ISSN: 0973-7510
E-ISSN: 2581-690X
Initial visual screening for most applicable strains visually based on cell-bound pigmented colonies from different sources in the Egyptian environment. Samples (n=25) of soil, milk, laban rayeb, and kareesh and Ras cheeses were screened for strains capable of producing carotenoids. Seven isolates showed fast growth rates and high content of carotenoids were further characterized. Physiological and molecular identification of the isolated yeast was done using API 32C and sequencing of 18S rRNA gene. The produced sequences were compared with reference 18S rRNA gene sequences available in NCBI GenBank database. Five yeasts strains (S-361, S-37, S-33, S-11 and S-10) out of seven were identified as R. diobovata [with accession no. KX866280, KX866279, KX866278, KX866276 and KX866275 respectively] and the rest were classified as R. mucilaginosa (S-5 and M-30) with accession no: KX866274 and KX866277. The carotenoid derivatives produced by R. diobovata S-361 and R. mucilaginosa S-5 strains were identified by chromatographic analysis (TLC and HPLC) to be b-carotene as a major fraction, torulene, torulene-like and torulahodine. The ability of R. diobovata S-361 strain to produce carotenoids from lactose hydrolyzed salt whey in bioreactor and high aeration led to promote growth resulted in high biomass production (22.7 g/l at day five). Also the production of yeast biomass was about 3.5 times higher yield than in Erlenmeyer flasks while high cellular carotenoids production obtained at day four (147 µg/g) and torulene was the most dominant carotenoids fraction, Cellular and volumetric carotenoids production in batch fermentation during 5 days was investigated. The highest cellular carotenoids were recorded in pellets of the culture of R. diobovata S-361.
Carotenoids, Rhodotorula diobovata, Rhodotorula mucilaginosa, Soil, Dairy Products, Salt Whey, Bioreactor
Carotenoids are one of the most important classes of pigments. More than 700 derivatives of carotenoid pigments are evidently occurring. Animals and human beings are not capable of synthesis carotenoids and diet is representing a sole source. Most frequent carotenoids detected in human plasma are a-carotene, b-carotene, lycopene, lutein, zeaxanthin, and b-cryptoxanthin1. Many meal ingredients such as vegetables, fruits, egg yolk, fish e.g. salmon and trout and crustaceans represent a great source of carotenoids.
In addition to be natural pigments, carotenoids also have crucial organic and curative activities. It’s widely recognized that few carotenoid types are act as precursors of vitamin A. Recently, carotenoids either in vitamin A active and inactive forms had been found to exert valuable effects on human health include immunity improvement and decrease of the risk for degenerative diseases such as cancers, cardiovascular diseases, macular degeneration and cataract2,3. Carotenoids were linked to the healthy conditions of the liver, prostate, breast, colon, and lungs 4. It is act predominantly as antioxidants; the antioxidant effect of carotenoids may also protect sperm health which improves male fertility5. Carotenoids have anti-inflammatory properties 6, promote cell-to-cell communication7 and improve mental acuity 8. Thus, carotenoids represent one of the highest valuable group of compounds for industrial utilization, e.g. within the pharmaceutical, chemical, food and feed industries.
Despite the availability of variety of natural and synthetic carotenoids, microbial carotenoids have attracted much attention in recent years 9. There are several microbes like algae, fungi, yeasts and bacteria reported to produce carotenoids10. Yeasts are ubiquitous unicellular eukaryotes, simply grown and naturally occurring in environment such as soil, fresh and marine water, animals, and on plants and also foods. Red yeasts are a group of yeast species able to accumulate carotenoid pigments include b-carotene, torulene, and thorularodin which responsible for coloring colonies by yellow, orange and red colors. Rhodotorula sp, Phaffia sp and recently Candida sp are the most dominant yeast species able to effectively synthetize these molecules with high growth potential 11.
Production of Egyptian white and hard cheeses results in high amount of waste salt whey. Salt whey contains lactose (4-4.5%), whey protein (0.8%), lactic acid (0.1– 0.8%) in addition to 1-4 % salt which are considered as dangerous organic pollutant. Annually it estimated that cheese manufacture produced more than 110 million metric worldwide12.In 2000, it is reported that the produced whey in Egypt is increasing steadily with amount reached to 1,452,500 metric tons in 2000 13. Domiati and Ras cheeses are the main source of salt whey while, the highest salt level is come from Domiati cheese (8- 15%) flowed by Ras cheese making as it is resulted in only about 2-5% salt in whey. High level of salinity represents an obstacle to consume salt whey as food product unlike sweet whey 14, 15. Furthermore, whey has a high biological (BOD) and chemical oxygen demand (COD) amounted to 35-40 g/l and 50 kg O2/t permeate respectively, resulted in disposal problem 16, 15. In Egypt, the most common practice of cheese manufacturing facilities is to get rid of salt whey by performing land spreading which lead to elevate soil salinity increasing the risk of crop damage 17. Salt whey is applied as an ingredient in processed cheese 18. Many applications have been proposed like using it as a medium for the cultivation of fungi producing milk clotting enzymes 15, in alcoholic and non-alcoholic beverage, bakery products, the production of single cell protein, and as an additive in dairy products 19. Ultrafiltration technology (UF) is a technique used to split whey into retentate containing proteins from permeates which is mostly lactose. Whey protein concentrate (WPC) is a highly nutritious part of diet become to be very popular worldwide. Furthermore, whey has been attracted food and pharmaceutical industry to be used as an inexpensive medium fermentation. Whey contains suitable amount of lactose which could be used as a low-cost carbon source for lactose fermented microorganisms. Moreover, it has a sufficient content of minerals and vitamins provide valuable nutrients able to enhance microbial growth and bio-products formation. Production of carotenoids from yeast by utilizing lactose as an effective cost carbon source looks to be a promising sustainable approach to handle salt whey waste 20. Production of microbial carotenoids is presently limited by the high cost of production. However, the cost of carotenoid production by fermentation can be minimized by selecting of powerful producing organisms, optimizing its process, and by using cheap industrial by-products as nutrient sources. In regard to the promising opportunities that Rhodotorula species might offer for the nutraceutical industry, the present study was to select and identify highly carotengenic strains of Rhodotorula sp., from Egyptian environment.
