ISSN: 0973-7510
E-ISSN: 2581-690X
Chronic suppurative otitis media (CSOM) is a disease of multiple etiology and is known for its persistence and recurrence despite the treatment. The antibiotic resistance of the micro-organisms due to its inappropriate use make this potential dangerous condition difficult to treat. Hence the knowledge of the local epidemiology of the organism and its susceptibility to an antibiotic is essential to initiate an effective treatment. Objectives: 1. To study the bacterial and fungal isolates of Chronic Suppurative Otitis Media (CSOM); 2. To study the antibiotic susceptibility pattern of the isolates. The study was conducted among 100 cases with CSOM and 20 controls without CSOM attending ENT outpatient department at KIMS hospital for a duration of one year. The standard conventional method of isolation and identification was followed for isolating the organisms. The antibiogram for the isolates were determined using Kirby Bauer disk diffusion method. The data was analyzed using standard statistical package. Majority 30.0% among the cases with CSOM were in the age group of 21-30 yrs. 76.0% yielded single organism and 18.0% yielded multiple isolates. Among the cases with single isolates, Pseudomonas aeruginosa (44.7%) was the commonest isolate followed by Staphylococcus aureus (34.2%). Proteus mirabilis + Klebsiella oxytoca was the highest yielded (27.7%) isolate among the multiple isolates. Aspergillus species predominated the fungal isolates. 94.6% of the Pseudomonas aeruginosa isolates was susceptible to piperacillin followed by 67.6% to Gentamycin and 58.5% to Ciprofloxacin. 90.0% of Staphylococcus aureus isolates were sensitive to Gentamycin and Cotrimoxazole followed by Ceftriaxone (87.8%) and 66.6% were sensitive to penicillin. Among all the drugs, gentamycin was found be the effective drug for majority of the bacterial isolates. The study suggests that the common etiological agents for CSOM were Pseudomonas aeruginosa and Aspergillus niger. Gentamycin was the most sensitive drug for treatment of CSOM.
Chronic Suppurative Otitis Media, Bacterial isolates, Fungal isolates, Antibiotic susceptibility, Antibiogram.
Chronic Suppurative Otitis Media (CSOM) is a commonly encountered infection of the middle ear. CSOM is defined as the chronic inflammation of middle ear and mastoid cavity that may present with recurrent ear discharges or otorrhoea through a tympanic perforation.1 Incidence of this disease is relatively higher in developing countries like India especially among low socio-economic society because of malnutrition, overcrowding, poor hygiene, inadequate health care and recurrent upper respiratory tract infection.2 It is one of the most common diseases of all age groups, especially of childhood.3 It is a disease with high risk of irreversible complications which may range from persistent otorrhoea, mastoiditis, labyrinthitis, facial palsy to more serious intracranial abscesses or thromboses. 4,5 Chronic suppurative otitis media (CSOM) is a disease of multiple etiology and is well known for its persistence and recurrence, despite treatment.1 Complications associated with CSOM were frequent in pre-antibiotic era, however, the introduction of antibiotics gave clinicians a tool to be used even without the precise etiological diagnosis and the irrational use of antibiotics led to the emergence of multi-drug resistant bacterial strains and disease complication in return. With the advent of anti-inflammatory and anti-histamine agents; and also with poor follow up of the patients have resulted in persistent low grade infections. All these factors have resulted in changes in bacterial flora in CSOM, which have been confirmed in the last decade and described by various authors. 6-10 With the development and widespread use of antibiotics, the prevalence and antibiogram of these organisms has been reported to vary with time and geographical area.10 All these factors increase the relevance of reappraisal of the modern day flora in CSOM and their in-vitro antibiotic sensitivity pattern. The knowledge of which is very important for the clinician to plan a treatment outline of the current day patients. It becomes necessary to decrease the potential risk of complications of CSOM by early institution of appropriate treatment. Hence the current study was done to study the bacterial and fungal isolates of the CSOM patients attending a tertiary care center.
Objectives
- To study the bacterial and fungal isolates of Chronic Suppurative Otitis Media (CSOM)
- To study the antibiotic susceptibility pattern of the isolates.
