Jaggery medium i.e 16.81g/l as compared to control. DCW of P. indica was relatively less in other Jaggery medium in comparison to Safal (Table 1). Now, diameter of leading hyphae of P.indica colonies growing on different Jaggery medium and Hill and Kaefer medium was also observed. This observation shows that again p.indica disc which was inoculated in the centre of Safal Jaggery medium plate leads to a significant increase in leading hyphae with diameter of 7.1 cm (Table 1) which is maximum out of all other media. This analysis of diameter of radial growth of fungus was measured every alternative day until the growth of fungus touched the edges of petriplate (Fig 2a-e). In terms of morphology of P.indica hyphae it was observed that the fungus showed same growth pattern of packed balls with coral morphology in jaggery medium this pattern confirms that this is suitable media1,21.
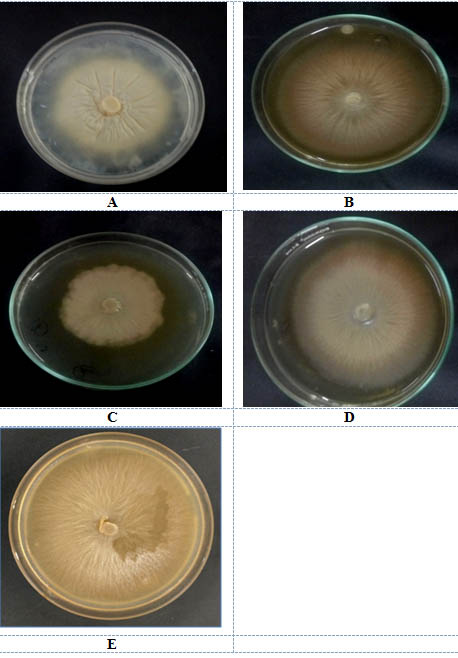
Fig. 2. Growth of P.indica on solid media A. Hill and kaefer B. Local C. Organic D Indian Institute of Sugarcane Research, Lucknow E. Safal, New delhi
Table (1):
Growth of P.indica on solid and liquid media
Medium |
DCW(g/l) |
Radial diameter (cm) |
|---|---|---|
Hill and Kaefer |
13.26 |
5.9 |
Local |
14.11 |
6.8 |
Indian Institute of Sugarcane Research, Lucknow |
13.81 |
6.9 |
Safal, New Delhi |
16.81 |
7.1 |
Organic |
12.16 |
5.6 |
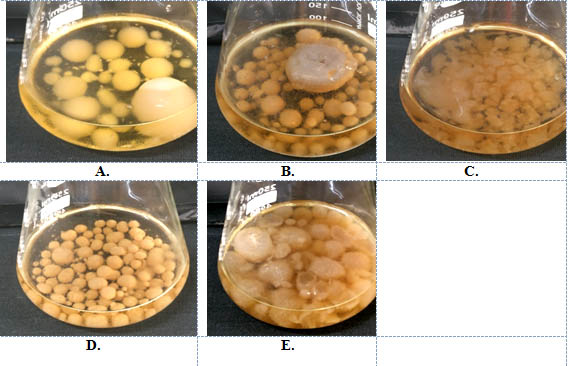
Fig. 3. Growth of P.indica in liquid media A. Hill and kaefer B. Local C. Organic D Indian Institute of Sugarcane Research, Lucknow E. Safal, New delhi
Spore count by using haemocytometer
Out of all five media, Safal Jaggery medium produced much higher spore yield of 1×109-1×1010 spores/ml. Similarly there is early start in sporulation i.e on 6th day. (Table 2). The least spore count of 1×106-1×107 (spores/ml) is obtained from Organic Jaggery and fungus start sporulated at 8th day. Fig 4 showed morphogenesis of chlamydospores grown on Hill and Kaefer and Safal Jaggery media. This figure depicts that in case of Hill and Kaefer the spores were small with thin walled hyphae and less in number on the other hand Safal Jaggery medium has more bigger spores with thick walled hyphae. Overall, Safal Jaggery media was found to be best medium as it gives highest number of bigger spores.