The present study describes finding and identifying yeasts from the Egyptian environment, capable of producing carotenoids of possible commercial importance with ability to resist high level of salt in waste salt whey and converting it to a valuable product rich in carotenoids in batch bioreactor fermentation process.
Materials
Dehydrated media used throughout this study were namely Sabouraud Dextrose Agar (SDA), Yeast Malt Extract Agar (YMEA), and Potato Dextrose Agar (PDA). The media were prepared according to the manufacturer’s instruction (Oxoid, UK and BD, USA). Other chemicals were of analytical grades obtained from different sources from local suppliers.
Sample collection
Twenty five samples were included ten samples of dairy products i.e. fresh cream (1), Laban rayeb (2), Kareish cheese (3) and Ras cheese (4) and fifteen soil samples which were collected from Alexandria (9), Port Said (1), Mansoura (1), Beheira (3) and Kafr El Sheikh (1) governorates, Egypt. Samples were collected transferred directly under aseptic conditions immediately to the laboratory and subjected to the isolation of carotenogenic yeasts.
Isolated samples processing
Collected sample (25g) was suspended in 225 ml of peptone buffer, then spread (100 µl) on Potato Dextrose Agar (PDA), Yeast Malt Extract Agar (YMEA) and Sabouraud Dextrose Agar (SDA) and incubated at 30°C for 3 day. Red, orange and pink pigmented colonies were picked, streaked and purified on Yeast Malt Extract Agar (YMEA) and Sabouraud Dextrose Agar (SDA). Single colonies were streaked on YMA slant, incubated at 30°C for 2 days and stored at 4°C as stocks for further investigations. For preservation of stock cultures, washed pellets were stored at -80°C in Malt Both-Glycerol (50/50, v/v).
Phenotypic characterizations
The yeast isolates were identified based on their morphological and physiological characteristics according to procedures described by Van der Walt and Yarrow 21.
Culture characterizations
YMEA and SDA were inoculated by fresh yeast culture and incubated at 25°C for 3 days. The colour, the surface appearance, margin and elevation were described
Cell colour estimation
The colour of pigmented cells on culture plates and after pellet separation by centrifugation were determine by Munsell colour charts22 ( Munsell colour Division, Maryland, USA)
Cell characteristics
The yeast isolates were inoculated in YMB medium and incubated for 2-3 days at 25°C and then examined the cell shape under the microscope.
Pellicle formation
The formation of film or pellicle on the surface of broth cultures was detected by inoculating the yeast in YMB medium and incubated for 2-3 days at 25°C.
Vegetative propagation
The yeast isolates were inoculated in YMB and incubated for 2-3 days at 25°C, and then investigated microscopically for the production of conidia and observing budding and fission.
True hyphae formation
The ability of isolates to produce true hyphae was examined microscopically after growth on YME agar at 25°C for 2-7 days.
Scanning electron microscopy (SEM)
Yeast pellets were removed and fixed by immediately immersing in 4F1G (Fixative, phosphate buffer solution) and left at pH 7.4, 4oC for 3 hours. Then fixed in 2% OsO4 using the phosphate buffer and kept at 4°C for 2 hours. Samples were washed in the buffer and dehydrated at 4°C using ethanol gradient of 30, 50, 75, and 100%. Then dried by means of a critical point method, mounted using carbon paste on an Aluminum- stub and then coated with gold up to a thickness of 400 Ao in a sputter – (coating unit JFC-1100 E). Specimen examination was performed using a Jeol JSM- 5300 Scanning Electron Microscope operated between 15 and 20 KeV 23 at the Electron Microscope Unit, Faculty of Science, Alexandria University.
Physiological characterization
Physiological identification of yeast isolates was done using the API ID 32C system (BioMerieux SA, Marcy L’Etoile, France). Strips were inoculated with a 72 h culture pre-grown at 25°C on YME agar. The results were expressed as numerical bio codes, and the isolates were identified through the use of the ID 32C Analytical Profile Index 24, 25.
Molecular Identification of isolated strains
Strains showing production of carotenoids were subjected to molecular characterization by sequencing of 18s rRNA gene at SolGent Company (34014 Daejeon, South Korea; http://www.solgent.com/eng/). Fresh cultures were cultivated on PDA at 25° C for 7 days. Isolates were individually scraped and suspended in 100µl distilled water in 2 ml sterile vials and boiled at 100°C for 15 minutes. DNA was extracted and isolated using SolGent purification bead. Prior to sequencing, the ribosomal rRNA gene was amplified using the polymerase chain reaction (PCR) technique in which two universal fungal primers ITS1 (forward) and ITS4 (reverse) were incorporated in the reaction mixture. Primers used for gene amplification have the following composition: ITS1 (5′ – TCC GTA GGT GAA CCT GCG G – 3′), and ITS4 (5′- TCC TCC GCT TAT TGA TAT GC -3′). PCR products were sequenced in the sense and antisense directions using ITS1 and ITS4 primers 26. The purified PCR products (amplicons) were reconfirmed using a size nucleotide marker (100 base pairs) by electrophoreses on 1% agarose gel. Then these bands were eluted and sequenced with the incorporation of dideoxynucleotides (dd NTPs) in the reaction mixture. Sequences were further analyzed using Basic Local Alignment Search Tool (BLAST) from the National Center of Biotechnology Information (NCBI) website and GenBank database. Phylogenetic analysis of sequences was done with the help of MegAlign (DNA Star) software version 5.05. The sequences of identified strains were deposited in the GenBank and received accession ID’s (https://www.ncbi.nlm.nih.gov/Genbank).