A comparative study was conducted in Kempegowda Institute of Medical Sciences, a private tertiary care center in Bangalore. By convenience sampling, 100 cases were selected for the study from the patients attending the ENT OPD of the hospital based on the inclusion and exclusion criteria. Inclusion criteria – The patients diagnosed as suffering from CSOM after thorough clinical evaluation by an ENT Surgeon. Exclusion criteria – 1.Patients suffering from any other ear infections like Otitis externa etc.,; 2.Patients who have used any local applications or on any treatment; 3.Patients suffering from any chronic systemic illnesses. For the study, 20 controls were selected from the patients attending the ENT OPD for complaints other than discharge from the ear and not suffering from CSOM. These patients had normal dry ear.
From all the study subjects, information regarding demographic and clinical details like side of the ear involved, presence of cholesteotoma, previously treated for the same condition was collected. Four samples from the ear was taken using sterile cotton swab sticks under aseptic precautions, labeled and taken to laboratory immediately for processing. Of the 4 swabs, one was used for Gram’s staining, by which pus cells, epithelial cells, gram’s positive and negative organisms were identified and recorded. If more than five epithelial cells were present, then the sample was discarded and repeat sample was taken. Second swab was used for streaking Blood agar (BA), Chocolate agar (CA)and Mac Conkey agar (MA), third one for KOH; forth one to streak Sabarauds Dextrose agar without cyclohexamide.
Isolation of bacteria
Isolation of fungi
One of the swabs was used for KOH mount and another for streaking SDA and incubated at room temperature and observed daily for growth upto 2 weeks. The growth was observed for – rate of growth, morphology, texture and surface pigmentation. Gram’s staining was done to identify yeast and yeast like cells. Sugar fermentation and assimilation, chlamydospore formation and germ tube tests were done to identify candida species. For confirmation of the particular fungal pathogen, a repeat sample was taken and only if the same isolate was repeatedly cultured, then it was considered as the etiological agent.
Antibiogram Testing
For antibiotic susceptibility testing, five colonies from the culture plate were inoculated into 2 ml of peptone water. It was incubated for 2 hrs at 370C. A cotton swab was immersed in this inoculum and then used for carpet streaking on Muller Hilton agar plate. The required antibiotic discs were then placed aseptically on this, which was incubated for 24 hrs at 370C. Next day, the zone size was recorded and reported as sensitive or resistant by comparing the zone size to the Kirby Bauer chart. If the organisms were not sensitive to any of the drugs, then a second line of antibiotic was used using the same procedure.
The drugs used were: Pencillin (10 U – P), Ampicillin (10 mg – A), Pipercillin (100mg – Pc), Erythromycin (15 mg – E), Co-trimoxazole (25 mg – Co), Gentamycin (10 mg – G), Ceftrioxone (10 mg), Ciprofloxacin (5 mg – Ci), Cloxacillin (5 mg – Cx), Tetracycline (30 mg – T), Cefotaxime (30 mg – Cf). Second line of drugs were: Amoxyclav (30 mg – Ac), Doxycycline (30 mg), Cefuroxime (30mg – Ce), Cefepime (30 mg), Cefdinir (5 mg), Ceftazidime (30 mg), Clindamycin (2 mg), Vancomycin (30 mg), Amikacin (30 mg – Ak), Netilmicin (30 mg).
Statistical analysis
The data collected was entered onto Microsoft excel. It was analyzed using standard statistical package for descriptive statistics and chi-square test was done with P value < 0.05 was set as level as significance
Majority i.e., 30/100, 30.0% among the cases and 7/20, 35.0% among the controls were in the age groups of 21-30 yrs and d 10 yrs respectively. The proportion of males (50/100) and females (50/100) were equally distributed among the cases and majority i.e., 14 (70.0%) among the controls were males. CSOM was most commonly seen in the Left ear among the cases (51/100. 51.0%) and right ear among the controls (11/20, 55.0%). The cases and controls were not significantly different with respect to age, gender and the side of involvement and hence comparable to each other (P>0.05). (Table 1)
Table (1):
Characteristics of study population.