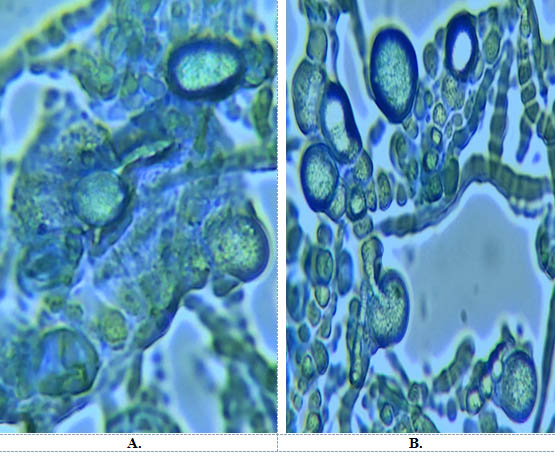
Fig. 4. Morphogenesis of Chlamydospores on A. Hill and Kaefer B. Safal jaggery medium
Table (2):
Growth and sporulation of P.indica
Medium |
Spore count |
Day of start of Sporulation |
|---|---|---|
Hill and Kaefer |
1×108–1×109 |
6 |
Local |
1×109 –1×1010 |
6 |
Indian Institute of Sugarcane Research, Lucknow |
1×108–1×109 |
7 |
Safal, New Delhi |
1×109-1×1010 |
5 |
Organic |
1×108–1×109 |
8 |
Sugar consumption rate of Jaggery Medium from 0hr to 9th day
The spore count and substrate consumption of P.indica in Safal Jaggery medium was observed and shown in Table 3 and Fig 5. Initially there is a lag phase for about 25h because the adaption of fungus to this environment. Now, there is exponential increase in spore count for next 7days as maximum spore count is of 1×108 –1×1010 at 7th day of cultivation. Then there is decrease in spore count after day 7 which is due to complete utilization of glucose. Due to this quick consumption of glucose colonies start getting aggregate and form clusters thats why there is increase in biomass but decrease in spore count.
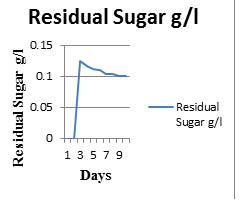
Fig. 5. Sugar consumption rate of Safal Jaggery medium
Table (3):
Sugar consumption rate by P.indica
Days |
Spore count |
Residual Sugar g/l |
|---|---|---|
0. |
– |
|
1. |
– |
|
2. |
1×102 –1×104 |
0.124 |
3. |
1×103 –1×105 |
0. 117 |
4. |
1×104 –1×105 |
0. 112 |
5. |
1×105 –1×107 |
0. 110 |
6. |
1×109 -1×1010 |
0. 104 |
7. |
1×108 –1×1010 |
0.103 |
8. |
1×108–1×1010 |
0.101 |
9. |
1×104 –1×105 |
0. 100 |
Confocal microscopy
P.indica spores grown on different jaggery sources were observed under CLSM (Confocal laser scanning microscopy) by using trypan blue dye (Fig 6 a-e). Laser excitation at 488 nm resulted in giving out in the observable range. In Safal Jaggery medium spores were bigger in size with thick and hyalin hyphae (Fig 6e). Moreover, it was observed that fungal spores are more in numbers with smooth surface as compare to spores grown on other media in which spore count was less with morphological deformities and disaggregation.
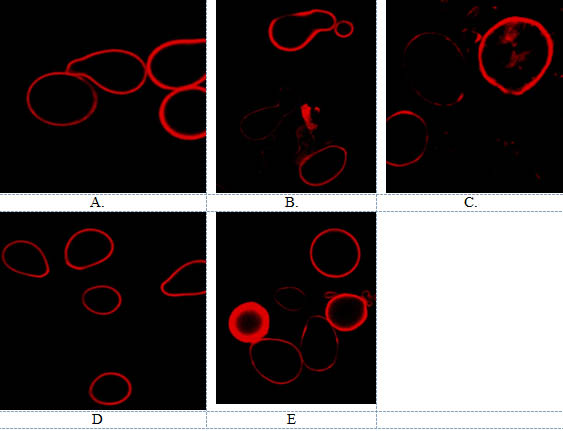
Fig. 6. Confocal Microscopy of P.indica A. Hill and kaefer B. Local C. Organic D Indian Institute of Sugarcane Research, Lucknow E. Safal, New delhi
Comparison of growth parameters
The P.indica biomass grown on Safal Jaggery medium was used to check its efficacy on Spinach (Spinacia oleracea). Germination rate of seeds inoculated with fungal biomass is increased by 13.8 % as compare to control (Fig 7 and Table 4). A visual representation of the plants shows the early flowering in case of treated as compare to control (Fig 8). The data observed indicates that fungal culture has positive effect on different growth parameters of plant as mentioned in Table 4 and Fig 9. This treatment results in an increase in root and shoot length, root and shoot dry and fresh weight by 56.08, 46.08, 27.77 and 47.22 % respectively as compare to plants which were not inoculated with P.indica.