Carotenoids extraction and identification
Carotenoids extraction
For carotenoid identification, isolates were cultivated in 100 ml of YME broth at pH 5.5 and incubated at 30°C in shaking incubator (120 rpm) for 3 days. Extraction of carotenoids was carried out according to Latha 27 and Yadav 28 with some modification. A portion of 100 ml of culture media was centrifuged at 6,000 g for 10 min at 10°C then cells washed twice with distilled water. Collected cell pellets were lysed in HCL 0.5 N (1:1) in water bath at 90°C for 20 min. Cells spine at 6000 g for 10 min, washed twice by distilled water then to obtain complete cell rupture a Sonicator (20 kHz) was applied with overall operation time of 10 min. Sonication was performed with bursts of 30-40 s and chill the suspension between ultrasonic bursts. Extraction of carotenoids was done by adding 10 ml of acetone to lysis cells which were then removed by centrifugation. Cells were examined under microscope to ensure complete cell lysis.
Determination of yeast dry weight
Yeast dry weight was determined by drying the yeast pellet obtained by centrifugation of broth culture at 3000 rpm at 85oC to a constant weight 29. Dry biomass was expressed as g/l.
Determination of total carotenoids content by UV-Visible Spectroscopy
The content of total carotenoids was determined by measuring the optical density of the extracted cell in acetone at 490 (lmax of our extraction solution) using helio alpha Thermo spectrophotometer (ThermoFisher Scientific, England). The total amount of carotenoids was calculated according to the equation proposed by Davies 30 :-
Total carotenoid (µg/g of yeast) as Torulene = (A max × D × V/ (E × W) …(1)
Where:
A max: the absorbency of total extract carotenoid at 490 nm
D: sample dilution ratio
V: volume of extraction solvent (ml)
W: dry weight of yeast (g)
E: The extinction coefficient of torulene in acetone [E ] 0.16 was used. Also the yield of total carotenoids (as torulene) obtained by one liter of culture was calculated as mg/l 31.
Volumetric carotenoids (mg/l) as Torulene = Dry biomass (g/l) × total carotenoids content (µg/g dry cells) ×10 -3 …(2)
Identification of carotenoids by Thin Layer Chromatography (TLC)
The carotenoids were separated by thin layer chromatography (TLC) on stationary phase of silica gel plates (Silica Gel G-60, 10 × 20 cm, 0.25 mm of thickness, Merck, Germany) using 5% benzene in petroleum ether (b.p. 80-100°C) as a mobile phase 27. The separation was developed for 1 h, the distance between baseline and spots was measured (Rf). The separated pigments were identified by their Rf values and measuring their maximum absorption 32. Further, the individual spots were sprayed with a saturated solution of antimony pentachloride (SbCl5) in chloroform (1:10 v/v) for confirmation 33.
HPLC analysis
The carotenoid extracts were re-dissolved in 1 ml acetone with the aid of sonication. The extract was filtered through cellulose acetate filters (0.45 mm; VWR International) before injection. The sample was analyzed using Agilent 1260 Infinity LC Separation Module (Agilent Technologies, Inc., USA) equipped with a Multiple Wavelength Detector (MWD). An aliquot of 10 µl extract was then injected into a reversed-phase C18 analytical column (Zorbax SB-C18 with 5µm particle size) while the column thermostat was set at ambient temperature. The isocratic mobile phase composed of methanol and acetonitrile (9:1, v/v) and run at a flow rate of 1.5 ml/min at room temperature. The detector was operated in scanning mode in the 390–700 nm wavelengths 34. b-Carotene purchased from Sigma–Aldrich (St.Louis, MO) was used an external standard.
Production of carotenoids by R. diobovata S-361 in batch bioreactor
Due to inability of R. diobovata S-361 (as all Rhodotorula sp) to assimilate lactose in whey as carbon source, whey is enzymatically hydrolyzed by b-galactosidase 35 into glucose and galactose. The fermentation was run in MS programmable bench top fermenter system MS-F1- 5L (MS Major Science ® USA) with 4 L working volume equipped with pH electrode (0-14; Finesse, USA); Antifoam probe 316 stainless steel with insulated tube; programmable temperature controller; PID cooling controller coil in the inner of the vessel with control valve; heating bottom plate Temp probe Pt 100; 0~90oC. Four built-in programmable pumps can be assigned to function via the keypad. These include addition of acid, base, chemical, defoamer and nutrients as well as the harvesting of culture. Adjustable flow rate for each easy load pump head programmable steps for each feeding application was applied. The fermentation conditions were set at 250 rpm agitation speed, 5 L/h aerated speed, 30oC and 5.5 pH adjusted with 1.5 M NaOH or HCl. Salt whey (3 %NaCl) was de-proteinized by autoclaving (10 min at 100°C), then filtered and finally hydrolyzed by b –galactosidase (Chr. Hansen, Germany) at pH of 6.5, temperature of 45 °C and 250 rpm of agitation for 4 hrs 36. The fermenter filled with prepared salt whey then autoclaved (15 min at 120o C). After cooling to 30 o C the filtrate sterilized 0.05/L MnSO4.H2O and 11g /L glucose was added then fermenter was inoculated inoculum size of 5 %. Cells were harvested after 2, 3, 4 and 5 days. Dry biomass, growth rate and total carotenoids were determined.