| Characteristics of the study participants | |||
|---|---|---|---|
| Age in years (%) | Cases (n=100) | Controls (n=20) | P-value |
| ≤ 10 | 23 (23.0) | 07 (35.0) | χ2 = 1.54
P>0.05 |
| 11-20 | 18 (18.0) | 03 (15.0) | |
| 21-30 | 30 (30.0) | 06 (30.0) | |
| >30 | 29 (29.0) | 04 (20.0) | |
| Gender (%) | |||
| Males | 50 (50.0) | 14 (70.0) | χ2 = 2.68
P>0.05 |
| Females | 50 (50.0) | 6 (30.0) | |
| CSOM (Side of the ear) (%) | |||
| Left | 51 (51.0) | 07 (35.0) | χ2 = 6.91
P>0.05 |
| Right | 35 (35.0) | 11 (55.0) | |
| Bilateral | 14 (14.0) | 02 (10.0) | |
Figures in the parenthesis indicates column percentage (%)
Among the cases, single organism was isolated in 76/100, 76.0% while 18/100, 18.0% yielded multiple isolates. In the remaining 6/100, 6.0% of the cases, the culture remained sterile. Among the cases with single isolates, Pseudomonas aeruginosa (34/100, 44.7%) was the commonest isolate followed by staphylococcus aureus (26/76, 34.2%), Proteus mirabilis (12/76, 15.7%), Klebsiella (2/76, 2.6%), Streptococcus pyogenes (1/76, 1.4%) and Aspergillus niger (1/76, 1.4%). Among the cases with multiple isolates, Proteus mirabilis + Klebsiella oxytoca was the highest yielded (5/18, 27.7%) isolate and Staphylococcus aureus + Klebsiella oxytoca, Staphylococcus aureus + NFGNB, Staphylococcus aureus + Aspergillus niger + Candida albicans was least yielded (1/18, 5.5%) each.
Among the controls, single organism was isolated in 16/20, 80.0% of the controls while 1/20, 5.0% yielded multiple isolates. In the remaining 3/20, 15.0% of the controls, the culture remained sterile. Among the controls with single isolates, Staphylococcus epidermidis (15/16, 93.7%) was the commonest isolate followed by Diphtheroids (01/16, 6.3%). The only multiple isolate which was isolated on culture was Staphylococcus epidermidis + Micrococci among the controls. (Table-2)
Table (2):
Distribution of bacterial and fungal isolates among the cases and controls with CSOM.
Organisms |
Cases (%) |
Controls (%) |
|---|---|---|
(n=100) |
(n=20) |
|
Sterile |
6 (6.0%) |
3 (15.0%) |
Single Isolates |
76 (76.0%) |
16 (80.0%) |
Pseudomonas aeruginosa |
34 (44.7) |
— |
Staphylococcus aureus |
26 (34.2) |
— |
Proteus mirabilis |
12 (15.7) |
— |
Klebsiella |
02 (2.6) |
— |
Streptococcus pyogenes |
01 (1.4) |
— |
Aspergillus niger |
01 (1.4) |
— |
Staphylococcus epidermidis |
— |
15 (93.7) |
Diphtheroids |
— |
01 (6.3) |
Multiple isolates |
18 (18.0%) |
01 (5.0%) |
Proteus mirabilis + Klebsiella oxytoca |
05 (27.7) |
— |
Proteus mirabilis + NFGNB |
03 (16.7) |
— |
Staphylococcus aureus + Streptococcus pyogenes |
03 (16.7) |
— |
Pseudomonas aeruginosa + NFGNB |
02 (11.2) |
— |
Pseudomonas aeruginosa + Klebsiella oxytoca |
02 (11.2) |
— |
Staphylococcus aureus + Klebsiella oxytoca |
01 (5.5) |
— |
Staphylococcus aureus + NFGNB |
01 (5.5) |
— |
Staphylococcus aureus + Aspergillus niger + Candida albicans |
01 (5.5) |
— |
Staphylococcus epidermidis + Micrococci |
— |
01 (100.0) |
Among the 11 cases who were treated earlier for the same condition of CSOM, Proteus mirabilis was the highest forming 45.4% followed by Pseudomonas aeruginosa, Staphylococcus aureus and NFGNB all forming 18.1% each, Klebsiella species, Streptococcus pyogenes and Aspergillus niger formed 9.1% each.