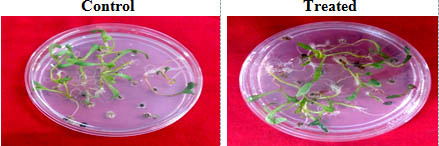
Fig. 7. Seed germination in petriplate

Fig. 8. Plant growth after 20 days of inoculation
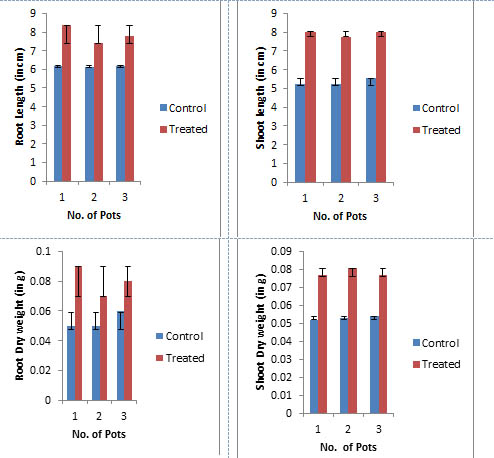
Fig. 9. Different Growth Parameters of Spinach (Spinacea oleracea) after 20days
Table (4):
Different Growth Parameters after 20 days
Characters |
Control |
Treated |
Changes (%) |
|---|---|---|---|
Seed germination |
66.6 |
88.8 |
13.8 |
Plant height |
5.49±0.06 |
8.02±0.01 |
46.08 |
Root length |
1.44±0.01 |
2.26±0.02 |
56.94 |
Shoot dry weight |
0.72±0.05 |
0.92±0.05 |
27.77 |
Root dry weight |
0.36±0.02 |
0.53±0.02 |
47.22 |
Root colonization
Root sample of treated plant stained were collected and stained with with lactophenol cotton blue and then observed under microscope. Microscopic observation showed the presence of inter and intracellular spores which proves that fungus enters in roots through root hairs and then grows in root cortex (Fig10).
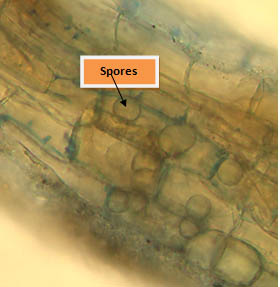
Fig. 10. Root colonization
In literature there are a variety of different media to grow different groups of fungus, but there is not any previous work which shows the axenic production of obligate symbiotic fungus as they can not grown on synthetic media without any plant host22. So, to grow AM fungi without a symbiotic association is a serious concern. But, P. indica is capable to grow axenically under laboratory conditions on a variety of readymade media. But these media is very expensive for mass production of fungus. As P. indica greatly improves the overall plant biomass and growth of different economically and medicinally important plants. Due to these beneficial properties of P. indica , there is a need to standardized a best possible nutrient medium for fungal growth23. This optimized media should be capable of providing all the nutrients for better growth24. To commercialize this magic fungus we have to focus on standardized the cost effective media. An advanced cost reduction technology is achieved by improving the better consumption of resources25. This cost effective method should not effect the quality and quantity of fungal spores and its positive effect on plant growth.23explain that substrate availability and consumption can drastically effect the spore count and biomass. Our results are also match with findings in all previous reports. In current work, the growth and spore count of fungus is different on different Jaggery media. But Safal Jaggery medium gives high number of effective spores.
The results of experiment of Co-cultivation of P.indica biomass with Spinach (Spinacia oleracea) indicates that P.indica biomass grown on Safal Jaggery medium has positive effect on different growth parameters of plant. As in treated plant there is increase in root and shoot length and weight as compare to uninoculated one. It means there is no change in the Viability of P.indica spores. This matches with the observations mentions in previous invesitigations5,27,28,4.
On the basis of all observations it is clear that Safal Jaggery medium is a highly cost effective energy source which is easily available. As cost of one litre Hill and Kaefer media is about 450/- rs on the other hand same quantity of Safal Jaggery medium is cost less than 2/- rs. Therefore, Safal Jaggery medium is a best and cheap energy source which provide all nutrients to fungus to produce highly active spores to induces plant growth.