Isolation of pigmented cultures
Initially, thirty eight pigmented cultures were isolated out of 25 samples included 15 soil samples (n= 35 isolates) and 10 dairy samples (n= 3 isolates) as shown in Table 1 and Figure 1. Many studies has been documented isolation of pigmented yeast from soil. Deak and Beuchat 37 reported that soil and plant surfaces are the natural habitats of Rhodotoula glutinis, Rhodotorula mucilaginosa and Rhodotorula minuta. They also mentioned that pink and red pigmented cells of Rhodotorula sp. were commonly found in air born isolates. Issa et al. (2015) isolated Rhodotorula strains from different sources (leaves trees, soils, meats, dairy products, pickle and traditional sweet). Yadav 38 isolated the pigmented of Rhodotorula sp strains from air, apple, milk and yoghurt.
Table (1):
Distributions of the total collected samples in the present study
Source |
No. of samples |
No. of isolates |
Percent of isolates |
Location |
|---|---|---|---|---|
Soil |
9 |
18 |
47.37 % |
Alexandria |
Soil |
1 |
3 |
7.9 % |
Kafr El Sheikh |
Soil |
3 |
10 |
26.32 % |
Beheira |
Soil |
1 |
0 |
0 |
Mansoura |
Soil |
1 |
0 |
0 |
Port said |
Ras cheese |
4 |
1 |
2.63 % |
Local market/ Alexandria |
Kareish cheese |
3 |
2 |
5.26 % |
Local market/ Alexandria |
Laban rayeb |
2 |
4 |
10.52 % |
Local market/ Alexandria |
Fresh cream |
1 |
0 |
0 |
Local market/ Alexandria |
Total |
25 |
38 |
100 % |

Fig. 1. Streaking of pigmented isolates on PDA, SDA and YMEA media
Seven isolates were selected on the basis of their ability to produce tangerine pigments, rapid growth in YME broth and agar with good biomass production and ease to separate by centrifugation. The selected isolates were subjected for further identification; Table 2 presented the results of the morphological, phenotypic and physiological characterizations of the yeast cultures by using of API ID 32 C. The morphological and microscopic features of selected pigmented isolates (n=7) showed a similarity of the main characteristics. The colonies of isolates appeared ovoidal, convex elevating with entire margin, and mucoid with smooth/glossy appearance. The isolates have a colony colour range from orange-red to dark-red 5R/7/8 to 10R/6/14) when compared by Munsell colour charts as shown in Figure 2. They don’t form pellicle on the surface of broth media or produce pseudohyphae and multilateral budding. All cultures were classified as Rhodotorula sp. These characteristics of the yeast isolates matched those of Rhodotorula sp described by Kurtezman and Fell 39. Al-Turki 40 also reported the multilateral budding and the absence of both ascospores and pseudohyphae in Rhodotorula sp.
Table 2. Morphological and phenotypic characterizations of selected pigmented yeast strains

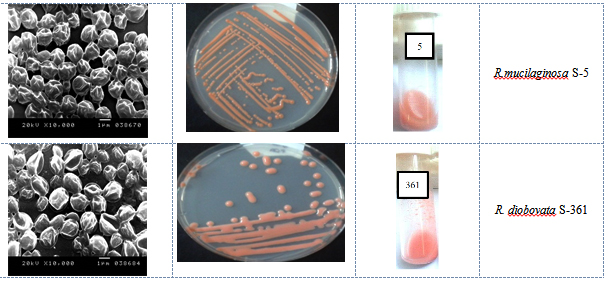
Fig. 2. Growth of yeast isolates grown on PDA and YME at 30°C for 3 days, and the morpholo-gy was monitored under SEM at 10000x magnitude
Seven yeast isolates were further subjected to molecular analysis using the polymerase chain reaction (PCR) technique and sequencing of 18S rRNA gene 26. Which were carried by Solgent Company, Daejeon (South Korea). Phylogenetic tree of partial 18S rRNA sequences of the seven isolates (S-5, S-10, S-11, R-30, S-33, S-37 and S-361) was constructed with closely related sequences accessed from the GenBank as shown in Fig. 3.
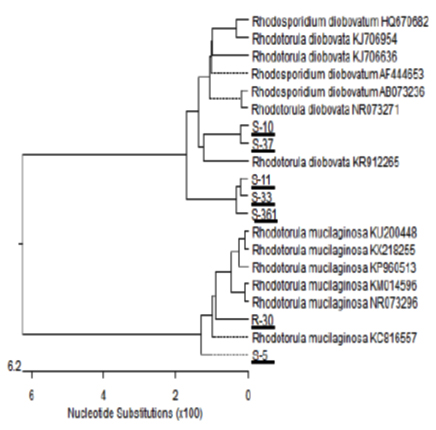
Fig. 3. Phylogenetic tree of 18S sequences of the isolates aligned with the closely re-lated sequences in the GenBank
Carotenoids production
After primary cultivation of isolates; Cells were harvested and the carotenoids were solvent extracted after using last modified method to obtain completely crude carotenoid extract. The maximum absorption spectra of total extracted carotenoids by isolates were shown that the absorbances were dependent on solvent phase and major carotenoid derivatives. In present study; acetone solvent was in range of 487 to 490 nm as shown in Fig.4. The dry biomass of yeast isolates and carotenoids concentration and yield ranged from 5.6 to 7.76 g/l, 0.73 to 1.261 mg/l and 105.5 to 217.4 µ/g respectively as shown in Table 3. Highest cellular carotenoids were observed in pellets of the culture cultivated R. diobovata S-361.