Out of 27 cases with cholesteatoma, Pseudomonas aeruginosa was isolated in 12 cases with 44.4 %. Second highest was Proteus mirabilis 11 i.e., 40.70% followed by Staphylococcus aureus 04 i.e., 14.80% and Klebsiella species 04 i.e., 14.80%. NFGNB was isolated only in 1 case forming 3.7%.
The organisms isolated were subjected to antibiotic susceptibility where they were susceptible to the following antibiotics: Amikacin (Ak), Gentamycin (G), Ciprofloxacin (Ci), cefotaxime (Cf), Polymyxin-B (Pb), Tetracycline (T), Erythromycin (E), Ampicillin (A), Pencillin (P), cloxacillin (Cx), cefuroxime (Ce), Cotrimoxazole (Co) and for Pseudomonas aeruginosa, piperacillin (Pc).
Table (3):
Antibiotic Susceptibility Pattern of the isolates.
Isolates |
A |
Co |
Cf |
G |
Ci |
Pc |
T |
Ce |
E |
P |
Cx |
Ak |
Pb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Pseudomonas aeruginosa |
12 |
12 |
22 |
25 |
19 |
35 |
12 |
18 |
— |
— |
— |
01 |
03 |
Staphylococcus aureus |
— |
30 |
28 |
30 |
29 |
— |
28 |
— |
06 |
22 |
29 |
— |
— |
Proteus mirabilis |
13 |
16 |
16 |
18 |
18 |
— |
07 |
15 |
— |
— |
— |
— |
— |
Kleibsiella |
00 |
08 |
08 |
09 |
06 |
— |
01 |
07 |
— |
— |
— |
— |
— |
NFGNB |
03 |
02 |
02 |
02 |
03 |
— |
02 |
03 |
— |
— |
— |
— |
— |
Streptococcus pyogenes |
— |
01 |
03 |
04 |
04 |
— |
05 |
— |
01 |
05 |
05 |
— |
— |
Total |
28 |
69 |
79 |
88 |
79 |
35 |
35 |
43 |
07 |
27 |
34 |
01 |
03 |
In the present study of 100 cases, 6 cases remained sterile on culture. Among the positive cases of 96/100, 94 were positive for bacterial growth and 2 cases were positive for fungal growth.
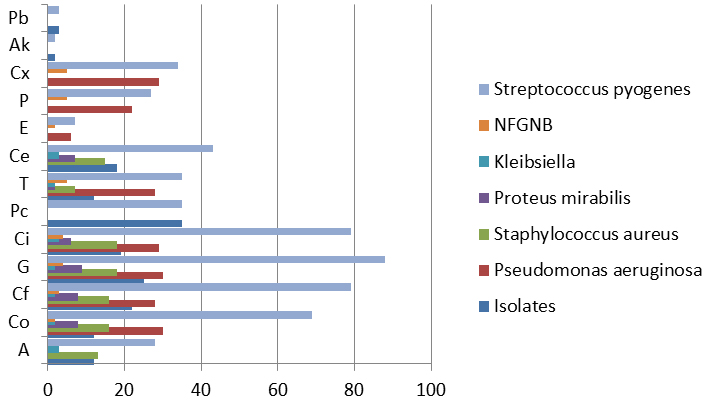
94.6% of the Pseudomonas aeruginosa isolates was susceptible to piperacillin followed by 67.6% to Gentamycin and 58.5% to Ciprofloxacin. 90.0% of Staphylococcus aureus isolates were sensitive to Gentamycin and Cotrimoxazole followed by Ceftriaxone (87.8%) and 66.6% were sensitive to penicillin. 90.0% of Proteus isolates were sensitive to Gentamycin and Ceftriaxone respectively followed by Cotrimoxazole and Ciprofloxacin of 80.0%.