P.indica is a well known endophytic fungus which is beneficial for a variety of plants as it increases plant growth rate. The present work has a aim to standardized an improved and cost effective energy source for mass cultivation of this magic fungus. Safal Jaggery medium which is a derivative of sugar cane was the cheap and cost effective source to grow P.indica on a large scale. It is a low priced substarte which is ecofriendly and appropriate to use as a biofertilizer to improve agriculture.
- Verma, S., Varma, A., Rexer, K. H., Hassel, A., Kost, G., Sarbhoy, A., Bisen, P., Bütehorn, B. and Franken, P. Piriformospora indica, gen. nov. sp. nov., a new root-colonizing fungus. Mycologia, 1998; 90: 896–903.
- Singh, A., Rajpal, K., Singh, M., Kharkwal, A.C., Arora, M. et al, 2013. Mass cultivation of P. indica & Sebacina Species. In: Varma A, Kost G and Oelmuller R (eds) Piriformospora indica, sebacinales & their biotechnological applications, (Springer-Verlag, Berlin Heidelberg), pp. 377-392.
- Bagde, U. S., Prasad, R., & Varma, A. K. Influence of culture filtrate of Piriformospora indica on growth and yield of seed oil in Helianthus annus. Symbiosis (USA), 2011; 53: 83-88. http://dx.doi.org/10.1007/s13199-011-0114-6.
- Pham, H. G. Interaction of Piriformospora indica with diverse microorganisms and plants. In: Varma A, Abbott L K, Werner D, Hampp R (eds) Plant Surface Microbiology (Berlin: Springer-Verlag), 2004; pp. 237-265.
- Varma, A., Singh, A., Sudha, S. N., Sharma, J., Roy, A., Kumari, … Kranner, I. Piriformospora indica: A cultivable mycorrhiza-like endosymbiotic fungus. In K. Esser, & P. A, Lemke (Eds.), Mycota IX (pp. 123-150). 2001; Germany: Springer-Verlag.
- Peškan-Berghöfer, T., Shahollari, B., Pham, H. G., Hehl, S., Markent, C., Blank, V., … Oelmüller R. Association of Piriformospora indica with Arabidopsis thaliana roots represent a novel system to study beneficial plant-microbe interactions and involve in early plant protein modifications in the endocytoplasmic reticulum and in the plasma membrane. Plant Physiology, 2004; 122: 465-471. http://dx.doi.org/10.1111/j.1399-3054.2004.00424.x.
- Waller, F., Baltruschat, H., Achatz, B., Becker, K., Fischer, M., Fodor, J., … Kogel, K. H. The endophytic fungus Piriformospora indica reprograms barley to salt stress tolerance, disease resistance and higher yield. Proceedings of the National Academy of Sciences (USA), 2005; 102: 13386-13391. http://dx.doi.org/10.1073/pnas.0504423102
- Barazani, O., Benderoth, M., Groten, K., Kuhlemeier, C., & Baldwin, I. T. Piriformospora indica and Sebacina vermifera increase growth performance at the expense of herbivore resistance in Nicotiana attenuata. Oecologia, 2005; 146: 234-243. http://dx.doi.org/10.1007/s00442-005-0193-2
- Varma, A., Verma, S., Sudha, S. N., Britta, B., & Franken, P. Piriformospora indica-a cultivable plant growth promoting root endophyte with similarities to arbuscular mycorrhizal fungi. Applied and Environmental Microbiology, 1999; 65: 2741-2744.
- Varma A, Sree KS, Arora M, Bajaj R, Prasad R, Kharkwal AC. Functions of novel symbiotic fungus Piriformospora indica. Proc Indian Nat Sci Acad. 2014; 80: 429 441.
- Prasad, R., Bagde, U. S., Pushpangadan, P., & Varma, A. Bacopa monniera L: Pharmacological aspects and case study involving Piriformospora indica. International Journal of Integrative Biology, Singapore, 2008a; 3: 100-110.
- Prasad, R., Sharma, M., Chatterjee, S., Chauhan, G., Tripathi, S., Das, K. S., … Varma, A. Interactions of Piriformospora indica with medicinal plants. In A. Varma, & B. Hock (Eds.), Mycorrhizae 3rd Edition 2008b; (pp. 655-678). Germany: Springer-Verlag.