Table 3. Dry biomass (g/l), volumetric carotenoids (mg /l) and cellular carotenoids (μg/g) produced by isolated strains
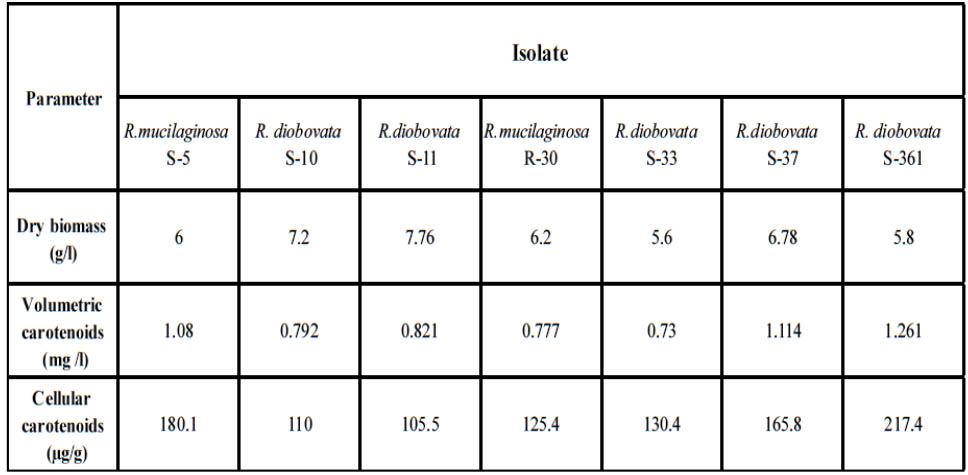
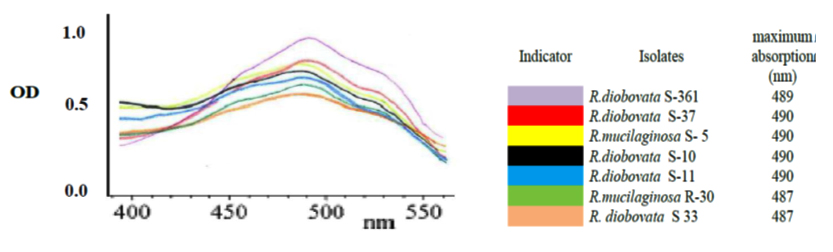
Fig. 4. The maximum absorption spectra of the total extracted carotenoids by the iso-lates using acetone as a solvent
Visual identification of carotenoids
As carotenoid pigments are rapidly disappearing after development of TLC, separated pigments were visible (Fig 5). The presence of carotenoids was confirmed by staining the plate with antimony pentachloride (SbCl5) dissolved in chloroform and this produced blue colours. Naziri 33 reported that staining of carotenoids by SbCl5 is resulted in blue spots. Further staining of the plate with iodine vapour in an airtight chamber resulted in brown colour. This method was reported to be one of the most sensitive methods for the identification of carotenoids 41.
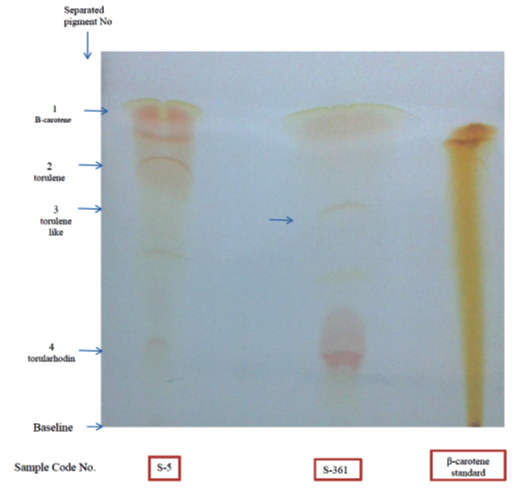
Fig. 5. Thin layer chromatogram of the extracted carotenoids from R. mucilagi-nosa S-5 and R. diobovata S-361 using mobile phase [benzene: petroleum ether (5:95 v/v)]
HPLC analysis of extracted carotenoids from R. mucilaginosa S-5 and R. diobovata S-361
Separation of extracted carotenoids from both selected stains R. mucilginosa (S-5) and R. diobovata (S-361) showed three components identified as b-carotene as a major fraction, torulene and torulahodine while later two components separated at retention times of 2.39 and 2.56 min respectively (Figure 6) Torulene and torulahodine (Thr) were identified by HPLC and separated on TLC. In accordance with the present results, the same sequence of separation of carotenoid ingredients was previously published by Al Turki 40 for R. mucilginosa. Meanwhile, a carotenoid unknown fraction (2.32 min) was detected in carotenoids extraction produced R. mucilginosa (S-5). It is reported that Urediniomycetes yeast including genus of Rhodotorula sp. and Sporobolomyces sp. and their teleomorphic analogs Rhodosporidium sp. and Sporidiobolus sp., mainly produced thee carotenoid types, called b-carotene, torulene, and Thr 42. The pigments composition was the same as determined in previous researches by Latha and Jeevaratnam 43 and Moliné 44 of Rhodotorula sp. Meanwhile, HPLC analysis of carotenoids produced by R. diobovatum (known now as R. diobovata) Sea 2–11 revealed of presence of five types of carotenoid included b-carotene, torularhodin, g-carotene, torulene and b-zeacarotene 45.