Chronic suppurative otitis media (CSOM) is an important cause of preventable hearing loss, particularly in the developing world which is also a reason of serious concern particularly in children because of long term effects on early communication, language development, auditory processing, educational process and physiological and cognitive development. Further CSOM can also progress and lead to the complications like intracranial or extracranial extension which needs an emergency mastoidectomy accounting for additional costs and expenditures for the patient and if not taken appropriate care might also take a death toll. Hence early microbiological diagnosis and testing for antibiotic susceptibility for bacterial isolates aids in initiating early prompt and effective treatment.11
Rani RU et al., has reported that the majority of the participants were in the age group 0-15 yrs and the age of the study participants ranged from 3-70 yrs and 47% of them were males and 53.0% were females which are similar to the current study where majority were in the age group 0-20 yrs.12 Similarly the disease was more common in the first and second decades of life as reported by Poorey VK and Iyer A.13
Prakash R et al., has observed mono-microbial growth in 57.8% samples, followed by 33.3% samples yielded polymicrobial growth, whereas, 8.82% samples showed no growth14 and Rangaiah ST et al., in their study single microbial isolate was found in 80%, multiple bacteria were isolated in 5.2% and 14.8% were reported with sterile culture or no growth of microbes similarly in the current study, 76.0% yielded single isolate, 18.0% yielded multiple isolates and remaining 6.0% of the cases reported with sterile culture or no growth of microbes.15
The study by Chandrashekhar M R et al., has reported in their study that Pseudomonas aeruginosa predominated (46.7%) the growth followed by Staphylococcus aureus 17.9%, Klebsiella species 12.2% and citrobacter species 11.51%. similar to the current study findings.16
Various studies have reported varied results with respect to the microbial culture. Some studies have reported Pseudomonas aeruginosa as the most commonly isolated organism in CSOM in few studies and Staphylococcus aureus as the most commonly isolated organism in CSOM in some other studies.14-23
Rangaiah ST et al., have found no anaerobes in the culture which is similar to the present study findings and similarly, studies by Maji et al., Ibekwe et al., and Indudharan et al., also showed negligible anaerobic isolates in their study.15. 24-26
In the current study, the most common species (27.7%) identified among the multiple isolates was Proteus and Klebsiella species, however in a study conducted by Garima et al.,the most common (75.0%) species were the combination of Pseudomonas and Klebsiella species. The proportions (14.0%) of Proteus and Klebsiella species identified by Garima et al., were less compared to the current study.27 The disparity could be attributed to difference in methodology, sample size and prior use of antibiotics and also it is noted that different pathogens cause otitis media in different geographic localities.28
Aspergillus species predominated of the fungal isolates, which was similar with the study findings conducted by Vaidya K et al and Shrestha et al. 29,30
Pseudomonas aeruginosa was found to be the most common isolate in CSOM with cholesteatoma in a study by Rathore VS et al., similarly in the current study Pseudomonas aeruginosa was the only isolated organism among cases of CSOM with cholesteatoma. 31
Upon analyzing the antibiotic sensitivity results it was evident that the antibiotics with the highest bacterial susceptibility in this study were gentamycin followed by ciprofloxacin and cefotaxime: this is in line with a study conducted by Garba BI et al who found highest bacterial susceptibility rate in this study were ciprofloxacin, ofloxacin and gentamicin. 28
Most common organisms isolated were Pseudomonas aeruginosa followed by Staphylococcus aureus. Among them, Pseudomonas aeruginosa had highest sensitivity to Piperacillin (94.6%) followed by Gentamycin and Ciprofloxacin. The findings were similar to various studies by Garima et al (80%)28, Malkappa et al (83%)32, Agarwal et al (85.4%)33 which showed high sensitivity to betalactam and beta-lactamase inhibitors whereas according to the study findings of Kumar S et al34 and Chakraborty et al35, Pseudomonas aeruginosa isolates had shown resistance to piperacillin but sensitivity was moderately good for Gentamicin, and ciprofloxacin. The difference in the antibiotic susceptibility pattern may be due to prior antibiotic therapy which might have caused antibiotic resistance.
Staphylococcus aureus was highly susceptible to Gentamycin and Cotrimoxazole which is similar to the findings of Malkappa SK et al32, Gulati et al.,36 and Mishra et al.37 who also found Staphylococcus aureus sensitive to gentamicin.32 In the present study, gentamicin with high susceptibility pattern is more effective in treating CSOM as majority of pathogens in CSOM cases are susceptible to it.38
It can be concluded that a variety of bacteria are responsible for CSOM with predominance of Pseudomonas aeruginosa either alone or in combination with other bacteria. The antimicrobial sensitivity of the isolates showed Gentamycin to be the drug of choice, followed by Ciprofloxacin for treating cases of CSOM due to either Gram positive or Gram negative organisms.