- Kumari, R., Pham, G.H., Prasad, R., et al, Piriformospora indica: fungus of the millennium. In: Podila G, Varma A (eds) Basic research and applications: mycorrhizae, Microbiology series (New York: IK International-India), 2004; pp. 259–295.
- Bagde, U. S., Prasad, R., & Varma, A. K. Influence of culture filtrate of Piriformospora indica on growth and yield of seed oil in Helianthus annus. Symbiosis (USA), 2011; 53: 83-88. http://dx.doi.org/10.1007/s13199-011-0114-6.
- Hill, T.W. and Kaefer, E. Improved protocols for aspergillus medium: trace elements and minimum medium salt stock solutions. Fungal Genet News Lett., 2001; 48: 20-21.
- Latifian, M., Rad, B., Amani, M. and Rahkhodaei, E. Mass production of entomopathogenic fungi Beauveria bassiano (Balsamo) by using agricultural products based on liquid-solid diphasic method for date palm pest control. International Journal of Agricultural and Crop Sciences, 2013; 5: 2337-2341.
- Alia N, Sardar K, Said M, Salma K, Sadia A, Sadaf S, Toqeer A and Miklas S. Toxicity and Bioaccumulation of Heavy Metals in Spinach (Spinacia oleracea) Grown in a Controlled Environment. Int. J. Environ. Res. Public Health ., 2015; 12: 7400-7416.
- Sharma J and Agarwal S. Impact Of Organic Fertilizers On Growth, Yield And Quality Of Spinach. Indian Journal of Plant Sciences, 2014; 3: 2319–3824.
- Prasad, R., Pham, H.G., Kumari, R., Singh, A., Yadav, V. et al., Sebacinacea: culturable mycorrhiza-like endosymbiotic fungi and their interaction with non transformed and transformed roots. In: Declerck S, Strullu D G, Fortin A (eds)In vitro culture of mycorrhizas. Soil Biology, 2005; 4 (Springer-Verlag, Berlin Heidelberg), pp. 291-312.
- Varma, A., Sherameti, I., Tripathi, S., Prasad, R., Das, A. et al., The Symbiotic Fungus Piriformospora indica: Review. In: Hock B (ed) Fungal Associations, 2nd edn, The Mycota, vol IX, (Springer-Verlag Berlin Heidelberg, New York), 2012; pp. 231-254.
- Singh, A. N., Singh, A. R., Kumari, M., Rai, M. K., & Varma, A. Biotechnological importance of Piriformospora indica. A novel symbiotic mycorrhiza-like fungus: An Overview. Indian Journal of Biotechnology, 2003; 2: 65-75.
- Hart, M.M. and Trevors, J.T. Microbe management: Application of mycorrhyzal fungi in sustainable Agriculture. Frontiers in Ecology and the Environment, 2005; 3: 533-539.
- Kumar, V., Sahai, V. and Bisaria, V.S. High density spore production of Piriformospora indica, a plant growth promoting endophyte by optimization of nutritional and cultural parameters, Bioresource Technology,2011; 102: 31693175.
- McGinnis, M.R. and Rinaldi, M. G. 1991. Antifungal Drugs: Mechanisms of action, drug resistance, susceptibility testing, and assays of activity in biological fluids, in Antibiotics in laboratory medicine. In: V. Lorian (ed) , 3rd edn, vol V, Williams and Wilkins Baltimore, Md), pp. 198–251.
- Savangikar, V. A. 2002. Role of low cost options in tissue culture. In: Low cost options for tissue culture technology in developing countries. Proceedings of a technical meeting organized by the Joint FAO/IAEA Division of Nuclear techniques in food and agriculture. Vienna, IAEA, pp. 11-15.
- Nautiyal, C. S., Chauhan, P. S., Mehta, D. S., Seem, K., Varma A., & Staddon, W. J. (2010). Tripartite interactions among Paenibacillus lentimorbus NRRL B-30488, Piriformospora indica DSM 11827, and Cicer arietinum L. World Journal of Microbiology and Biotechnology, 26, 1393-1399. http://dx.doi.org/10.1007/s11274-010-0312
- Bagde, U. S. Impact of Bio inoculants on economically important plants: Development and formulations. D. Sc. thesis submitted to Amity University Uttar Pradesh, India 2010a.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