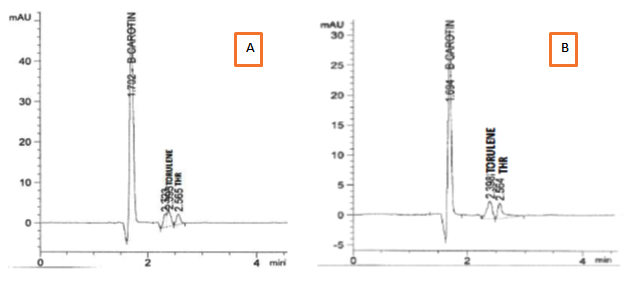
Fig. 6. HPLC spectra of the extracted carotenoids from : A) R. mucilagi-nosa S-5 . B) R. diobovata S-361
Production of carotenoids from salt whey in batch –fermentation
In the present study R. doivbovata (S-361) is selected to utilize salt whey since it showed higher capability to produce carotenoids and more tolerable to salt compared to R. mucilaginosa (S-5). Pre-processing of salted whey with
b-galactosidase enzyme was indispensable step to break down lactose into monosaccharides (glucose and galactose). Preliminary flask fermentation trials confirmed inability of the organism to initiate growth in whey as members of Rhodotorula sp lack of lactase enzyme. Hence, innovative step applied using hydrolysis of lactose by adding of b-galactosidase for 45 °C and 250 rpm of agitation for 4 hours. The growth and carotenoids production were both monitored in the batch bioreactor under dimed light and aerated conditions. The results showed the high ability of R. doivbovata (S-361) to utilize hydrolyzed salt whey under cultivated conditions and produced high biomass and carotenoids.
The growth curve characteristic of R. doivbovata (S-361) exhibited typical two-phase character with prolonged stationary phase (Fig. 7). This might be due to the ability of the yeast cells to utilize lipid storages formed during growth as additional energy source 46. In comparing to shaking flask trial (present study) high aeration in fermentor led to promote growth resulted in high biomass production (22.7 g/l at day five). In laboratory fermentor (4 L) experiment with R. doivbovata (S-361), the production of yeast biomass was about 3.5 times higher yield than in Erlenmeyer flasks. The growth rate was high as 0.21 (1/h) with R2 =0.98 which reflects the suitability of salt whey medium and culture conditions to support growth. Along stable stationary phase, noticed from second day till the end of experiment at (120 hrs) day five (Fig. 7 and 8).
In fermenter, 0.18 mg/l carotenoids were reached after 48 hrs of incubation. The production proceeded and reached its maximum after 96 hrs to record 2.1 mg/l. Meanwhile, carotenoids production showed fluctuations as it is noticed in Figures 7 and 8, as the production tends to decrease from the fifth day of incubation (1.5 mg/l). The carotenoids content changed during fermentation period. At 48 and 72 hr the maximum absorbance of carotenoids extracts was at 460 nm wavelength indicating the production of b-carotene latter on the torulene became most dominant carotenoids fraction as the maximum absorbance was at 488 nm (Figures 7 and 8 ). In agreement with the present results, Tkáèová 47 found that R. glutinis JMT 21978 they found b-carotene was the predominant molecule synthesized as long as glucose is still presented in growth medium. El-Banna 48 found that R. glutinis grown on a medium at a low C/N ratio and low glucose concentration synthesized torulene and Thr as the main carotenoids meanwhile, high C/N ratio led to produce b-carotene as a major pigment. In contradiction, it is reported that the maximum production of b-carotene occurred when R. glutinis was grown in a medium with a low C/N ratio containing a high concentration of both carbon and nitrogen sources 49.
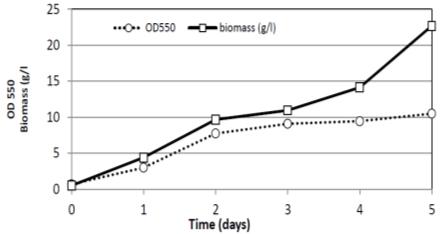
Fig. 7. Growth and biomass production of R. doivbovata (S-361) grown on salt whey in batch fermentation during 5 days of incubation under aerated condition at 30°C
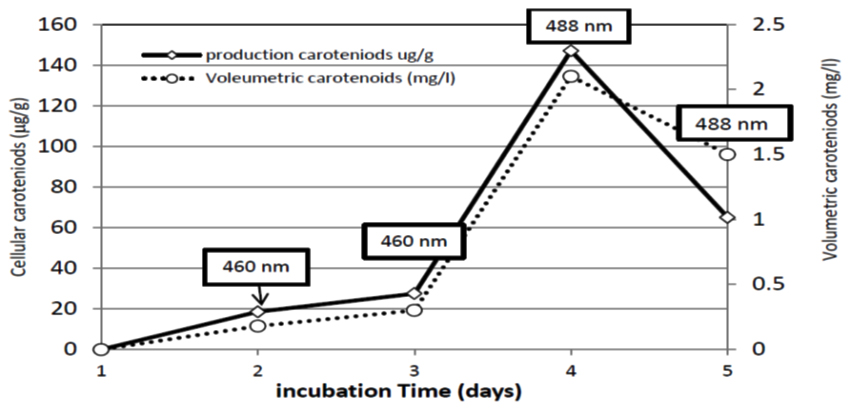
Fig. 8. Cellular and volumetric carotenoids production by R. doivbovata (S-361) grown on salt whey in batch fermentation during 5 days of incubation under aerated conditions at 30°C. Values in box indicate maximum absorbance wavelength of carotenoids extracts at each time interval
- Peng, J., Yuan, J.P., Wu, C.F., Wang, J.H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: metabolism and bioactivities relevant to human health. Mar Drugs., 2011; 9(10):1806-28.