More comprehensive studies are required to define the true magnitude of CSOM, to determine the microbiological profile of isolates and produce data for policy decision on optimal intervention modalities.
- Acuin J. Geneva: World Health Organisation; 2004. Global burden of disease due to chronic suppurative otitis media: Disease, deafness, deaths and DALYs Chronic Suppurative Otitis Media–Burden of Illness and Management Options; pp. 9–23.
- Zhang, Y., Xu, M., Zhang, J., Zeng, L., Wang, Y., Zhang, Q.Y. Risk Factors for Chronic and Recurrent Otitis Media A Meta Analysis. PloS ONE., 2014; 9 (1): e86397.
- Daly K, Hunter L, Levine S, Lindgren B, Giebink G. Relationships between otitis media sequelae and age. Laryngoscope. 1998; 108:1306-1310.
- Loy AHC, Tan AL, Lu PKS. Microbiology of chronic suppurative otitis media in Singapore. Singapore Med J. 2002; 43:296–299.
- Verhoeff M, Veen EL, Rovers MM, Sanders EAM, Schilder AGM. Chronic suppurative otitis media: a review. Int J Pediatr Otorhinolaryngol. 2006; 70:1–12.
- Kristo B, Buljan M. Microbiology of the chronic suppurative otitis media. Med Glas (Zenica). 2011; 8(2):284-6.
- Nandy A, Mully PS, Sivarajan K. Chronic suppurative otitis media – A bacteriological study. Indian J Otol 1991; 43: 136-8.
- Rout MR, Mohanty D, Vijaylaxmi Y, Kamalesh B, Chakradhar M. Prevalence of cholesteatoma in chronic suppurative otitis media with central perforation. Indian J Otol 2012; 18:7-10.
- Kumar H, Seth S. Bacterial and fungal study of 100 cases of chronic suppurative otitis media. J Clin Diagn Res 2011; 5:1224-7.
- Hassan O, Adeyemi. A Study of Bacterial Isolates In Cases of Otitis Media in patients Attending Oautch, Ile- Ife. Afr J Exper Microbiol 2007; 8: 130-6.
- Chronic suppurative otitis media burden of illness and management options, World Health Organisation,Geneva, Switzerland 2004.
- Rani RU, Satyanarayana O, Suryakirani KRL. Bacterial and Fungal Study of 100 Cases of Chronic Suppurative Otitis Media. IJSR 2015; 4(8):125-6.
- Poorey VK. Study of bacterial flora in CSOM and its clinical significance. Indian Journal of Otolaryngology and Head and neck surgery. 2002; 1; 54(2):91-5.
- Prakash R, Juyal D, Negi V, Pal S, Adekhandi S, Sharma M, Sharma N. Microbiology of chronic suppurative otitis media in a tertiary care setup of Uttarakhand state, India. North American journal of medical Sciences. 2013; 5(4):282.
- Rangaiah ST, Dudda R, Prasad MH, Balaji NK, Sumangala B, Gudikote MM. Bacteriological profile of chronic suppurative otitis media in a tertiary care hospital. International Journal of Otorhinolaryngology and Head and Neck Surgery. 2017; 3(3):601-5.
- Chandrasekhar MR, Krishna BV, Patil BA. A bacteriological profile of CSOM with pseudomonas aeruginosa as the prime pathogen. Ind J Otol. 2004; 10:10-3.
- Kumar S, Sharma R, Saxena A, Pandey A, Gautam P, Taneja V. Bacterial flora of infected unsafe CSOM. Indian J Otol. 2012; 18:208-11.
- Kulkarni G, Damle A. Bacteriology of chronic suppurative otitis media and its complications. MRIMS J Health Sciences. 2015; 3(2):125-9.
- Bhan C, Purohit K, Purohit JP, Kumar V, Yadav HS. Clinical vs Bacteriological and Mycological Evaluation in Chronic Suppurative Otitis Media. Int J Contemporary Med Res. 2016; 3(5):1443-7.