- Astorg, P. Food carotenoids and cancer prevention. An overview of current research. Trends Food Sci. Technol. 1997; 8: 406-413.
- Krinsky, N.I., Johnson, E.J. Carotenoid actions and their relation to health and disease. Mol Aspects Med., 2005; 26:459–516.
- Trejo-Solís, C., Pedraza-Chaverrí, J., Torres-Ramos, M., Jiménez-Farfán, D., Cruz, S. A., Serrano-García, N., Osorio-Rico, L., Sotelo, J., Multiple Molecular and Cellular Mechanisms of Action of Lycopene in Cancer Inhibition. Evidence-Based Complementary and Alternative Medicine 2013:705121.
- Sayo, T., Sugiyama, Y., Inoue, S., Lutein, a nonprovitamin A, activates the retinoic acid receptor to induce HAS3-dependent hyaluronan synthesis in keratinocytes. Biosci Biotechnol Biochem, 2013; 77: 1282–1286.
- Khachik, F., Pfander, H., Traber, B. Dietary caroternoids and their metabolites as potentially useful chemoprotective agents against cancer. In: Antioxidant Food Supplements in Human Health, L. Packer et al., eds., Academic Press , 1999; pp. 203–229.
- Gartner, C., Stahl, W., Sies, H., Lycopene is more bioavailable from tomato ¨ paste than from fresh tomatoes. Am J Clin Nutr 1997; 66:116–22
- Akbaraly, N.T., Faure, H., Gourlet, V., Favier, A., Berr, C., Plasma carotenoid levels and cognitive performance in an elderly population: Results of the EVA study. J. Gerontol. A Biol. Sci. Med. Sci., 2007; 62: 308–316.
- Agnieszka, K., Malgorzata , B. Nutrition, Analysis and Technology Edt., 1ed Edt. Carotenoids John Wiley & Sons, Ltd. 2016.
- Ralf , M.S., Reinhold,C., Carotenoid production by bacteria, microalgae, and fungi, IN: Carotenoids: Nutrition, Analysis and Technology Edt. Agnieszka Kaczor and Malgorzata Baranska, 1ed John Wiley & Sons, Ltd., 2016; 217-240.
- Aksu, Z., Eren, A.T., Carotenoids production by the yeast Rhodotorula mucilaginosa: Use of agricultural wastes as a carbon source. Process Biochemistry, 2005; 40: 2551-2557.
- Briczinski, E.P., Roberts, R.F., Production of an Exopolysaccharide-Containing Whey Protein Concentrate by Fermentation of Whey. J Dairy Sci., 2002; 12: 3189-3197.
- Zhang, X., Kilmer, R.L., Muhammed, A. A., descriptive analysis of Egypt and Saudi Arabia import United States dairy products. Int. Agric. Trade Policy Center, Florida University; 2003.
- Sanderson, W.B., Brady, A.P., Whitehead, G.F., Oldham, I.J., Brockwell, I.P., Recycling salt solution in cheese processing and apparatus therefore. Murray Goulburn Co-Operative. Australia, assignee US Pat.,1996; No. 5, 73,237.
- El-Tanboly, E., El-Hof, M., Youssef, Y.B., El-Desoki, W., Ismail, A., Utilization of salt whey from Egyptian Ras (cephalotyre) cheese in microbial milk clotting enzymes production. Acta Sci. Pol., Technol. Aliment, 2013; 12: 9-19.
- Zayed, G., Winter, J., Batch and continuous production of lactic acid from salt whey using free and immobilized cultures of lactobacilli., 1995; 44 (3): 362-366.
- Awad, S., Ehoda, N., Elsoda, M., Application of salt whey from Egyptian ras cheese in processed cheese making. Food and nutrition, 2013; 4: 79-86.
- Code of regulation, US Dept. Health Human Services, Washington DC. section 113.169,2003;
- Gomashe, A. V., Minakshi, A.P., Pranita, G., Liquid Whey: A Potential Substrate for Single Cell Protein Production from Bacillus subtilis NCIM 2010. Int. J. of Life Sciences., 2014; 2: 119-123.
- Aspasia, Anastassios , Biotechnological Utilization with a Focus on Anaerobic Treatment of Cheese Whey: Current Status and Prospects. Energies., 2012; 5: 3492-3525.
- Van der Walt, J.P., Yarrow, D., The genus Arxiozyma gen. nov. ( Saccharomycetaceae). S Afr J Bot, 1984; 3: 340–342.
- Anonymous, Munsell color charts for plant tissues. Munsell color division. Kollmorgen corporation. Baltimore.1972
- Tahmasebi, P., Javadpour, F., Sahimi, M., Three-Dimensional Stochastic Characterization of Shale SEM Images. Transport in Porous Media, 2015; 110(3): 521–531.
- Ramani, R., Gromadzki, S., Pincus, D. H., Salkin, I.F., Chaturvedi, V., Efficacy of API 20C and ID 32C systems for identification of common and rare clinical yeast isolates. J Clin Microbiol., 1998; 36: 3396–3398.
- Deak, T., Beuchat, L.R., Comparison of the SIM, API 20C, and ID 32C Systems for Identification of Yeasts Isolated from Fruit Juice Concentrates and Beverages. Journal of Food Protection. 1993; 56 (7): 585-592.
- White, T.J., Bruns, T., Lee, S., Taylor, J., Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols a guide to methods and applications. Academic Press; San Diego, CA, USA,1990; 315–322.
- Latha, B.V., Jeevaratnam, K., Murali, H.S., Manja, K.S., Influence of growth factors on carotenoid pigmentation of Rhodotorula glutinis DFR-DPY from natural sources. IJBT, 2005; 4:353–357.
- Yadav, S., Manjunatha, K.H., Ramachandra, B., Suchitra, N., Prabha, R., Characterization of pigment producing Rhodotorula from dairy environmental samples. Asian J. Dairying Foods Res., 2014; 33(1): 1-4.
- Shih, C.T., Hang, Y.D., Production of Carotenoids by Rhodotorula rubra from Sauerkraut Brine. Lebensmit-tel-Wissenschaft und-Technologie., 1996; 29( 5-6): 570-572.
- Davies, B.H., Carotenoids. In: Goodwin, T W. (ed.) Chemistry and biochemistry of plant pigments. 2nd edn. Academic Press, London, 1976;38- 165.
- Cheng, Y., Chu-Fang, Yang., Using strain Rhodotorula mucilaginosa to produce carotenoids using food wastes. Journal of the Taiwan Institute of Chemical Engineers, 2016; 61: 270–275.
- El-Banna, A.A., El-Razek, A.M., El-Mahdy, A.R., Isolation, Identification and Screening of Carotenoid-Producing Strains of Rhodotorula glutinis. Food and Nutrition Sciences., 2012; (3): 627-633.
- Naziri, D., Hamidi, M., Hassanzadeh, S., Tarhriz, V., Zanjani, B.M., Nazemyieh, H., Analysis of carotenoid production by Halorubrum sp. TBZ126;an extremely halophilic archeon from Urmia Lake. Adv Pharm Bull, 2014;.4:61–67.
- Malisorn, C., Suntornsuk, W., Optimization of b-carotene production by Rhodotorula glutinis DM28 in fermented radish brine. Bioresource Technology., 2008; 99: 2281–2287.
- Domingues ,L., Dantas, M.M., Lima, N., Teixeira, J.A., Continuous ethanol fermentation of lactose by a recombinant flocculating Saccharomyces cerevisiae strain. Biotechnology and Bioengineering., 1999; 64(6): 692-697.
- Mata-Gomez, L.C., Mendez-Zavala, A., Aguilar, C.N., Montantez, J.C., Effect of Nitrogen Source on Carotenoid Production By Rhodotorula glutinis P4M422 in a medium based on goat milk whey. AIChE Annual Meeting. 2014; 384609
- Deak, T., Beuchat, L.R., Handbook of Food Spoilage Yeasts. CRS Press, New York, 1996; 111-154.
- Yadav, S., Prabha, R., Production of Intracellular Carotenoid Pigment from Wild Strains of Rhodotorula. International Journal of Current Microbiology and Applied Sciences., 2017; 6: 679-683.
- Kurtzman, C. P., Fell, J. W., (editors). The Yeasts, a Taxonomic Study, 4th edn. Amsterdam: Elsevier, 1998.
- Al-Turki, A.I., Al-Hassan, A.A., Abdel-Razik, M.M., Isolation and characterization of carotenoid producing yeasts from Qassim region. Journal of Food, Agriculture Environment., 2016; 14: 20-23.
- Davies, B.H., Jones, D., Goodwin, T.W., Studies in carotenogenesis. The problem of lycopersene formation in Neurospora Crassa. Biochem J., 1963; 87: 326.
- Wang, Q.M., Yurkov, A.M., Göker, M., Lumbsch, H.T., Leavitt, S.D., Groenewald, M., Phylogenetic classification of yeasts and related taxa within Pucciniomycotina. Stud. Mycol., 2015; 81: 149–189.
- Latha, B.V., Jeevaratnam, K., Purification and characterization of the pigments from Rhodotorula glutinis DFR-PDY isolated from natural source. Global J. Biotechnol. Biochem., 2010; 5: 166-174.
- Moliné, M., Libkind, D., Broock, M.V., Production of torularhodin, torulene and b- carotene by Rhodotorula yeasts. Methods Mol Biol., 2012; 898:275–83.
- Ueno, R., Hamada-Sato, N., Ishida, M., Naoto Uranon, N., Potential of carotenoids in aquatic yeasts as a phylogenetically reliable marker and natural colorant for aquaculture. Bioscience, Biotechnology and Biochemistry., 2011; 75: 1654-1661.
- Marova, I., Carnecka, M., Halienova, A., Koci, R., Breierova, E., Production of carotenoid/ ergosterol-supplemented biomass by red yeast Rhodotorula glutinis grown under external stress. In Food Technology and Biotechnology., 2010; 48(1): 56-61.
- Tkáová, J., Taplová, J., Klempová, T., Tertík, M., Correlation between lipid and carotenoid synthesis in torularhodin-producing Rhodotorula glutinis. Ann Microbiol. , 2017; 67:541–551.
- El-Banna, A.A., Abd El-Razek, A.M., El-Mahdy, A.R., Some Factors Affecting the Production of Carotenoids by Rhodotorula glutinis var. glutinis. Food and Nutrition Sciences, 2012; 3: 64-71.
- Cutzu, A., Clemente, A., Reis, A., Nobre, B., Mannazzu, I., Roseiro, J., da Silva, T.L., Assessment of b-carotene content, cell physiology and morphology of the yellow yeast Rhodotorula glutinis mutant 400A15 using flow cytometry. J Ind Microbiol Biotechnol., 2013; 40: 865–875.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