- Prakash M, Lakshmi K Anuradha S, Swathi GN. Bacteriological profile and their antibiotic susceptibility pattern of cases of chronic suppurative otitis media. Asian J Pharm Clin Res. 2013; 6(3):210-2.
- Singh AH, Basu R, Venkatesh A. Aerobic bacteriology of chronic suppurative otitis media in Rajahmundry, Andhra Pradesh, India. Biol Med. 2012; 4(2):73-9.
- Nazir A, Kadri SM. Aerobic bacteriology of chronic suppurative otitis media: a hospital based study. Int J Res Med Sci. 2014; 2(4):1521-5.
- Kusuma Bai S, Venkateswarulu K, BalaKrishna, Ashokareddy, Rao N. Study of bacteriology in chronic suppurative otitis media. Int J Med Res Health Sci. 2013; 2(3):510-3.
- Ibekwe AO, al Shareef Z, Benayam A. Anaerobes and fungi in chronic suppurative otitis media. Ann Otol Rhinol Laryngol. 1997; 106:649-52.
- Maji PK, Chatterjee TK, Chatterjee S, Chakrabarty J, Mukhopadhyay BB. The investigation of bacteriology of chronic suppurative otitis media in patients attending a tertiary care hospital with special emphasis on seasonal variation. Indian J Otolaryngol Head Neck Surg. 2007; 59:128-31.
- Indudharan R, Haq JA, Aiyar S. Antibiotics in chronic suppurative otitis media: A bacteriologic study. Ann Otol Rhinol Laryngol. 1999; 108:440-5.
- Garima K, Chaurasia D, Poorey VK. Antimicrobial susceptibility pattern of bacterial isolates from chronic suppurative otitis media patients in Central India. Ind. J. Microbiol. Res. 2016; 3(4):373-382.
- Garba BI, Mohammed BA, Mohammed F, Rabiu M, Sani UM, Isezuo KO, Waziri UM. Antibiotic susceptibility pattern of bacterial isolates in children with otitis media in Zamfara, North-Western Nigeria. African Journal of Microbiology Research. 2017; 11(43):1558-63.
- Vaidya K, Madhup SK, Shrestha BL, Gautam A, Tuladha NR. Bacteriological and mycological profile of chronic suppurative otitis media among patients visiting Dhulikhel Hospital. Annals of Clinical Chemistry and Laboratory Medicine. 2015; 1(1):37-41.
- Shrestha BL, Amatya RCM, Shrestha I and Ghosh I. Microbiological profile of chronic suppurative otitis media. Nepalese J ENT Head Neck Surg 2011; 2(2):6-7.
- Rathore VS, Shekhawat KK. Prevalence of Pseudomonas aeruginosa in cholesteatoma patients in tertiary care hospital in North India. Int J Otorhinolaryngol Head Neck Surg 2018; 4:76-9.
- Malkappa SK, Kondapaneni S, Surpam RB, Chakraverti TK. Study of aerobic bacterial isolates and their antibiotic susceptibility pattern in Chronic Suppurative Otitis Media. Indian Journal of Otology, 2012; 18:136-139.
- Agrawal A, Dharmendra K, Ankur G et al. Microbiological profile and their antimicrobial sensitivity pattern in patients of otitis media with ear discharge. Indian J Otol 2013; 19:1.
- Kumar S, Sharma R, Saxena AK, Pandey A, Gautam P, Jain R. A study of bacterial flora and sensitivity to antibiotics in cases of CSOM TTD in western UP. Indian J Otol 2008; 14:20-4.
- Chakraborty A, Bhattacharjee A, Purkaystha P. Microbiological profile of chronic suppurative otitis media: Its significance in North-East India. Indian J Otol 2005; 11:39-44.
- Gulati SK. Investigative profile in patients of chronic suppurative otitis media. Indian J Otol 1997; 3:59-62.
- Anupam M, Girish S, Devika N, Chandra MS. Bacteriological study of chronic suppurative otitis media. Indian J Otol 1999; 5:87-91.
- Hegde MC, Bhat GK, Sreedharan S, Ninan GP. Bacteriological study of tubotympanic type of chronic suppurative otitis media. Indian J Otol 2005; 11:13-6.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


